Abstract
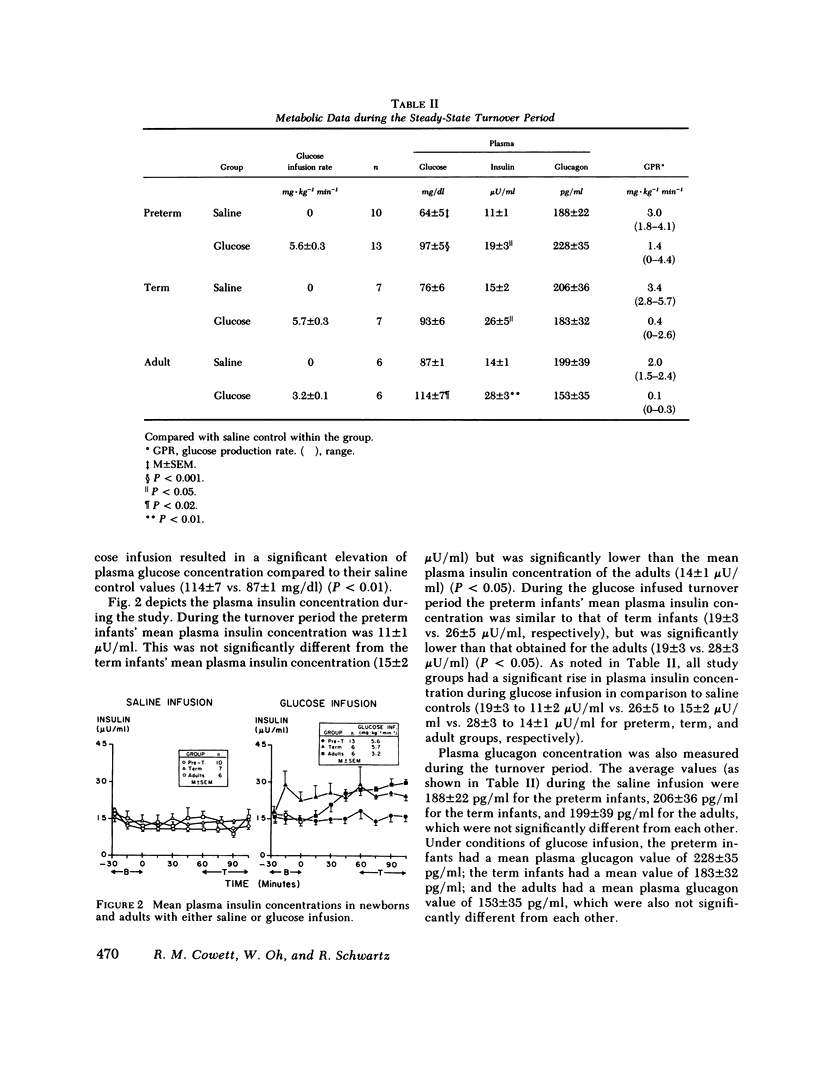
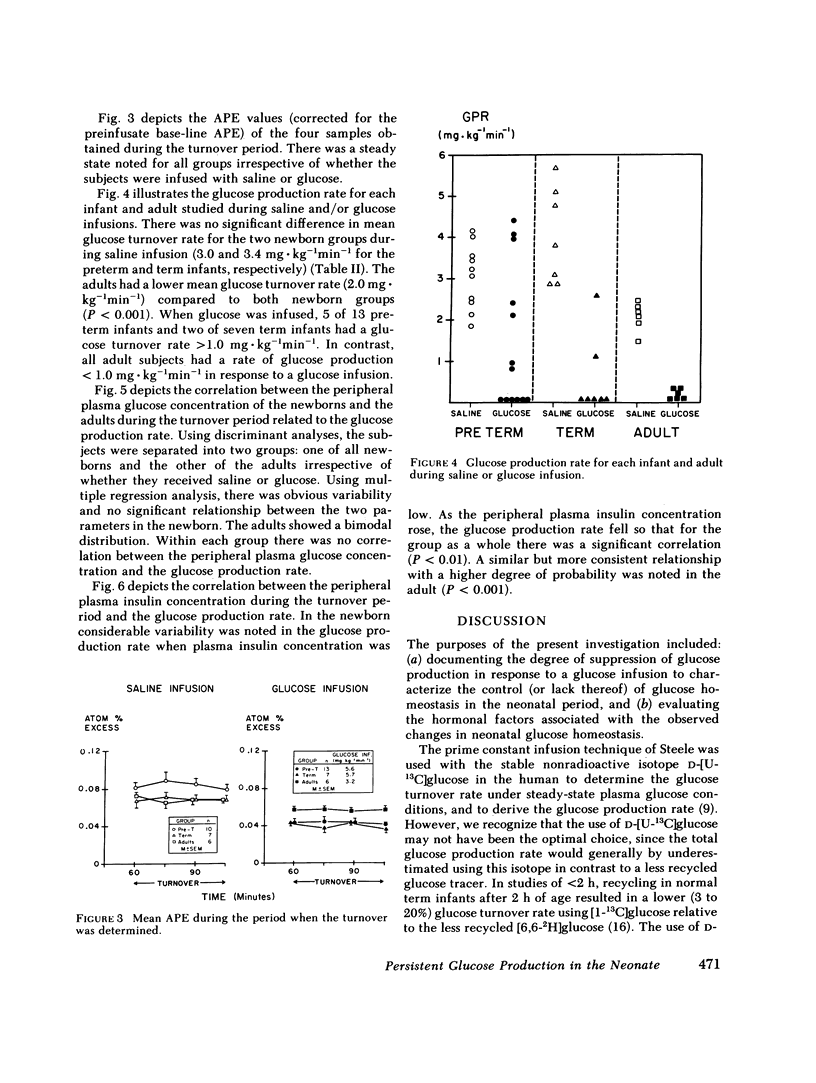
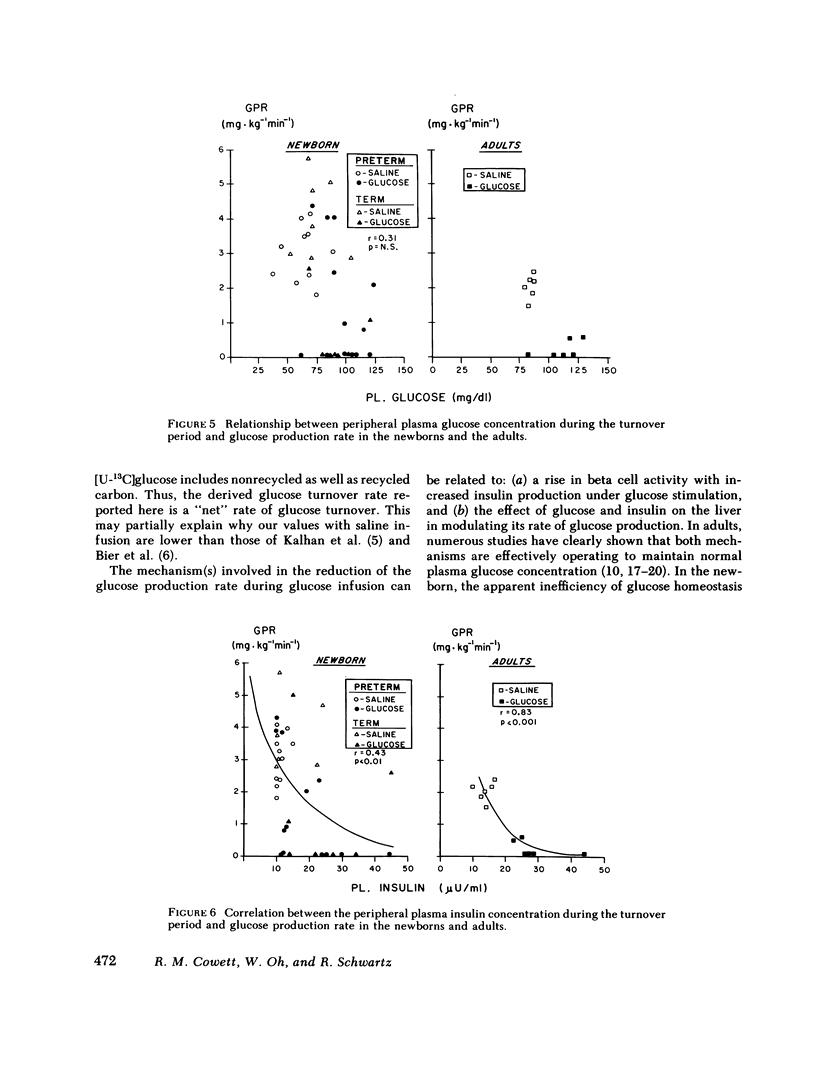
In adults, glucose infusion results in a decreased glucose production rate (GPR) as a mechanism for maintaining euglycemia. To document the development of glucose homeostasis, we derived the GPR in 23 preterm appropriate for gestational age infants, 14 term appropriate for gestational age infants, and in 6 adults. After a 3-h fast, the average plasma glucose and insulin concentration was measured and the GPR was derived. During glucose infusion (5.6 +/- 0.3 mg X kg-1 min-1), compared with saline controls, the preterms had a rise in plasma glucose and plasma insulin, and the GPR was 1.4 mg X kg-1 min-1 (range, 0-4.4) vs. 3.0 mg X kg-1 min-1 (range, 1.8-4.1) (saline controls). In the term infants, only the plasma insulin concentration was elevated when the glucose infused (5.7 +/- 0.3 mg X kg-1 min-1) infants were compared with the saline controls and GPR was 0.4 X kg-1 min-1 (range, 0-2.6) vs. 3.4 mg X kg-1 min-1 (range, 2.8-5.7) (saline controls). In comparison to saline infused adults, glucose infusion (3.2 +/- 0.1 mg X kg-1 min-1) resulted in a significant rise in plasma glucose and in plasma insulin; and the GPR was reduced to 0.1 mg X kg-1 min-1 (range, 0-0.3) from 2.0 mg X kg-1 min-1 (range, 1.5-2.4). 5 of 13 preterms and 2 of 7 term infants had persistent GPR during glucose infusion; in contrast, the GPR in all adults was unmeasurable. There was no correlation between the plasma glucose concentration and the GPR in the newborn or in the adult. Both newborns and adults did have a correlation between plasma insulin concentration and the GPR; however, there was considerable variability in the neonate. We conclude that there are significant developmental differences in neonatal glucose homeostasis and that insulin is important in neonatal hormonal control of glucose production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., King K., Schwartz R. Model for the investigation of intractable hypoglycemia: insulin-glucose interrelationships during steady state infusions. Pediatrics. 