Abstract
A 61-year-old man presented with a 1-month history of breathlessness, chest pain and lethargy. He had been taking adalimumab for ankylosing spondylitis for 2 years. Pleural and pericardial effusions were both found. A video-assisted thorascopic (VATS) pleural and lung biopsy were performed. The pleural pathology showed eosinophils, acute inflammatory cells and lymphoid aggregates. The patient was positive for antinuclear, antidouble-stranded and antihistone antibodies consistent with drug-induced lupus due to adalimumab. His serositis resolved on withdrawal of the drug. Drug-induced lupus can occur as a consequence of anti-TNF-α agents from induction of autoimmunity in a predisposed host.
Background
Anti-tumour-necrosis factor (TNF) α agents are now increasingly being used for a variety of conditions including rheumatoid arthritis (RA), psoriatic arthritis (PA), ankylosing spondylitis (AS), juvenile idiopathic arthritis (JIA) and inflammatory bowel disease (IBD). They are also rarely used as third-line therapy in the treatment of sarcoidosis.1 However, these agents have a variety of serious adverse effects including infection, reactivation of tuberculosis (TB) and immunogenicity. Induction of autoantibodies, particularly antinuclear antibodies, is a relatively common side effect post treatment, but the development of drug-induced lupus with overt clinical manifestations remains rare. Its recognition is a challenge in a patient population where its symptoms may mimic a flare of their underlying disease, associated autoimmune conditions or overlap syndromes. Adalimumab is a fully humanised monoclonal antibody against TNF-α purported to be less immunogenic with a safer side effect profile. Here we describe a case of anti-TNF-induced lupus (ATIL) secondary to adalimumab therapy.
Case presentation
A 61-year-old man presented to the emergency department of Cork University Hospital in Ireland with a 4-week history of exertional dyspnoea, chest tightness and poor exercise tolerance. He also reported anorexia and involuntary weight loss of 6 kg in 2 months.
He had a background of AS diagnosed 20 years earlier for which he was treated with adalimumab for the past 2 years. He had previously failed to respond to two different non-steroidal anti-inflammatory drugs (NSAIDs) including ibuprofen and diclofenac. No trial of sulfasalazine was attempted, as it is no longer a mandatory pretreatment in this group.2 Prior to start of adalimumab 40 mg every alternate week, the patient had negative testing for latent TB infection including Quantiferon and Mantoux tests along with a normal chest X-ray (CXR) as per the American College of Rheumatology (ACR) guidelines.3
He had been recently treated for recurrent lower respiratory tract infections (LRTI) by his primary care physician. He had a CXR at the time, which showed a small right pleural effusion with patchy atelectasis. His adalimumab had been temporarily withheld for 4 weeks and he was treated with antibiotics.
He was an active smoker with a 50-pack year history and he consumed 12 units of alcohol per week. He was a former army engineer. There was no known history of asbestos exposure or other risk factors for respiratory disease.
On examination, he was tachypnoic with a respiratory rate (RR) of 28 breaths per minute and his oxygen saturations were reduced at 92% on room air. He was apyrexial. His blood pressure (BP) was 101/59 mm Hg and his heart rate (HR) was 82 bpm. Auscultation of his chest elicited reduced air entry bibasally with inspiratory coarse crackles.
Investigations
Arterial blood gas (ABG) showed a pH of 7.47, pCO2 of 4.6 kPa, pO2 of 7.7 kPa, SO2 of 91.5% and HCO3 of 25.5 (normal ranges: pH 7.35–7.45, pCO2 4.5–6 kPa, pO2 11–15kPa, sO2 94–100%, HCO3 22–28 mmol/L), consistent with type 1 respiratory failure. Collected sputum and blood cultures were all sterile. The patient's white cell count (WCC) was 11.3×109/L (normal range: 4.0–11.0×109/L) with a differential of 9.28×109/L neutrophils (normal range: 1.4–6.6×109/L) and 1.37×109/L lymphocytes (normal range: 0.9–3.2×109/L). He had a normochromic normocytic anaemia with a haemoglobin (Hb) level of 12.3 g/dL (normal range: 14–17.4 g/dL). Direct Coombs test was not performed as there was no evidence of haemolysis. The patient's platelets were 331×109/L (normal range: 140–440×109/L). His C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were both elevated at 116.1 mg/L (normal range: 0–5 mg/L) and 68 mm/h (normal range: 0–10 mm/h), respectively. His renal and liver function tests were unremarkable. There were bilateral pleural effusions and bibasal consolidation on his CXR (figure 1).
