Abstract
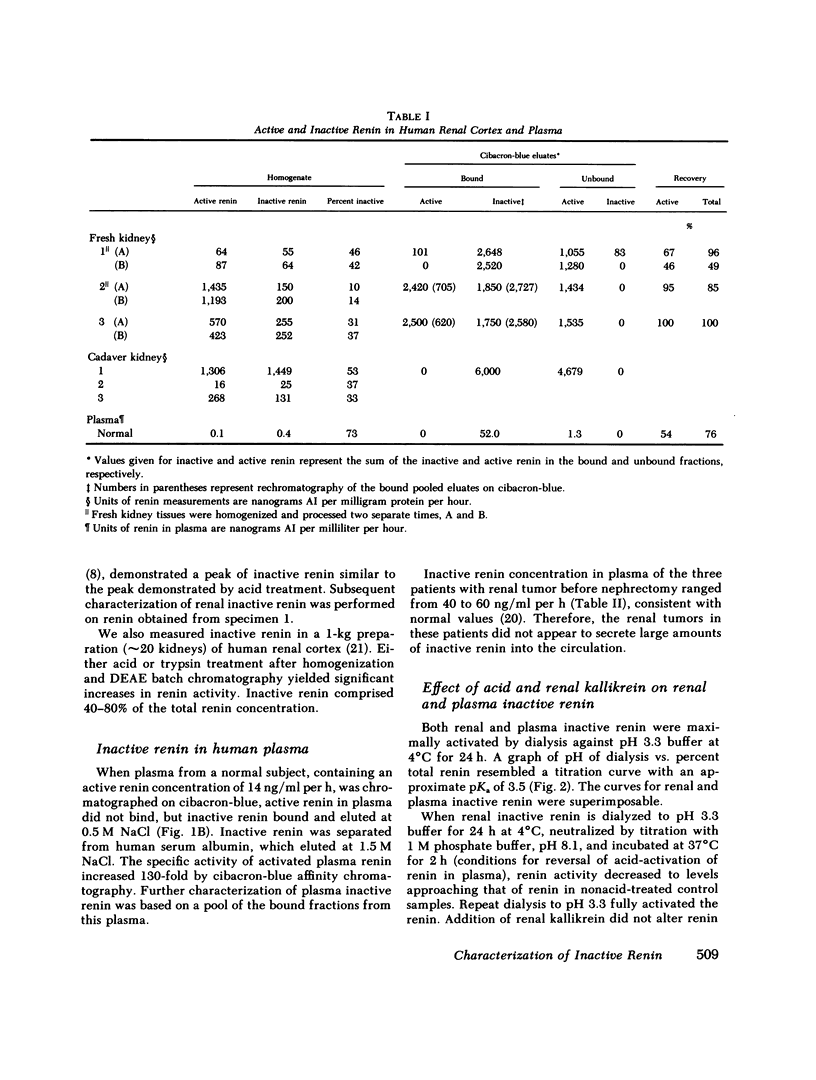
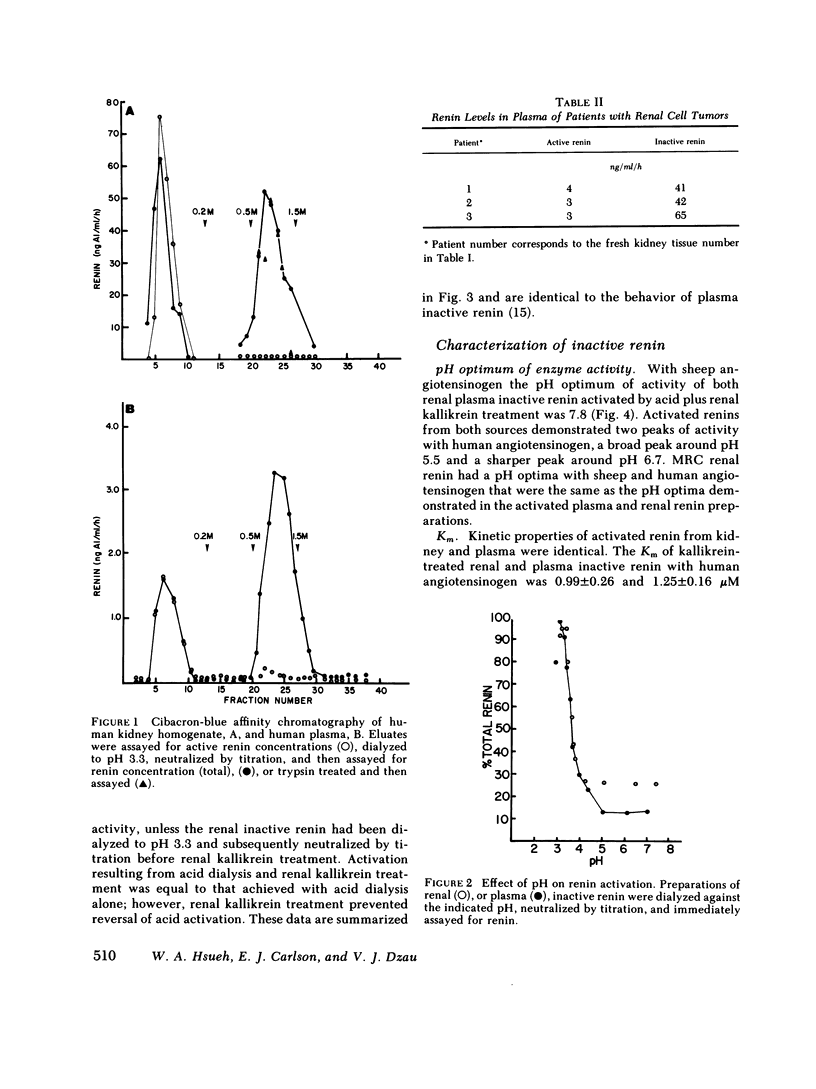
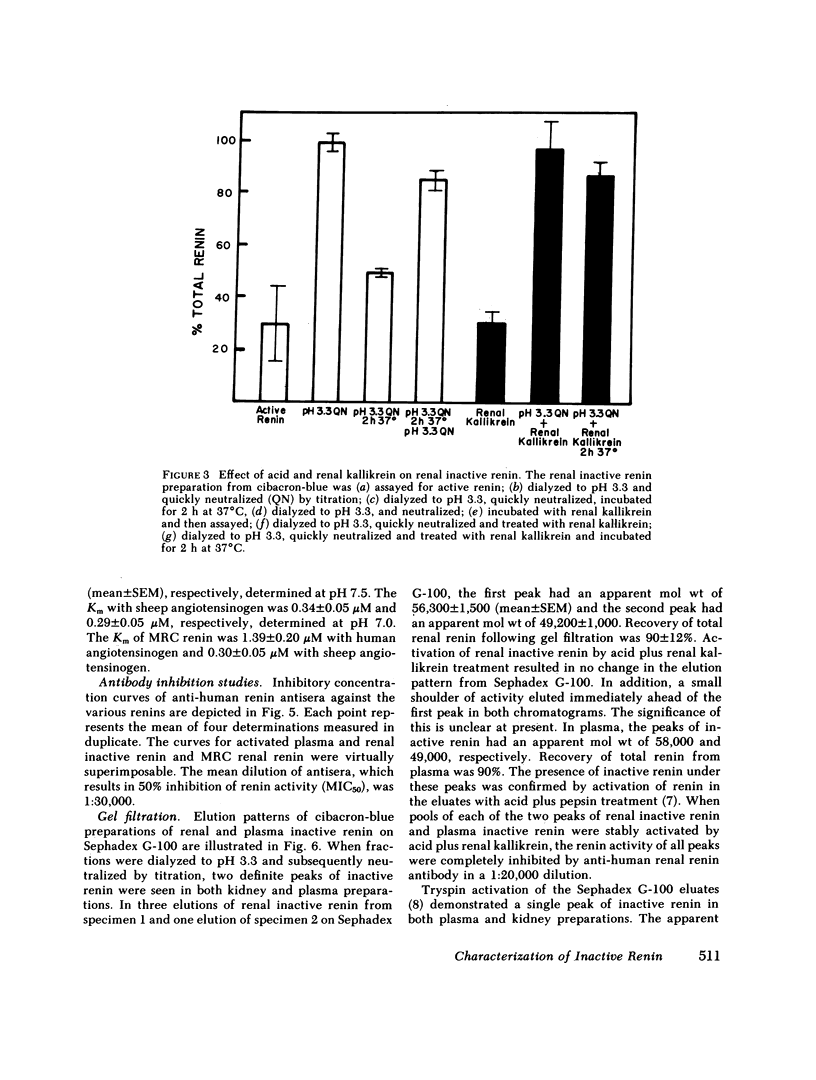
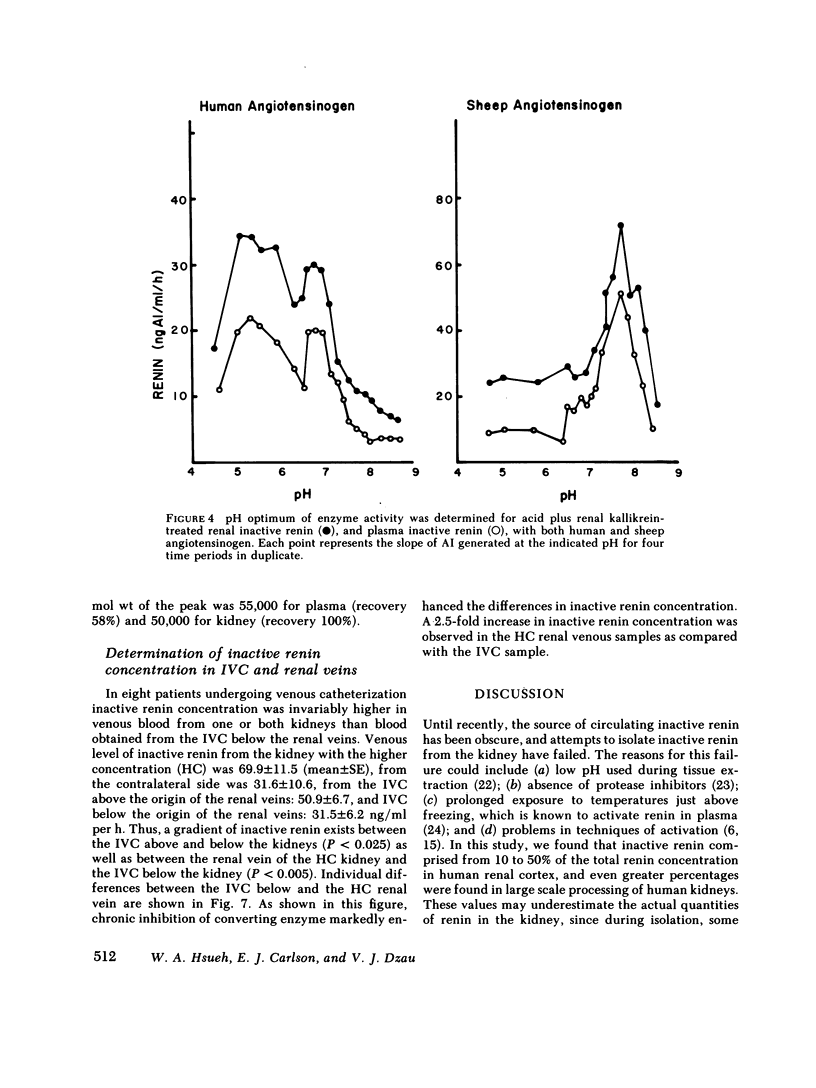
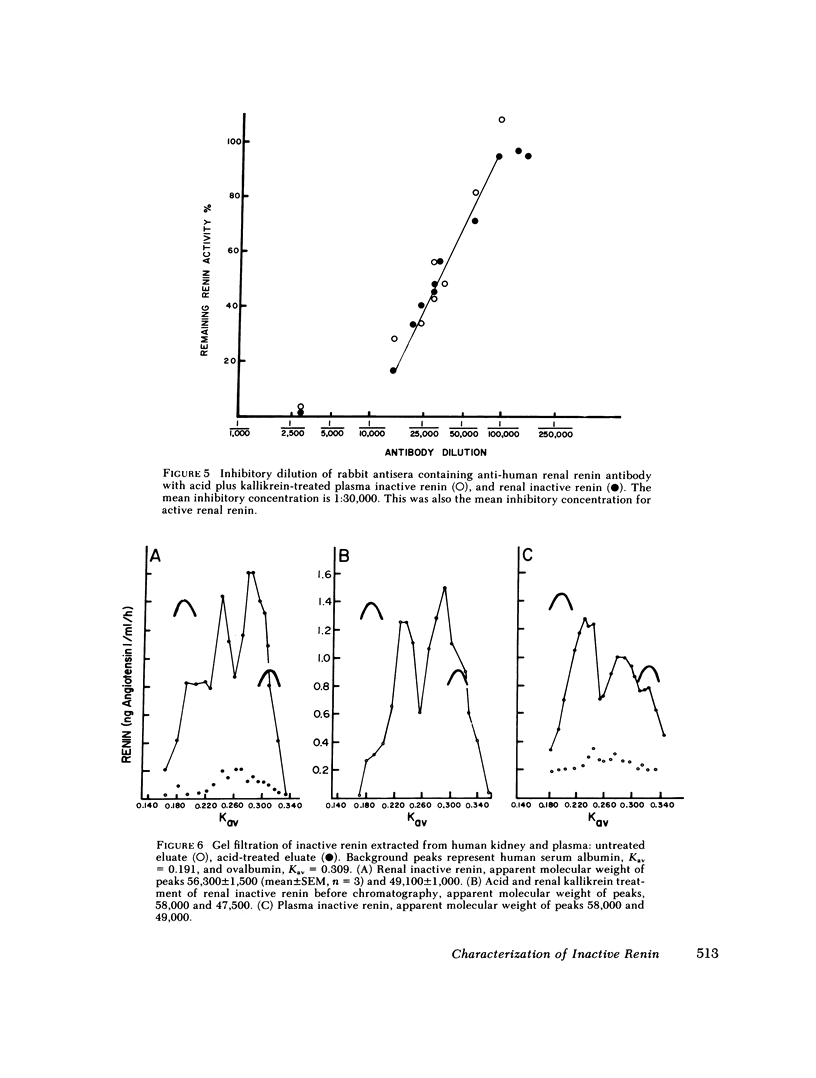
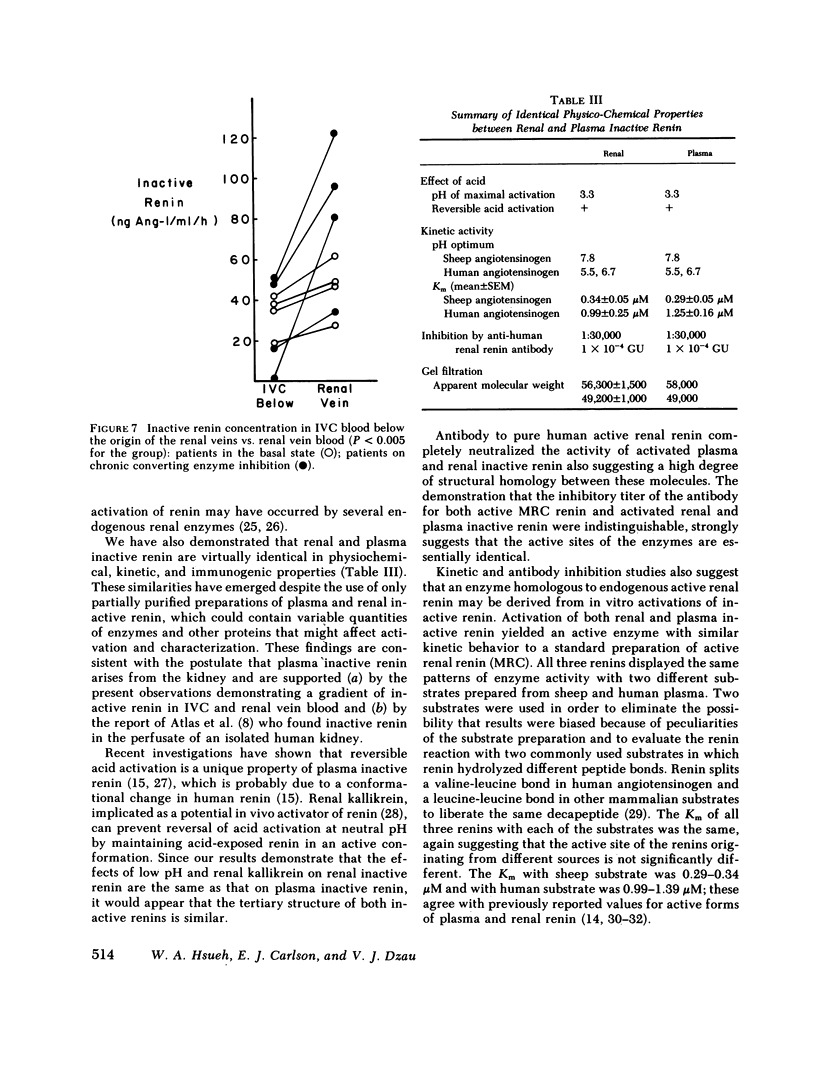
An inactive form of renin has been isolated from human plasma. It has been suggested that this may represent renin precursor secreted from the kidney. However, early studies failed to isolate inactive renin from human renal tissue. In this investigation, rapid processing of human kidney cortex at temperatures below 4 degrees C in the presence of protease inhibitors followed by cibacron-blue affinity chromatography allowed us to extract a totally inactive form of renal renin. Furthermore, we found that in kidney inactive renin constituted from 10 to as much as 50% of the total renin concentration. Biochemical characterization of the inactive renin from plasma and from kidney indicates that they are structural homologues and, when activated, have enzymatic properties that resemble active renal renin. Renal and plasma inactive renin were found to have the following properties in common: (a) a pH optimum of activation of 3.3; (b) reversible activation by acid dialysis on return to pH 7.4, 37 degrees C; (c) pH optima of enzyme activity of 7.8 with