Abstract
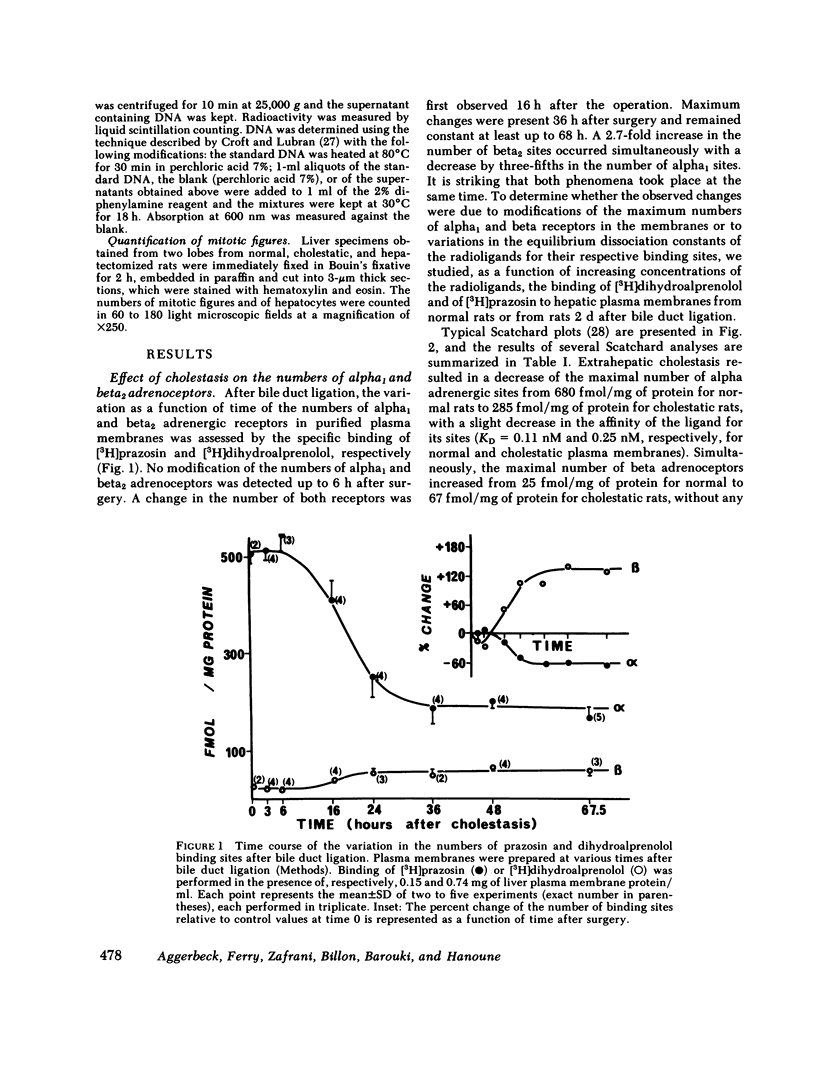
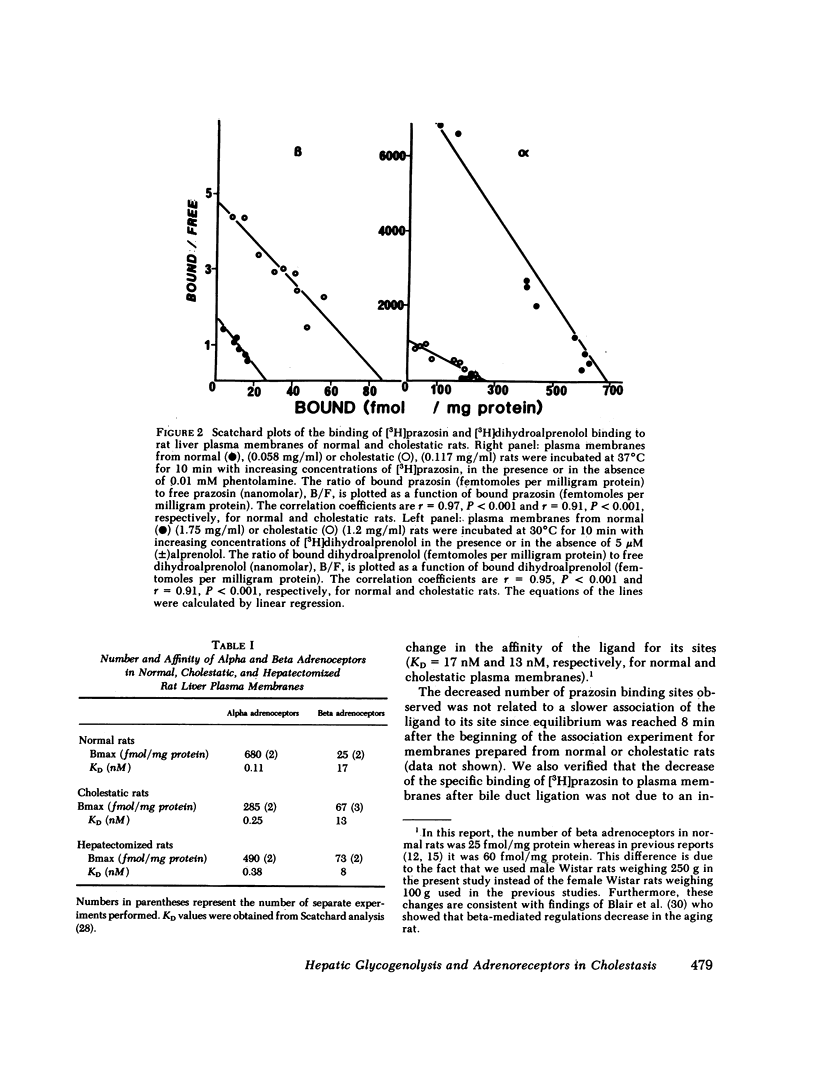
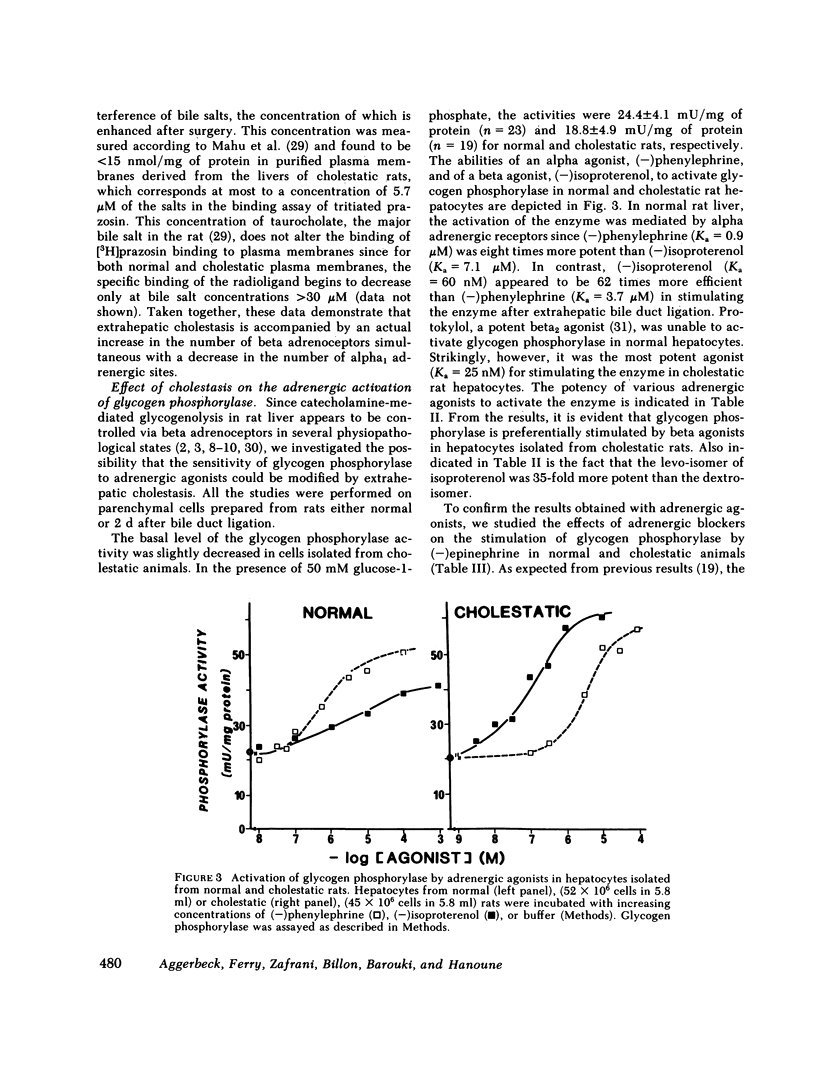
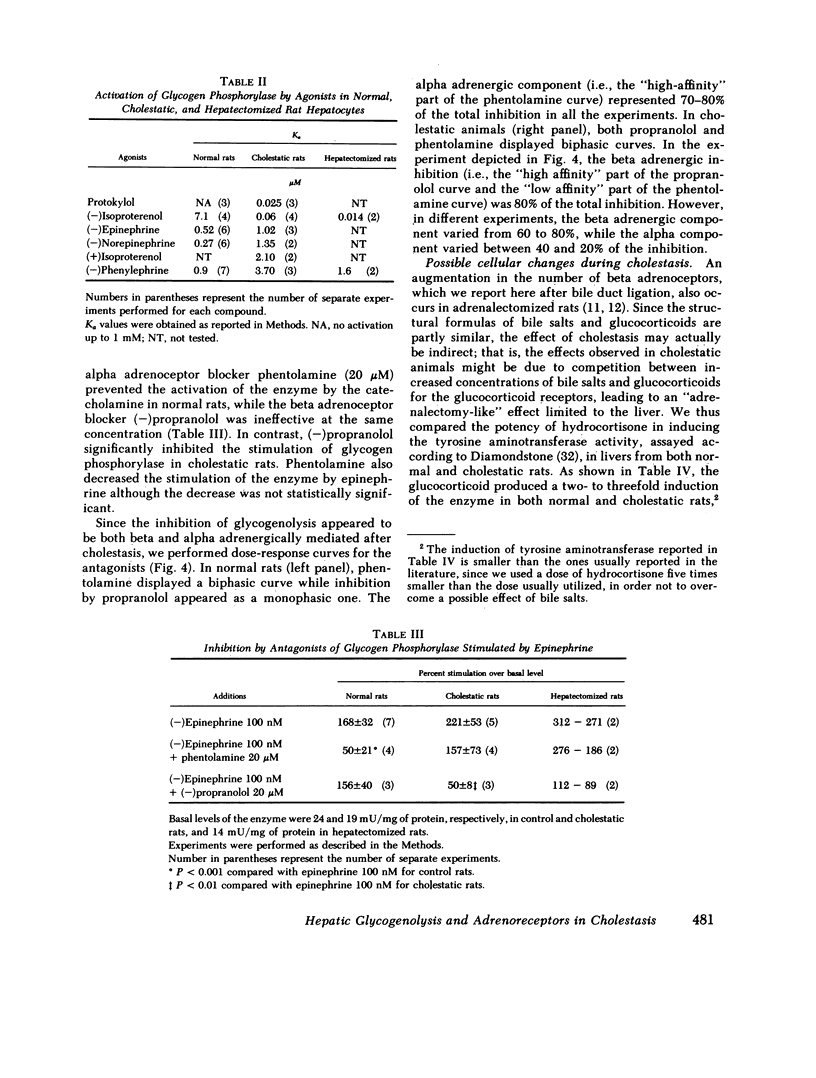
The effects of extrahepatic cholestasis upon adrenergic regulation of glycogenolysis and upon the numbers of adrenoceptors in rat liver were studied using isolated hepatocytes and plasma membranes, respectively. A 60% decrease in the number of alpha 1 adrenoceptors (285 vs. 680 fmol/mg protein) and a simultaneous 2.7-fold increase in the number of beta adrenergic sites (67 vs. 25 fmol/mg protein) were observed beginning 36 h after bile flow obstruction and persisted for at least 68 h. The reciprocal modification of the numbers of alpha 1 and beta adrenoceptors was accompanied by a change in the manner of stimulation of glycogen phosphorylase by catecholamines in hepatocytes; originally alpha 1 adrenergic in normal rats (phenylephrine Ka = 0.9 microM, isoproterenol Ka = 7.1 microM), the stimulation became predominantly beta adrenergic in cholestatic animals (phenylephrine Ka = 3.7 microM, isoproterenol Ka = 0.06 microM). In normal rats, activation of the enzyme by epinephrine was inhibited by the alpha blocker phentolamine, without inhibition by the beta blocker propranolol. In contrast, propranolol was more effective than phentolamine in cholestatic rat hepatocytes. Modification of the regulation of glycogenolysis after cholestasis did not seem to be secondary to an alteration in the metabolism of thyroid hormones or in the action of glucocorticoids. However, cholestasis provoked a 10-fold increase in the number of hepatic mitoses and in the incorporation of thymidine into liver DNA of cholestatic animals. Similar changes were observed in regenerating