Abstract
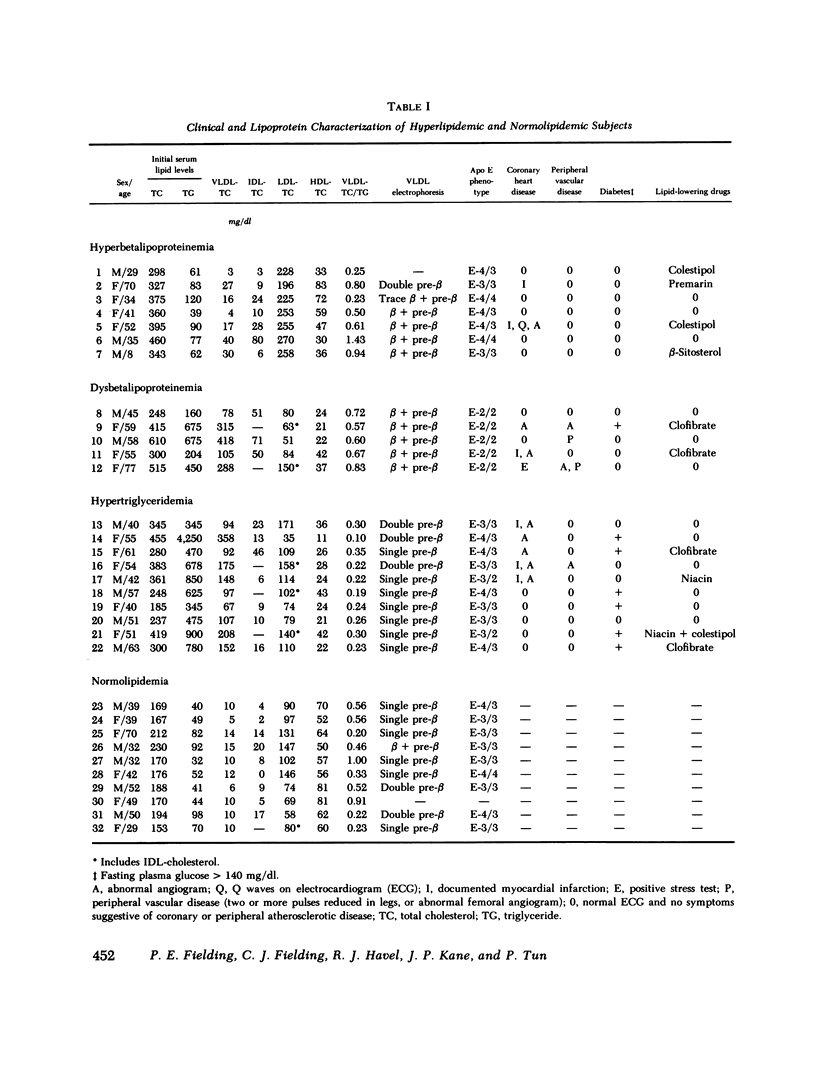
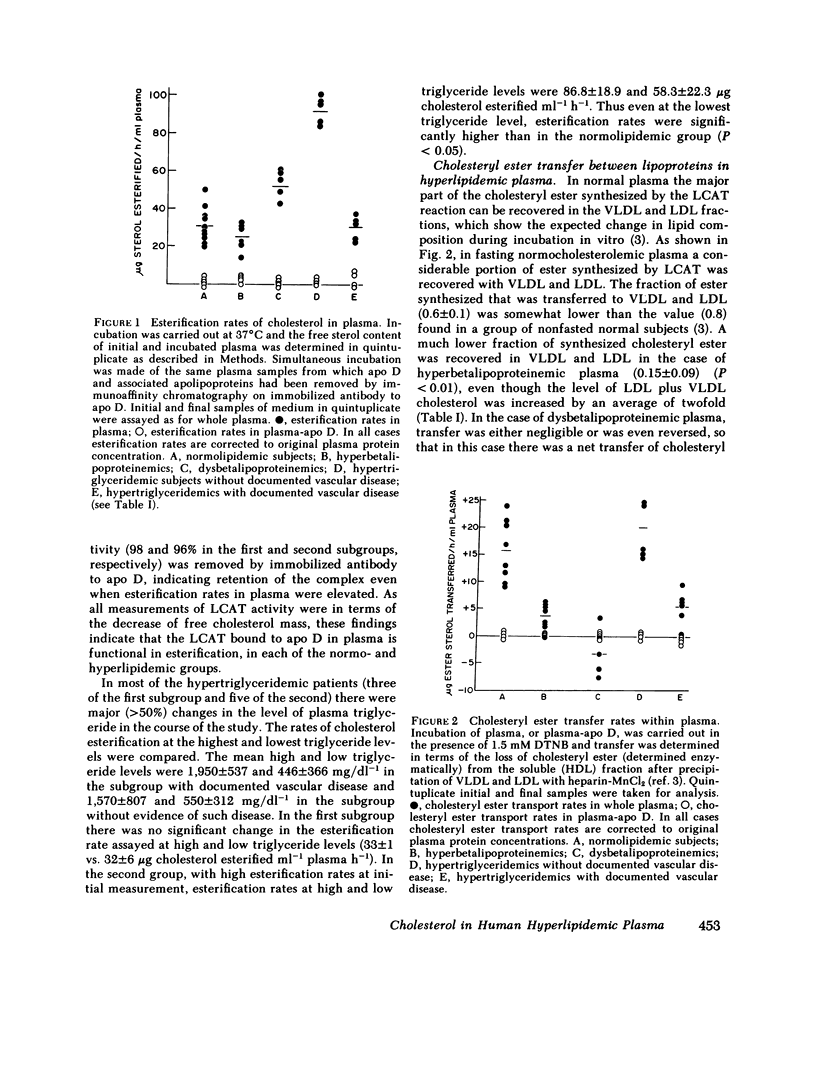
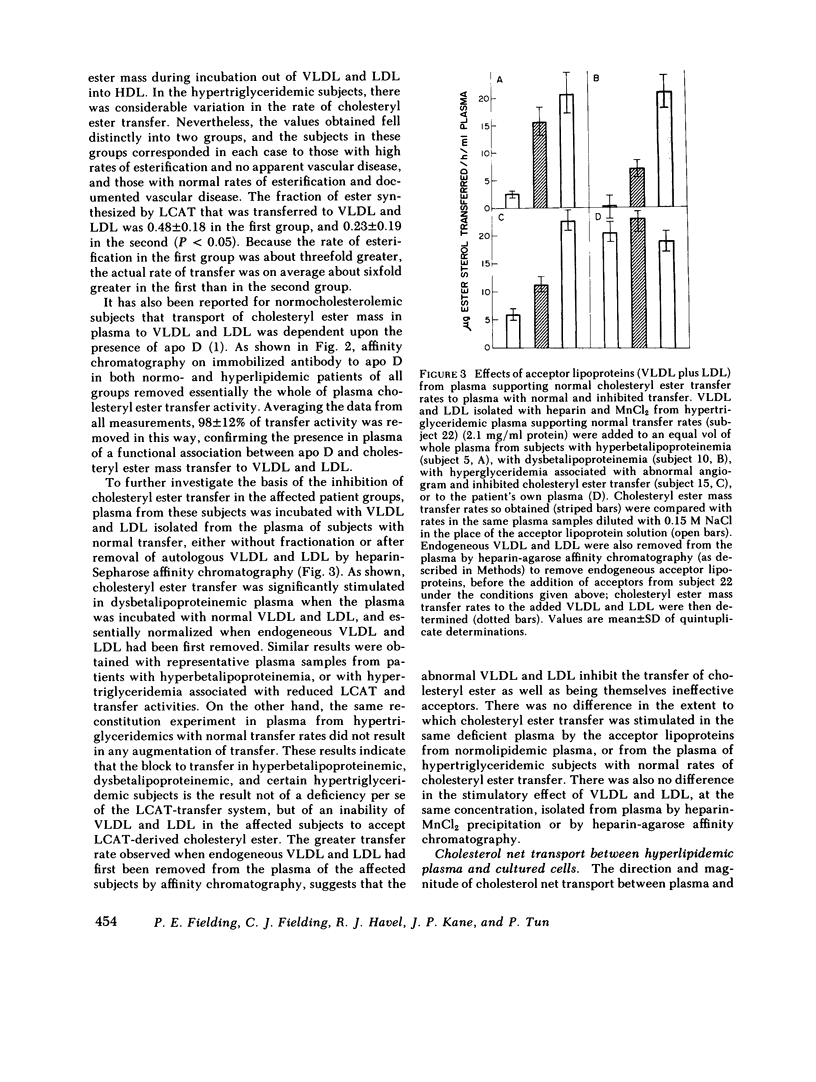
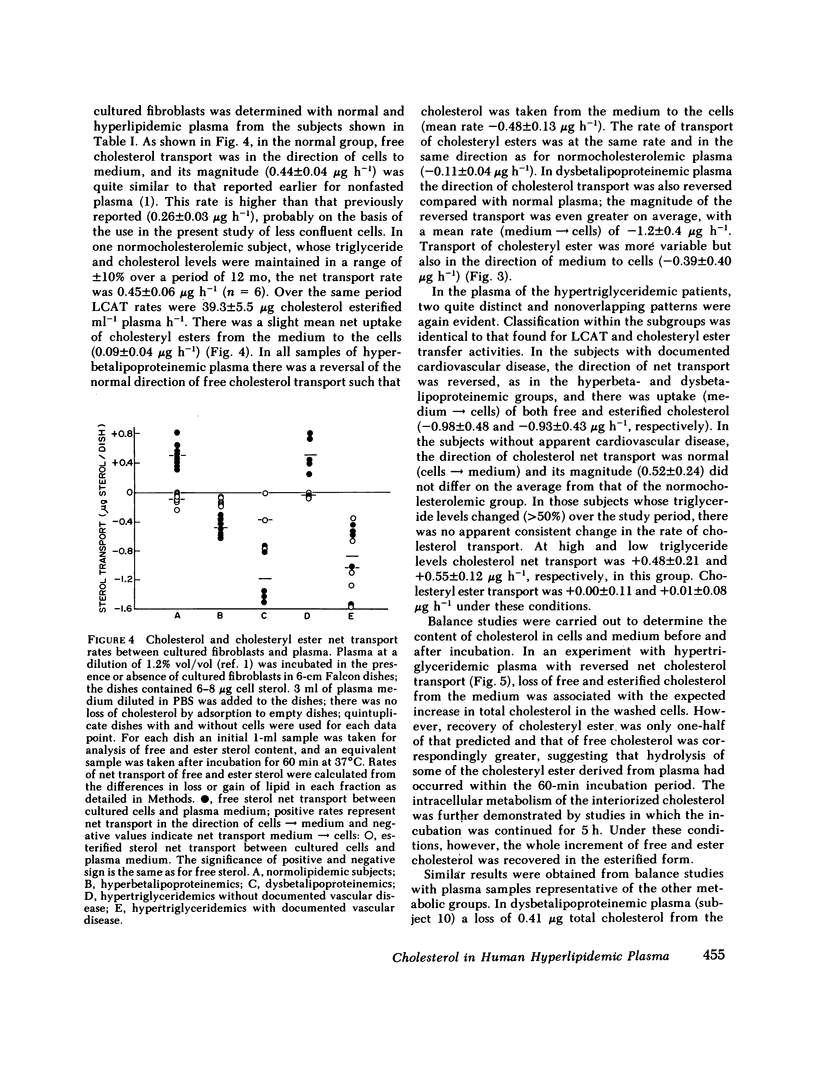
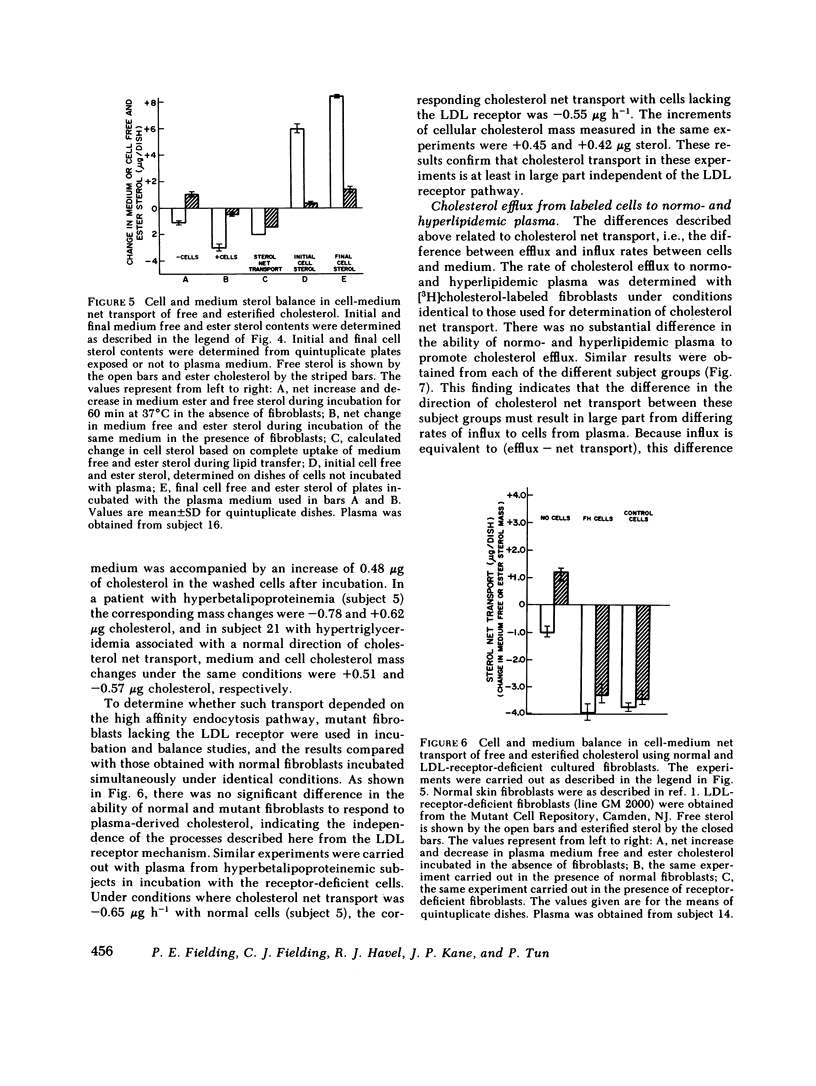
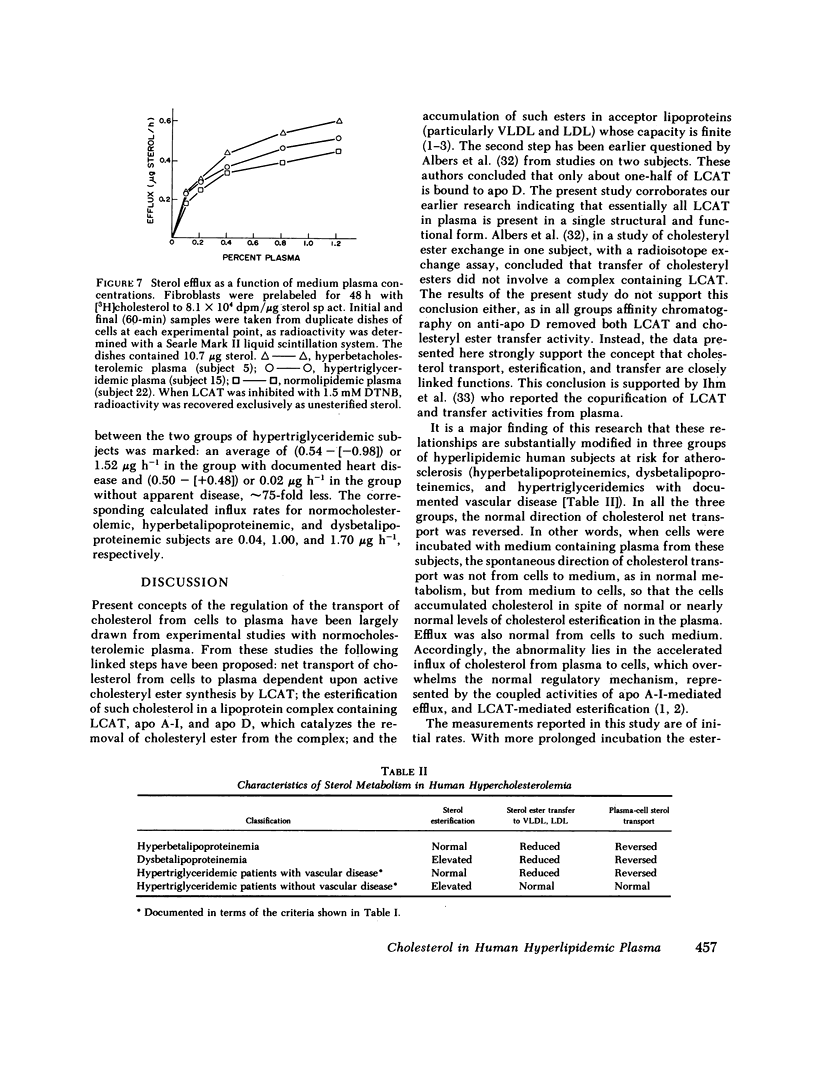
Cholesterol esterification, cholesteryl ester transfer between lipoproteins, and cholesterol transport between lipoproteins and cultured cells have been measured in the plasma of 22 patients with primary hyperlipidemia and 10 normolipidemic subjects. In hyperbetalipoproteinemia, increase in plasma low density lipoprotein levels was associated with a reduction of cholesteryl ester transfer rates, and with a reversal of the normal direction of sterol transport between fibroblasts and their plasma culture medium. Instead of net transport from cells to medium there was a net uptake of sterol from plasma by the cells, despite a level of plasma lecithin/cholesterol acyltransferase activity that was within the normal range. In dysbetalipoproteinemia, esterification rates were increased above normal levels, but cholesteryl ester transfer was reduced and the direction of sterol transport between the cells and plasma medium was reversed, as in the hyperbetalipoproteinemic group. In hypertriglyceridemia, those subjects with cardiovascular disease showed a metabolic pattern similar to the hyperbetalipoproteinemic group. The subjects in this group without symptoms of cardiovascular disease showed a normal