sheep angiotensinogen and 5.5 and 6.7 (biphasic) with human angiotensinogen; (d) Michaelis-Menten constants, Km, of 0.29-0.34 microM with sheep angiotensinogen, and 0.99-1.25 microM with human angiotensinogen; (e) an antibody to human renal renin mean inhibitory titer of 1:30,000 with 1 X 10(-4) Goldblatt units of activated renal or plasma inactive renin; (f) gel filtration profiles consisting of two peaks with apparent molecular weights of 56,000 +/- 1,500 and 49,200 +/- 1,000. Activation of plasma and kidney inactive renin by acid plus renal kallikrein was not accompanied by a change in gel filtration elution patterns. To determine whether inactive renin is released by the kidney, we measured inactive renin in samples obtained simultaneously from both the renal veins and inferior vena cava below the origin of the renal veins. In eight consecutive patients, inactive renin concentration was significantly higher in renal venous blood than in inferior vena caval blood. These data indicate that human kidney contains and secretes significant quantities of inactive renin. Thus, the kidney appears to be a major source of inactive renin in human plasma.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoi W., Doi Y., Seto S., Suzuki S., Hashiba K. Dynamic responses of active and inactive renin in patients with essential and renovascular hypertension. Hypertension. 1981 Jan-Feb;3(1):126–133. doi: 10.1161/01.hyp.3.1.126. [DOI] [PubMed] [Google Scholar]