livers, following two-thirds hepatectomy. We propose that the changes following extrahepatic cholestasis might, as well, be explained by a regenerative process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggerbeck M., Guellaen G., Hanoune J. Adrenergic receptor of the alpha 1-subtype mediates the activation of the glycogen phosphorylase in normal rat liver. Biochem Pharmacol. 1980 Feb 15;29(4):643–645. doi: 10.1016/0006-2952(80)90389-5. [DOI] [PubMed] [Google Scholar]
- Aggerbeck M., Guellaen G., Hanoune J. The alpha-adrenergic mediated effect in rat liver. Correlation between [3H]-dihydroergocryptine binding to plasma membranes and glycogen phosphorylase activation in isolated hepatocytes. Biochem Pharmacol. 1980 Jun 15;29(12):1653–1662. doi: 10.1016/0006-2952(80)90120-3. [DOI] [PubMed] [Google Scholar]
- Aggerbeck M., Guellaën G., Hanoune J. N-Aralkyl substitution increases the affinity of adrenergic drugs for the alpha-adrenoceptor in rat liver. Br J Pharmacol. 1979 Jan;65(1):155–159. doi: 10.1111/j.1476-5381.1979.tb17344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri S. A., Bolt M. G., Boyer J. L., Palmer R. H. Stimulation of thymidine incorporation in mouse liver and biliary tract epithelium by lithocholate and deoxycholate. Gastroenterology. 1978 Feb;74(2 Pt 1):188–192. [PubMed] [Google Scholar]
- Barbiroli B., Potter V. R. DNA synthesis and interaction between controlled feeding schedules and partial hepatectomy in rats. Science. 1971 May 14;172(3984):738–741. doi: 10.1126/science.172.3984.738. [DOI] [PubMed] [Google Scholar]
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Brønstad G., Christoffersen T. Increased effect of adrenaline on cyclic AMP formation and positive beta-adrenergic modulation of DNA-synthesis in regenerating hepatocytes. FEBS Lett. 1980 Oct 20;120(1):89–93. doi: 10.1016/0014-5793(80)81053-2. [DOI] [PubMed] [Google Scholar]