Figure 1.
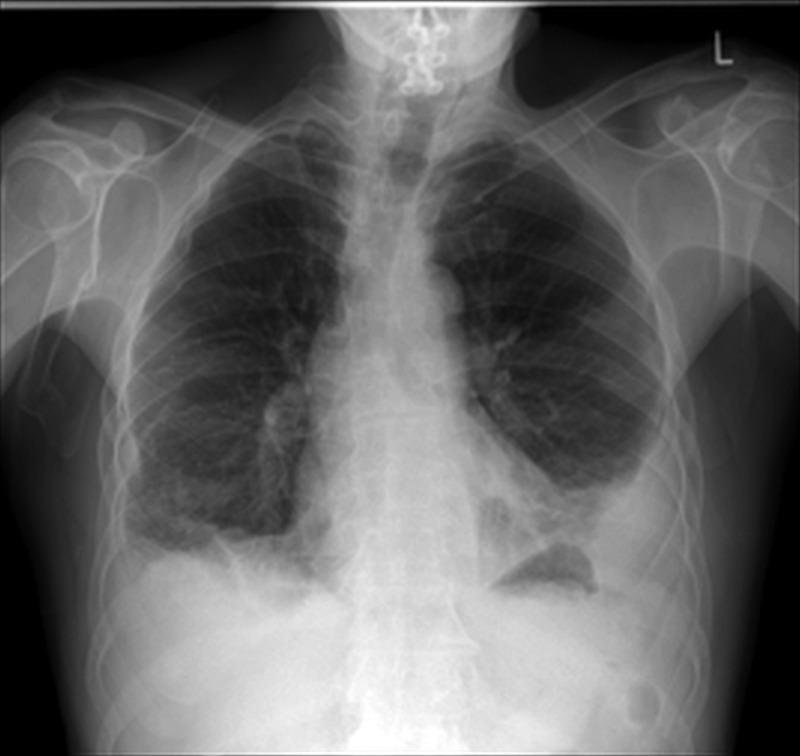
Bilateral effusions and consolidation on chest X-ray.
Ultrasound-guided left pleural drainage was performed, with fluid analysis showing a pH of 7.24, glucose 4 mmol/L (serum 6.5 mmol/L), total protein 53 g/L (serum total protein 62 g/L), lactate dehydrogenase 2737 U/L and 7390 white cells/cm2 with 70% neutrophils. No organisms were grown after 48 h incubation. There were no acid-fast bacilli seen and the TB culture was negative. The cytology showed degenerating lymphocytes, polymorphs and eosinophils. At this time, the clinical impression was community-acquired pneumonia with a complex parapneumonic effusion. Adalimumab was withheld and antibiotics (tazobactam, clarithromycin and metronidazole) were started as per hospital guidelines.
The patient's echocardiogram showed a structurally and functionally normal heart with an ejection fraction (EF) of 60%. His pulmonary function tests showed a reduced forced expiratory volume in 1 s FEV1 (56% predicted) and forced vital capacity FVC (55.3% predicted) with a preserved ratio (80.4%) and reduced diffusing capacity of the lungs for carbon monoxide (49.6% predicted) correcting for lung volumes (91.5%), all consistent with extrathoracic restriction due to reduced chest expansion in the setting of AS.
A high-resolution CT of the thorax demonstrated right lower lobe and lingular atelectasis along with mild biapical scarring and upper lobe paraseptal emphysema. There was no evidence of interstitial lung disease (ILD). A trace right pleural effusion was seen.
The patient clinically improved after 10 days of intravenous antibiotics and was discharged home. Adalimumab was held for 2 weeks post-treatment of his pneumonia.
One month later, the patient re-presented to the emergency department with acute severe respiratory distress, chest pain and hypotension. He was tachypnoeic with a RR of 36 breaths per minute and his oxygen saturations were 94% on room air. He had a visibly raised jugular venous pressure but no substantial peripheral oedema. There were no murmurs or rub on auscultation. He had bi-basal inspiratory coarse crackles.