- Chang J. J., Kisaragi M., Okamoto H., Inagami T. Isolation and activation of inactive renin from human kidney and plasma. Plasma and renal inactive renins have different molecular weights. Hypertension. 1981 Sep-Oct;3(5):509–515. doi: 10.1161/01.hyp.3.5.509. [DOI] [PubMed] [Google Scholar]
- Day R. P., Luetscher J. A. Big renin: a possible prohormone in kidney and plasma of a patient with Wilm's tumor. J Clin Endocrinol Metab. 1974 May;38(5):923–926. doi: 10.1210/jcem-38-5-923. [DOI] [PubMed] [Google Scholar]
- Derkx F. H., Wenting G. J., Man in 't Veld A. J., Verhoeven R. P., Schalekamp M. A. Control of enzymatically inactive renin in man under various pathological conditions: implications for the interpretation of renin measurements in peripheral and renal venous plasma. Clin Sci Mol Med. 1978 May;54(5):529–538. doi: 10.1042/cs0540529. [DOI] [PubMed] [Google Scholar]
- Eggena P., Chu C. L., Barrett J. D., Sambhi M. P. Purification and partial characterization of human angiotensinogen. Biochim Biophys Acta. 1976 Mar 18;427(1):208–217. doi: 10.1016/0005-2795(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Galen F. X., Devaux C., Guyenne T., Menard J., Corvol P. Multiple forms of human renin. Purification and characterization. J Biol Chem. 1979 Jun 10;254(11):4848–4855. [PubMed] [Google Scholar]