- Bucher N. L. Experimental aspects of hepatic regeneration. N Engl J Med. 1967 Oct 5;277(14):738–concl. doi: 10.1056/NEJM196710052771405. [DOI] [PubMed] [Google Scholar]
- CROFT D. N., LUBRAN M. THE ESTIMATION OF DEOXYRIBONUCLEIC ACID IN THE PRESENCE OF SIALIC ACID: APPLICATION TO ANALYSIS OF HUMAN GASTRIC WASHINGS. Biochem J. 1965 Jun;95:612–620. doi: 10.1042/bj0950612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Blackmore P. F., Steiner K. E., Exton J. H. Effects of adrenalectomy on hormone action on hepatic glucose metabolism. Reciprocal change in alpha- and beta-adrenergic activation of hepatic glycogen phosphorylase and calcium mobilization in adrenalectomized rats. J Biol Chem. 1979 Apr 10;254(7):2428–2433. [PubMed] [Google Scholar]
- El-Refai M. F., Exton J. H. Subclassification of two types of alpha-adrenergic binding sites in rat liver. Eur J Pharmacol. 1980 Mar 21;62(2-3):201–204. doi: 10.1016/0014-2999(80)90276-9. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Guellaen G., Hanoune J. Thiol reactivity and the molecular individuality of alpha- and beta-adrenoreceptors in rat liver plasma membranes. Biochim Biophys Acta. 1979 Nov 1;587(4):618–627. doi: 10.1016/0304-4165(79)90013-8. [DOI] [PubMed] [Google Scholar]
- Guellaen G., Yates-Aggerbeck M., Vauquelin G., Strosberg D., Hanoune J. Characterization with [3H] dihydroergocryptine of the alpha-adrenergic receptor of the hepatic plasma membrane. Comparison with the beta-adrenergic receptor in normal and adrenalectomized rats. J Biol Chem. 1978 Feb 25;253(4):1114–1120. [PubMed] [Google Scholar]