An ABG performed on 3 L of oxygen revealed moderate hypoxaemia (pO2 10.8 mm Hg). The patient's white cell count was slightly raised at 11.4×109/L (9.41×109/L neutrophils, 1.46×109/L lymphocytes). His Hb level was unchanged at 12.3 g/dL and his platelets were 299×109/L. His CRP was notably elevated at 144.7 mg/L. Renal and liver function tests were again within the normal range. A CXR (figure 2) showed recurrence of bilateral pleural effusions and new cardiomegaly.
Figure 2.
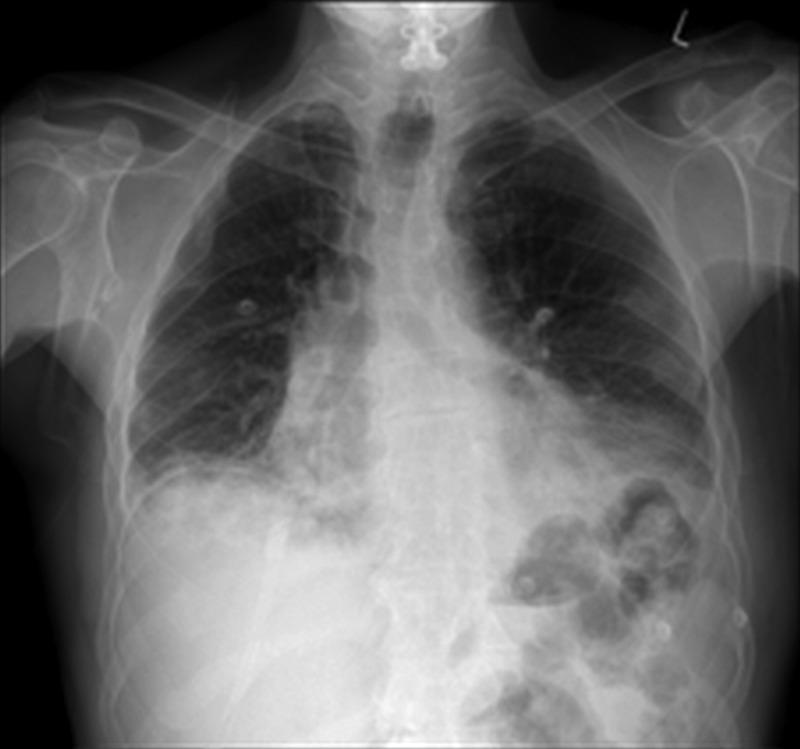
Chest X-ray: increased heart size, bibasal inflammatory change, worse on left side with small bilateral pleural effusions.
A CT pulmonary angiogram (CTPA) was performed to exclude pulmonary embolism (PE; figure 3). This instead showed a moderate-sized pericardial effusion and bilateral pleural effusion. A repeat echocardiogram demonstrated a 1.5 cm circumferential pericardial effusion with no evidence of cardiac tamponade. Left and right ventricular size and function were normal.
Figure 3.
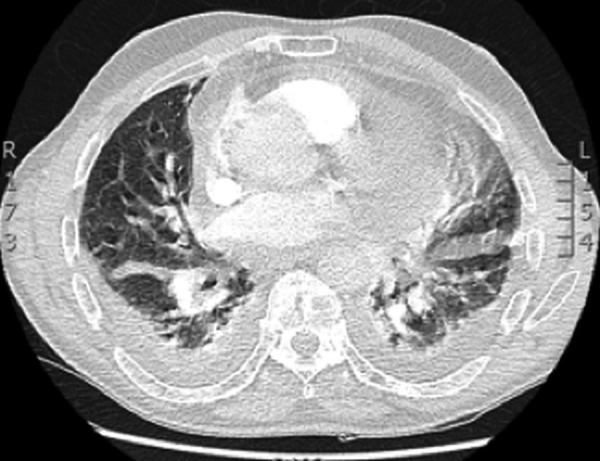
CT pulmonary angiogram: new global cardiomegaly with moderate pericardial effusion.
The patient's blood cultures were sterile. Viral serologies including cytomegalovirus, Epstein-Barr virus, HIV, herpes simplex virus type 1, varicella-zoster virus and echovirus were all negative. Repeat Quantiferon and Mantoux tests were also negative. A video-assisted thorascopic pleural and lung biopsy were performed and samples were sent for microbiological and histopathological analysis to exclude TB and other causes of recurrent exudative pleural effusions. The pathology report from the patient's pleural biopsy showed eosinophils, acute inflammatory cells and lymphoid aggregates at the junction with the adipose tissue. No granulomas were seen. Cultures were negative.