- Haas E., Goldblatt H., Gipson E. C. Extraction, purification, and acetylation of human renin and the production of antirenin to human renin. Arch Biochem Biophys. 1965 Jun;110(3):534–543. doi: 10.1016/0003-9861(65)90447-9. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Carlson E. J., O'Connor D., Warren S. Renin requires a structural alteration prior to activation by renal kallikrein. J Clin Endocrinol Metab. 1980 Oct;51(4):942–944. doi: 10.1210/jcem-51-4-942. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Luetscher J. A., Carlson E. J., Grislis G. Big renin in plasma of healthy subjects on high sodium intake. Lancet. 1978 Jun 17;1(8077):1281–1284. doi: 10.1016/s0140-6736(78)91267-9. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Luetscher J. A., Carlson E. J., Grislis G. Inactive renin of high molecular weight (big renin) in normal human plasma. Activation by pepsin, trypsin, or dialysis to pH 3.3 and 7.5. Hypertension. 1980 Nov-Dec;2(6):750–756. doi: 10.1161/01.hyp.2.6.750. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Luetscher J. A., Carlson E., Grislis G., Elbaum D., Chavarri M. A comparison of cold and acid activation of big renin and of inactive renin in normal plasma. J Clin Endocrinol Metab. 1978 Oct;47(4):792–799. doi: 10.1210/jcem-47-4-792. [DOI] [PubMed] [Google Scholar]