1968 Jan;41(1):91–105. [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Leake R. D., Haymond M. W., Arnold K. J., Gruenke L. D., Sperling M. A., Kipnis D. M. Measurement of "true" glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977 Nov;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- Carroll J. J., Smith N., Babson A. L. A colorimetric serum glucose determination using hexokinase and glucose-6-phosphate dehydrogenase. Biochem Med. 1970 Sep;4(2):171–180. doi: 10.1016/0006-2944(70)90093-1. [DOI] [PubMed] [Google Scholar]
- Cowett R. M., Oh W., Pollak A., Schwartz R., Stonestreet B. S. Glucose disposal of low birth weight infants: steady state hyperglycemia produced by constant intravenous glucose infusion. Pediatrics. 1979 Mar;63(3):389–396. [PubMed] [Google Scholar]
- Cowett R. M., Susa J. B., Oh W., Schwartz R. Endogenous glucose production during constant glucose infusion in the newborn lamb. Pediatr Res. 1978 Aug;12(8):853–857. doi: 10.1203/00006450-197808000-00010. [DOI] [PubMed] [Google Scholar]
- Dweck H. S., Cassady G. Glucose intolerance in infants of very low birth weight. I. Incidence of hyperglycemia in infants of birth weights 1,100 grams or less. Pediatrics. 1974 Feb;53(2):189–195. [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Varma S., Cowan J. S. Relations between blood glucose and hepatic glucose production in newborn dogs. Br Med J. 1972 Jun 10;2(5814):625–627. doi: 10.1136/bmj.2.5814.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan S. C., Bier D. M., Savin S. M., Adam P. A. Estimation of glucose turnover and 13C recycling in the human newborn by simultaneous [1-13C]glucose and [6,6-1H2]glucose tracers. J Clin Endocrinol Metab. 1980 Mar;50(3):456–460. doi: 10.1210/jcem-50-3-456. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Savin S. M., Adam P. A. Measurement of glucose turnover in the human newborn with glucose-1-13C. J Clin Endocrinol Metab. 1976 Sep;43(3):704–707. doi: 10.1210/jcem-43-3-704. [DOI] [PubMed] [Google Scholar]