Concurrently, an autoantibody screen was checked. One week later, the patient's antinuclear antibody (ANA) result returned with a strongly positive homogeneous pattern. The Phadia Elia antidouble-stranded (ds) DNA titre was only 1.2 IU/mL (reference range: 0–10 IU/mL) but the Crithida anti-dsDNA was strongly positive (titre unavailable retrospectively). The antihistone antibodies were 109 U/mL (reference range: 0–40 U/mL). These results were consistent with a diagnosis of drug-induced lupus.
Treatment
The patient was diagnosed with adalimumab-induced lupus. He received treatment with colcichine and NSAIDs for his pleuropericardial syndrome. His adalimumab was stopped permanently. Glucocorticoids were not required as he responded readily to discontinuation of the offending agent.
Outcome and follow-up
On follow-up at 2 months, the patient's symptoms had resolved. He was no longer breathless and his CXR had returned to normal (figure 4). His peripheral arthritis was now controlled with intermittent use of NSAIDs only. Although he remains at significant risk of disease relapse after stopping his anti-TNF therapy, he may be part of the 1% of patients who develop a ‘burn-out’ of disease activity and enter long-term remission.4
Figure 4.
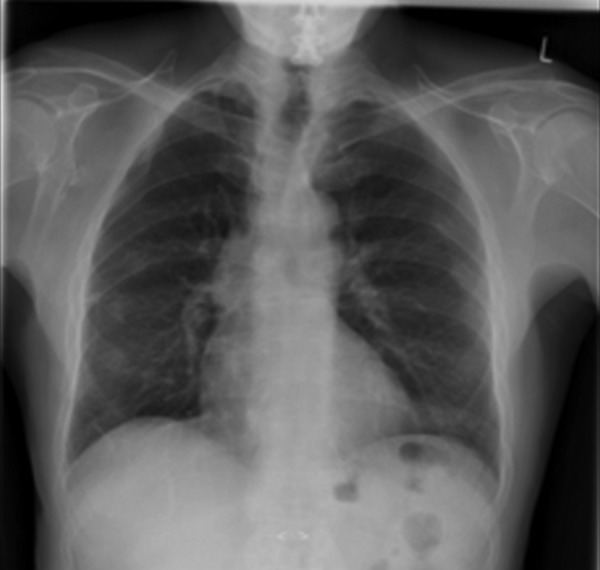
Chest X-ray 2 months after stopping adalimumab.
Discussion
This case outlines the issues involved in the diagnosis and management of a patient with autoimmune disease in receipt of biological therapy, in what was ultimately a case of Adalimumab-induced lupus. In particular it highlights the key temporal sequence of events that often underpins the diagnosis of drug-induced lupus. This patient acutely decompensated following re-introduction of adalimumab after it had been initially held for a presumed LRTI. His illness resolved within 10 days after withdrawing this agent in keeping with its mean terminal half life.5
There are no consensus diagnostic criteria for drug-induced lupus. It is typically characterised by the onset of systemic lupus erythaematosus (SLE) features according to ACR criteria6 associated with the use of a lupus-inducing drug. The gold standard diagnostic criterion is symptom resolution after stopping the offending medication.