- Leckie B. J., McGhee N. K. Reversible activation-inactivation of renin in human plasma. Nature. 1980 Dec 25;288(5792):702–705. doi: 10.1038/288702a0. [DOI] [PubMed] [Google Scholar]
- Morris B. J. Activation of human inactive ("pro-") renin by cathepsin D and pepsin. J Clin Endocrinol Metab. 1978 Jan;46(1):153–157. doi: 10.1210/jcem-46-1-153. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A. Role of proteolytic enzymes in biological regulation (a review). Proc Natl Acad Sci U S A. 1976 Nov;73(11):3825–3832. doi: 10.1073/pnas.73.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K., Vuust J., Lund T. Renin precursor from mouse kidney identified by cell-free translation of messenger RNA. Clin Sci (Lond) 1980 Oct;59(4):297–299. doi: 10.1042/cs0590297. [DOI] [PubMed] [Google Scholar]
- Pratt R. E., Dzau V. J., Ouellette A. J. Abundant androgen regulated mRNAs in mouse submandibular gland: cell-free translation of renin precursor mRNA. Nucleic Acids Res. 1981 Jul 24;9(14):3433–3449. doi: 10.1093/nar/9.14.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H., Oza N. B., Ryan J. W. Human urinary kallikrein converts inactive to active renin and is a possible physiological activator of renin. Nature. 