- MADISON L. L., COMBES B., ADAMS R., STRICKLAND W. The physiological significance of the secretion of endogenous insulin into the portal circulation. III. Evidence for a direct immediate effect of insulin on the balance of glucose across the liver. J Clin Invest. 1960 Mar;39:507–522. doi: 10.1172/JCI104065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison L. L. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969 Mar;123(3):284–292. [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- STEELE R., BISHOP J. S., DUNN A., ALTSZULER N., RATHBEB I., DEBODO R. C. INHIBITION BY INSULIN OF HEPATIC GLUCOSE PRODUCTION IN THE NORMAL DOG. Am J Physiol. 1965 Feb;208:301–306. doi: 10.1152/ajplegacy.1965.208.2.301. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Shelley H. J., Bassett J. M., Milner R. D. Control of carbohydrate metabolism in the fetus and newborn. Br Med Bull. 1975 Jan;31(1):37–43. doi: 10.1093/oxfordjournals.bmb.a071239. [DOI] [PubMed] [Google Scholar]
- Susa J. B., Cowett R. M., Oh W., Schwartz R. Suppression of gluconeogenesis and endogenous glucose production by exogenous insulin administration in the newborn lamb. Pediatr Res. 1979 May;13(5 Pt 1):594–598. doi: 10.1203/00006450-197905000-00003. [DOI] [PubMed] [Google Scholar]
- Varma S., Nickerson H., Cowan J. S., Hetenyi G., Jr Homeostatic responses to glucose loading in newborn and young dogs. Metabolism. 1973 Nov;23(2):1367–1375. doi: 10.1016/0026-0495(73)90252-7. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- Zarif M., Pildes R. S., Vidyasagar D. Insulin and growth-hormone responses in neonatal hyperglycemia. Diabetes. 1976 May;25(5):428–433. doi: 10.2337/diab.25.5.428. [DOI] [PubMed] [Google Scholar]