The most frequent clinical signs and symptoms of drug-induced lupus are arthralgia, myalgia, fever, anorexia, weight loss and serositis. The interval between medication exposure and symptom onset may range from 1 month to 12 years.7 Symptoms tend to be milder in drug-induced lupus when compared with classic SLE, and major organ involvement is rare. Unlike classic SLE, malar rash, photosensitivity, oral ulcers and alopecia are rare in drug-induced lupus. Agents often implicated in drug-induced lupus include procainamide, quinidine and hydralazine.8 Commonly prescribed medications such as angiotensin-converting enzyme inhibitors, thiazides and statins may be associated with a subacute cutaneous form.9
The laboratory findings in drug-induced lupus most commonly include an elevated ESR in 80% of patients and a positive ANA.10 Most frequently, the ANA fluorescence-staining pattern is homogeneous and is caused by antibodies to histones. These target the histone-DNA macromolecular complex in the cell nucleus and are present in approximately 50–90% of patients with drug-induced lupus. Anti-dsDNA antibodies occur in less than 5% of patients with drug-induced lupus and complement levels are typically normal.11
ATIL is regarded as a distinct subgroup of drug-induced lupus.12 The autoantibody profile of ATIL differs from that of classical drug-induced lupus as there is a higher prevalence of anti-dsDNA antibodies and a lower prevalence of antihistone antibodies.13
A large review published in 2008 evaluated 33 cases of ATIL.14 Of the included cases, 21 were due to infliximab, 10 were due to etanercept, and only two cases were related to adalimumab. The relative infrequency of cases associated with adalimumab may simply reflect fewer years of patient exposure to this agent. The authors concluded that ATIL has significant clinical and laboratory manifestations distinguishing it from that due to other drugs. In total 91% of ATIL cases were positive for anti-dsDNA versus less than 1% of drug-induced lupus cases overall.15 Fifty-nine per cent were noted to have hypocomplementemia. Only 57% of cases were antihistone positive. Classical drug-induced lupus does not tend to have renal involvement and has less frequent cutaneous involvement than idiopathic SLE. However, in this review, 72% of patients with ATIL reported a rash, and the authors noted several reports of glomerulonephritis. Pericardial or pleural effusion occurred in only three cases.
There are a number of proposed mechanisms for drug-induced lupus in this context, including a disturbance in TNF-α-controlled homeostasis of interferon (IFN)-α.16 Over-expression of TNF-α in RA has an inhibitory effect on plasmatocytoid dendritic cell expansion and systemic release of IFN-α, a culprit for SLE. The occurrence of a lupus-like syndrome with either TNF-α blockade or interferon-α treatment supports this hypothesis.17 There is seroconversion of SLE markers,18 as demonstrated in this case. Murine studies suggest that the induction of cytotoxic T lymphocytes that would typically suppress autoreactive B cells is selectively inhibited by TNF blockade, allowing unchecked humoral autoimmunity.19 Another theory is that anti-TNF-α agents induce apoptosis, releasing nucleosomes that promote autoantibody formation.20
From a retrospective national study of 7700 French patients treated with anti-TNF agents for rheumatic disease in 2003, there appears to be an incidence of 0.19% of drug-induced lupus among infliximab and etanercept users.13 There are less data available for ATIL incidence secondary to adalimumab. The Spanish Study Group of Biological Agents in Autoimmune Diseases (BIOGEAS project) reported a total of 92 cases of lupus secondary to biological agents from January 1990 to December 2006, 15 of them associated with adalimumab.21
In keeping with these statistics, there are relatively few case reports of drug-induced lupus by adalimumab in the literature. The majority present with mucocutaneous, joint or constitutional symptoms.14 22–26 The affected participants were almost invariably female with a background of RA or Crohn's disease. The onset of symptoms ranged from 5 weeks to 2 years post treatment. There does not appear to be any association with pretreatment antibody profiles. Several case reports of serositis associated with anti-TNF-α agents have already been described.27 28
Primary treatment of drug-induced lupus involves discontinuation of the medication and, in severe cases, use of corticosteroids.12 In the Spanish study,21 47% of patients recovered on withdrawal of the anti-TNF-α antagonist alone. However, 40% required steroids and an additional 12% needed further immunosuppression. It may be difficult to evaluate remission if there is return of symptoms of the underlying rheumatic condition. There are conflicting reports as to whether concurrent use of disease-modifying anti-rheumatic drugs might reduce the incidence of autoantibody formation and thereby reduce the incidence of ATIL.29 30
Several other autoimmune disorders have been implicated with use of anti-TNF-α agents, including vasculitic and demyelination syndromes, sarcoidosis, psoriasis and granulomatous interstitial nephritis.21 31 32
With novel use of these biological agents to treat conditions such as sarcoidosis,1 in addition to their approved use for RA, PA, AS, JIA and IBD, it is imperative that specialists are aware of the risk of a lupus-like syndrome. It may be prudent to introduce pretreatment serological evaluation for SLE. However, there are no studies on its usefulness as a predictor for the development of drug-induced lupus. Re-challenge with the same TNF-α antagonist, as demonstrated in this case, may provoke disease recurrence. There is anecdotal evidence that there is a class effect and that the syndrome will recur when challenged with another agent.25 However, successful switching to alternative biological therapy has been reported in other TNF-α-mediated autoimmune diseases.33 In one case series, six patients with Crohn's disease were successfully switched to certolizumab with symptom relief and reversal of ANA positivity following lupus-reactions with either infliximab or adalimumab.34
Learning points.