1978 Sep 14;275(5676):144–145. doi: 10.1038/275144a0. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H. Prorenin and other large molecular weight forms of renin. Endocr Rev. 1980 Fall;1(4):365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Bühler F. R., Laragh J. H., Vaughan E. D., Jr The physiology of renin secretion in essential hypertension. Estimation of renin secretion rate and renal plasma flow from peripheral and renal vein renin levels. Am J Med. 1973 Sep;55(3):391–401. doi: 10.1016/0002-9343(73)90138-1. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Moon C., Laragh J. H., Alderman M. Plasma prorenin: cryoactivation and relationship to renin substrate in normal subjects. Am J Med. 1976 Nov;61(5):731–738. doi: 10.1016/0002-9343(76)90154-6. [DOI] [PubMed] [Google Scholar]
- Skeggs L. T., Lentz K. E., Kahn J. R., Hochstrasser H. Studies on the preparation and properties of renin. Circ Res. 1967 Jul;21(1 Suppl):91+–91+. [PubMed] [Google Scholar]
- Skinner S. L., Cran E. J., Gibson R., Taylor R., Walters W. A., Catt K. J. Angiotensins I and II, active and inactive renin, renin substrate, renin activity, and angiotensinase in human liquor amnii and plasma. Am J Obstet Gynecol. 1975 Mar 1;121(5):626–630. doi: 10.1016/0002-9378(75)90463-9. [DOI] [PubMed] [Google Scholar]