- Hoffman B. B., Dukes D. F., Lefkowitz R. J. Alpha-adrenergic receptors in liver membranes: delineation with subtype selective radioligands. Life Sci. 1981 Jan 19;28(3):265–272. doi: 10.1016/0024-3205(81)90732-3. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Michel T., Kilpatrick D. M., Lefkowitz R. J., Tolbert M. E., Gilman H., Fain J. N. Agonist versus antagonist binding to alpha-adrenergic receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4569–4573. doi: 10.1073/pnas.77.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallner A. Determination of phosphate in serum and urine by a single step malachite-green method. Clin Chim Acta. 1975 Feb 22;59(1):35–39. doi: 10.1016/0009-8981(75)90215-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacombe M. L., Rene E., Guellaen G., Hanoune J. Transformation of the beta2 adrenoceptor in normal rat liver into a beta1 type in Zajdela hepatoma. Nature. 1976 Jul 1;262(5563):70–72. doi: 10.1038/262070a0. [DOI] [PubMed] [Google Scholar]
- Lafarge-Frayssinet C., Morel-Chany E., Trincal G., Frayssinet C. Enhancement of DNA synthesis by biliverdin in a non-transformed liver cell strain. Cell Mol Biol Incl Cyto Enzymol. 1981;27(2-3):77–82. [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Li S., Fain J. N. Hormonal activation of glycogen phosphorylase in hepatocytes from hypothyroid rats. J Biol Chem. 1978 Dec 25;253(24):8820–8825. [PubMed] [Google Scholar]
- Malbon C. C. Liver cell adenylate cyclase and beta-adrenergic receptors. Increased beta-adrenergic receptor number and responsiveness in the hypothyroid rat. J Biol Chem. 1980 Sep 25;255(18):8692–8699. [PubMed] [Google Scholar]
- Malbon C. C., LoPresti J. J. Hyperthyroidism impairs the activation of glycogen phosphorylase by epinephrine in rat hepatocytes. J Biol Chem. 1981 Dec 10;256(23):12199–12204. [PubMed] [Google Scholar]