The occurrence of pleural and of pericardial effusions may be evidence of polyserositis, which can be infective or inflammatory in origin. Drug-induced lupus can present with such a pleuropericardial syndrome.
The typical pleural effusion in lupus is mildly exudative with an elevated lactate dehydrogenase and a predominance of lymphocytes or neutrophils. Pleural fluid antinuclear antibody (ANA) titre and serum C reactive protein levels are significantly increased in lupus pleuritis.35
Drug-induced lupus can occur as a consequence of anti-tumour-necrosis factor-α agents from induction of autoimmunity in a predisposed host.
Checking for baseline ANA prior to treatment with a repeat ANA and antihistone antibody test if anti-TNF-induced lupus (ATIL) is suspected may aid in the diagnosis. Unlike other forms of drug-induced lupus, antibodies against double-stranded DNA antibodies may also be positive in ATIL.
Clinical and radiological resolution should occur with discontinuation of the offending drug. If not the diagnosis needs to be re-assessed.
Footnotes
Contributors: DK researched and prepared the manuscript. OO and MH reviewed, revised and edited it.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Korsten P, Mirsaeidi M, Sweiss NJ. Nonsteroidal therapy of sarcoidosis. Curr Opin Pulm Med 2013;19:516–23. 10.1097/MCP.0b013e3283642ad0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D, Sieper J, Maksymowych WP et al. Assessment of SpondyloArthritis International Society. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:905 10.1136/ard.2011.151563 [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Furst DE, Bharat A et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. 10.1002/acr.21641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy LG, Edmunds L, Calin A. The natural history of ankylosing spondylitis. Does it burn out? J Rheumatol 1993;20:688. [PubMed] [Google Scholar]
- 5.den Broeder AA, van de Putte LBA, Rau R et al. A single-dose, placebo-controlled study of the fully human anti-TNF antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol 2002;29:2288–9. [PubMed] [Google Scholar]
- 6.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 7.Hess E. Drug-related lupus. N Engl J Med 1988;318:1460–2. 10.1056/NEJM198806023182209 [DOI] [PubMed] [Google Scholar]
- 8.Burlingame RW, Rubin RL. Drug-induced anti-histone autoantibodies display two patterns of reactivity with substructures of chromatin. J Clin Invest 1991;88:680 10.1172/JCI115353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C, Gershwin ME. Drug-induced lupus erythematosus. Drug Saf 2011;34:357–74. 10.2165/11588500-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10.Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci 2007;1108:166–82. 10.1196/annals.1422.019 [DOI] [PubMed] [Google Scholar]
- 11.Vasoo S. Drug-induced lupus: an update. Lupus 2006;15:757–61. 10.1177/0961203306070000 [DOI] [PubMed] [Google Scholar]
- 12.Williams EL, Gadola S, Edwards CJ et al. Anti-TNF-induced lupus. Rheumatology (Oxford) 2008;48:716–20. 10.1093/rheumatology/kep080 [DOI] [PubMed] [Google Scholar]
- 13.DeBandt M, Sibilia J, Le Loet X et al. Systemic lupus erythematosus induced by anti-tumor necrosis factor alpha therapy: a French national survey. Arthritis Res Ther 2005;7:R545–51. 10.1186/ar1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin Arthritis Rheum 2008;37:381–7. 10.1016/j.semarthrit.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 15.Yung RL, Richardson BC. Drug-induced lupus. Rheum Dis Clin North Am 1994;20:61–86. [PubMed] [Google Scholar]
- 16.Palucka AK, Blanck JP, Bennett L et al. Cross-regulation of TNF and IFN-a in autoimmune diseases. Proc Natl Acad Sci USA 2005;102:3372–7. 10.1073/pnas.0408506102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum 2000;43:1431–42. [DOI] [PubMed] [Google Scholar]