- Skinner S. L., Dunn J. R., Mazzetti J., Campbell D. J., Fidge N. H. Purification, properties and kinetics of sheep and human renin substrates. Aust J Exp Biol Med Sci. 1975 Feb;53(1):77–88. doi: 10.1038/icb.1975.8. [DOI] [PubMed] [Google Scholar]
- Slater E. E., Strout H. V., Jr Pure human renin. Identification and characterization and of two major molecular weight forms. J Biol Chem. 1981 Aug 10;256(15):8164–8171. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosawa H., Holladay L. A., Inagami T., Haas E., Murakami K. Human renal renin. Complete purification and characterization. J Biol Chem. 1980 Apr 25;255(8):3498–3502. [PubMed] [Google Scholar]
- Yokosawa N., Takahashi N., Inagami T., Page D. L. Isolation of completely inactive plasma prorenin and its activation by kallikreins. A possible new link between renin and kallikrein. Biochim Biophys Acta. 1979 Aug 15;569(2):211–219. doi: 10.1016/0005-2744(79)90056-1. [DOI] [PubMed] [Google Scholar]
- deLeiva A., Christlieb A. R., Melby J. C., Graham C. A., Day R. P., Luetscher J. A., Zager P. G. Big renin and biosynthetic defect of aldosterone in diabetes mellitus. N Engl J Med. 1976 Sep 16;295(12):639–643. doi: 10.1056/NEJM197609162951203. [DOI] [PubMed] [Google Scholar]