- Moncany M. L., Plas C. Interaction of glucagon and epinephrine in the regulation of adenosine 3',5'-monophosphate-dependent glycogenolysis in the cultured fetal hepatocyte. Endocrinology. 1980 Dec;107(6):1667–1675. doi: 10.1210/endo-107-6-1667. [DOI] [PubMed] [Google Scholar]
- Morley C. G., Royse V. L. Adrenergic agents as possible regulators of liver regeneration. Int J Biochem. 1981;13(9):969–973. doi: 10.1016/0020-711x(81)90001-x. [DOI] [PubMed] [Google Scholar]
- Mourelle M., Rubalcava B. Regeneration of the liver after carbon tetrachloride. Differences in adenylate cyclase and pancreatic hormone receptors. J Biol Chem. 1981 Feb 25;256(4):1656–1660. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Nickerson M., Kunos G. Discussion of evidence regarding induced changes in adrenoceptors. Fed Proc. 1977 Nov;36(12):2580–2583. [PubMed] [Google Scholar]
- Okajima F., Ui M. Conversion of adrenergic regulation of glycogen phosphorylase and synthase from an alpha to a beta type during primary culture of rat hepatocytes. Arch Biochem Biophys. 1982 Feb;213(2):658–668. doi: 10.1016/0003-9861(82)90596-3. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Nishimura H., Arizono H., Nishimura N., Suzuki Y. Biliverdin initiates the liver regeneration in the rat--a hypothesis. Biochem Biophys Res Commun. 1978 Mar 30;81(2):512–520. doi: 10.1016/0006-291x(78)91564-4. [DOI] [PubMed] [Google Scholar]
- Preiksaitis H. G., Kan W. H., Kunos G. Decreased alpha 1-adrenoceptor responsiveness and density in liver cells of thyroidectomized rats. J Biol Chem. 1982 Apr 25;257(8):4321–4327. [PubMed] [Google Scholar]