direction of sterol transport, normal or raised rates of cholesteryl ester transfer between lipoproteins, and an increased rate of sterol esterification in plasma that decreased towards normal levels as plasma triglyceride levels decreased. Despite their quite distinct metabolic patterns there was no consistent difference between the two hypertriglyceridemic groups in triglyceride or cholesterol levels, very low density lipoprotein composition, or electrophoretic or isoelectric focussing patterns. All hypertriglyceridemic subjects with documented cardiovascular disease showed reversed cell-plasma sterol transport and all subjects without such disease showed a normal direction of cell-plasma sterol transport. The results of this study indicate major and reproducible abnormalities in plasma cholesterol metabolism in several groups of subjects with genetically distinct hyperlipidemias, who are at risk for atherosclerotic vascular disease. The possible predictive value of sterol metabolic measurements in the analysis of cardiovascular disease is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Cheung M. C., Ewens S. L., Tollefson J. H. Characterization and immunoassay of apolipoprotein D. Atherosclerosis. 1981 Jun;39(3):395–409. doi: 10.1016/0021-9150(81)90025-3. [DOI] [PubMed] [Google Scholar]
- Burstein M., Scholnick H. R., Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970 Nov;11(6):583–595. [PubMed] [Google Scholar]
- Castelli W. P., Doyle J. T., Gordon T., Hames C. G., Hjortland M. C., Hulley S. B., Kagan A., Zukel W. J. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977 May;55(5):767–772. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Cholesterol transport between cells and body fluids. Role of plasma lipoproteins and the plasma cholesterol esterification system. Med Clin North Am. 1982 Mar;66(2):363–373. doi: 10.1016/s0025-7125(16)31425-0. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Evidence for a lipoprotein carrier in human plasma catalyzing sterol efflux from cultured fibroblasts and its relationship to lecithin:cholesterol acyltransferase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3911–3914. doi: 10.1073/pnas.78.6.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Regulation of human plasma lecithin:cholesterol acyltransferase activity by lipoprotein acceptor cholesteryl ester content. J Biol Chem. 1981 Mar 10;256(5):2102–2104. [PubMed] [Google Scholar]
- Fielding C. J. Metabolism of cholesterol-rich chylomicroms. Mechanism of binding and uptake of cholesteryl esters by the vascular bed of the perfused rat heart. J Clin Invest. 1978 Jul;62(1):141–151. doi: 10.1172/JCI109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Vlodavsky I., Fielding P. E., Gospodarowicz D. Characteristics of chylomicron binding and lipid uptake by endothelial cells in culture. J Biol Chem. 1979 Sep 25;254(18):8861–8868. [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. A cholesteryl ester transfer complex in human plasma. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3327–3330. doi: 10.1073/pnas.77.6.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Hess B. The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J Biol Chem. 1980 Nov 25;255(22):10875–10883. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P. Primary dysbetalipoproteinemia: predominance of a specific apoprotein species in triglyceride-rich lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2015–2019. doi: 10.1073/pnas.70.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J Clin Invest. 1980 Dec;66(6):1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Ihm J., Harmony J. A., Ellsworth J., Jackson R. L. Simultaneous transfer of cholesteryl ester and phospholipid by protein(s) isolated from human lipoprotein-free plasma. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1114–1120. doi: 10.1016/0006-291x(80)90604-x. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Malloy M. J., Tun P., Phillips N. R., Freedman D. D., Williams M. L., Rowe J. S., Havel R. J. Normalization of low-density-lipoprotein levels in heterozygous familial hypercholesterolemia with a combined drug regimen. N Engl J Med. 1981 Jan 29;304(5):251–258. doi: 10.1056/NEJM198101293040502. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T., McNamara P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971 Jan;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis B., Chait A., Oakley C. M., Wootton I. D., Krikler D. M., Onitiri A., Sigurdsson G., February A. Serum lipoprotein abnormalities in patients with ischaemic heart disease: comparisons with a control population. Br Med J. 1974 Aug 24;3(5929):489–493. doi: 10.1136/bmj.3.5929.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippel K., Tyroler H., Eder H., Gotto A., Jr, Vahouny G. Relationship of hypertriglyceridemia to atherosclerosis. Arteriosclerosis. 1981 Nov-Dec;1(6):406–417. doi: 10.1161/01.atv.1.6.406. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., Vezina C. A method for the determination of the initial rate of reaction of lecithin: cholesterol acyltransferase in human plasma. Biochim Biophys Acta. 1973 Jun 21;306(3):497–504. doi: 10.1016/0005-2760(73)90188-4. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Rao S. N., Magill P. J., Miller N. E., Lewis B. Plasma high-density lipoprotein metabolism in subjects with primary hypertriglyceridaemia: altered metabolism of apoproteins AI and AII. Clin Sci (Lond) 1980 Nov;59(5):359–367. doi: 10.1042/cs0590359. [DOI] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Slack J., Mills G. L. Anomalous low density lipoproteins in familial hyperbetalipoproteinaemia. Clin Chim Acta. 1970 Jul;29(1):15–25. doi: 10.1016/0009-8981(70)90215-9. [DOI] [PubMed] [Google Scholar]