- 18.Bobbio-Pallavicini F, Alpini C, Caporali R et al. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment. Arthritis Res Ther 2004;6:R264–72. 10.1186/ar1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Via CS, Shustov A, Rus V et al. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol 2001;167:6821–6. 10.4049/jimmunol.167.12.6821 [DOI] [PubMed] [Google Scholar]
- 20.D'Auria F, Rovere-Querini P, Giazzon M et al. Accumulation of plasma nucleosomes upon treatment with anti-tumour necrosis factor-alpha antibodies. J Intern Med 2004;255:409–18. 10.1111/j.1365-2796.2003.01298.x [DOI] [PubMed] [Google Scholar]
- 21.Ramos-Casals M, Brito-Zer NP, Munoz S et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86:242–51. 10.1097/MD.0b013e3181441a68 [DOI] [PubMed] [Google Scholar]
- 22.Al-Niaimi F. Adalimumab-induced lupus erythematosus. Eur J Dermatol 2009;19:380–412. [DOI] [PubMed] [Google Scholar]
- 23.Vezzoli P, Violetti SA, Serini SM et al. Cutaneous lupus erythematosus induced by adalimumab. J Dermatol 2011;38:283–4. 10.1111/j.1346-8138.2010.00951.x [DOI] [PubMed] [Google Scholar]
- 24.Mañosa M, Domeènech E, Mariín L et al. Adalimumab-induced lupus erythematosus in Crohn's disease patients previously treated with infliximab. Gut 2008;57:559. [PubMed] [Google Scholar]
- 25.van Rijthoven AW, Bijlsma JW, Canninga-van Dijk M et al. Onset of systemic lupus erythematosus after conversion of infliximab to adalimumab treatment in rheumatoid arthritis with a pre-existing anti-dsDNA antibody level. Rheumatology (Oxford) 2006;45:1317–19. doi:10.1093/rheumatology/kel227 [DOI] [PubMed] [Google Scholar]
- 26.Zella GC, Weinblatt ME, Winter HS. Drug-induced lupus associated with infliximab and adalimumab in an adolescent with Crohn disease. J Pediatr Gastroenterol Nutr 2009;49:355–8. 10.1097/MPG.0b013e3181837289 [DOI] [PubMed] [Google Scholar]
- 27.Abunasser J, Forouhar FA, Metersky ML. Etanercept-induced lupus erythematosus presenting as a unilateral pleural effusion. Chest 2008;134:850–63. 10.1378/chest.08-0034 [DOI] [PubMed] [Google Scholar]
- 28.Porfyridis I, Kalomenidis I, Psallidas I et al. Etanercept-induced pleuropericardial lupus-like syndrome. Eur Respir J 2009;33:939–41. 10.1183/09031936.00182308 [DOI] [PubMed] [Google Scholar]
- 29.Charles PJ, Smeenk RJT, De Jong J et al. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor. Arthritis Rheum 2000;43:2383–90. [DOI] [PubMed] [Google Scholar]
- 30.Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int 1998;18:59–62. 10.1007/s002960050058 [DOI] [PubMed] [Google Scholar]
- 31.Korsten P, Sweiss NJ, Nagorsnik U et al. Drug-induced granulomatous interstitial nephritis in a patient with ankylosing spondylitis during therapy with adalimumab. Am J Kidney Dis 2010;56:e17–21. 10.1053/j.ajkd.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohan N, Edwards ET, Cupps TR et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001;44:2862 [DOI] [PubMed] [Google Scholar]
- 33.Tong D, Manolios N, Howe G et al. New onset sarcoid-like granulomatosis developing during anti-TNF therapy: an under-recognised complication. Intern Med J 2012;42:89–94. 10.1111/j.1445-5994.2011.02612.x [DOI] [PubMed] [Google Scholar]
- 34.Verma HD, Scherl EJ, Jacob VE et al. Anti-nuclear antibody positivity and the use of certolizumab in inflammatory bowel disease patients who have had arthralgias or lupus-like reactions from infliximab or adalimumab. J Dig Dis 2011;12:379–83. 10.1111/j.1751-2980.2011.00522.x [DOI] [PubMed] [Google Scholar]
- 35.Choi BY, Yoon MJ, Shin K et al. Characteristics of pleural effusions in systemic lupus erythematosus: differential diagnosis of lupus pleuritis. Lupus 2014;24:321–6. 10.1177/0961203314555171 [DOI] [PubMed] [Google Scholar]