- Preiksaitis H. G., Kunos G. Adrenoceptor-mediated activation of liver glycogen phosphorylase: effects of thyroid state. Life Sci. 1979 Jan 1;24(1):35–41. doi: 10.1016/0024-3205(79)90277-7. [DOI] [PubMed] [Google Scholar]
- Schmelck P. H., Billon M. C., Munnich A., Geynet P., Houssin D., Hanoune J. The effects of common bile duct ligation upon the rat liver beta-adrenergic receptor-adenylate cylase system. FEBS Lett. 1979 Nov 1;107(1):259–263. doi: 10.1016/0014-5793(79)80509-8. [DOI] [PubMed] [Google Scholar]
- Schmelck P. H., Hanoune J. The hepatic adrenergic receptors. Mol Cell Biochem. 1980 Dec 10;33(1-2):35–48. doi: 10.1007/BF00224570. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sherline P., Eisen H., Glinsmann W. Acute hormonal regulation of cyclic AMP content and glycogen phosphorylase activity in fetal liver in organ culture. Endocrinology. 1974 Apr;94(4):935–939. doi: 10.1210/endo-94-4-935. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Richards W., Tsukada Y., Sattler C. A., Pitot H. C. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer R. K., Borle A. B. Differences between male and female rats in the regulation of hepatic glycogenolysis. The relative role of calcium and cAMP in phosphorylase activation by catecholamines. J Biol Chem. 1982 Jul 25;257(14):7987–7993. [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K., Molinoff P. B. beta-adrenergic receptors in rat liver: effects of adrenalectomy. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1343–1347. doi: 10.1073/pnas.73.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. H. Changes in plasma membrane enzyme activities during liver regeneration in the rat. Biochim Biophys Acta. 1977 Nov 1;470(3):368–381. doi: 10.1016/0005-2736(77)90128-6. [DOI] [PubMed] [Google Scholar]