- St Clair R. W., Mitschelen J. J., Leight M. Metabolism by cells in culture of low-density lipoproteins of abnormal composition from non-human primates with diet-induced hypercholesterolemia. Biochim Biophys Acta. 1980 Apr 18;618(1):63–79. doi: 10.1016/0005-2760(80)90054-5. [DOI] [PubMed] [Google Scholar]
- Stewart C. P., Hendry E. B. The phospholipins of blood. Biochem J. 1935 Jul;29(7):1683–1689. doi: 10.1042/bj0291683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke K. T., Norum K. R. Determination of lecithin: cholesterol acyltransfer in human blood plasma. Scand J Clin Lab Invest. 1971 Feb;27(1):21–27. doi: 10.3109/00365517109080184. [DOI] [PubMed] [Google Scholar]
- Utermann G., Jaeschke M., Menzel J. Familial hyperlipoproteinemia type III: deficiency of a specific apolipoprotein (apo E-III) in the very-low-density lipoproteins. FEBS Lett. 1975 Aug 15;56(2):352–355. doi: 10.1016/0014-5793(75)81125-2. [DOI] [PubMed] [Google Scholar]
- Wilson D. E., Lees R. S. Metabolic relationships among the plasma lipoproteins. Reciprocal changes in the concentrations of very low and low density lipoproteins in man. J Clin Invest. 1972 May;51(5):1051–1057. doi: 10.1172/JCI106896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem. 1980 Sep 10;255(17):8303–8307. [PubMed] [Google Scholar]
- Witztum J. L., Schonfeld G., Weidman S. W., Giese W. E., Dillingham M. A. Bile sequestrant therapy alters the compositions of low-density and high-density lipoproteins. Metabolism. 1979 Mar;28(3):221–229. doi: 10.1016/0026-0495(79)90067-2. [DOI] [PubMed] [Google Scholar]