Abstract
Objective
To analyze the durability of monotherapy with different classes of oral hypoglycemic agents (OHAs) in drug naïve patients with type 2 diabetes mellitus (T2DM) in real life.
Methods
Men and women with T2DM, who were new users of OHA monotherapy and registered in the Swedish National Diabetes Register July 2005–December 2011, were available (n=17 309) and followed for up to 5.5 years. Time to monotherapy failure, defined as discontinuation of continuous use with the initial agent, switch to a new agent, or add-on treatment of a second agent, was analyzed as a measure of durability. Baseline characteristics were balanced by propensity score matching 1:5 between groups of sulfonylurea (SU) versus metformin (n=4303) and meglitinide versus metformin (n=1308). HRs with 95% CIs were calculated using Cox regression models.
Results
SU and meglitinide, as compared with metformin, were associated with increased risk of monotherapy failure (HR 1.74; 95% CI 1.56 to 1.94 and 1.66; 1.37 to 2.00 for SU and meglitinide, respectively). When broken down by type of monotherapy failure, SU and meglitinide were associated with an increased risk of add-on treatment of a second agent (HR 3.14; 95% CI 2.66 to 3.69 and 2.52; 1.89 to 3.37 for SU and meglitinide, respectively) and of switch to a new agent (HR 2.81; 95% CI 2.01 to 3.92 and 3.78; 2.25 to 6.32 for SU and meglitinide, respectively). The risk of discontinuation did not differ significantly between the groups.
Conclusions
In this nationwide observational study reflecting clinical practice, SU and meglitinide showed substantially increased risk of switch to a new agent or add on of a second agent compared with metformin. These results indicate superior glycemic durability with metformin compared with SU and also meglitinide in real life.
Keywords: Pharmacoepidemiology, Oral Hypoglycaemic Agents, Type 2 Diabetes
Key messages.
Almost half of the patients with type 2 diabetes mellitus who were new users of metformin, sulfonylurea (SU), or meglitinide got add on of a second agent, switched to a new agent, or discontinued the initial agent when followed for up to 5.5 years.
SU and meglitinide were associated with a 2.5-fold to 3-fold increased risk of add-on treatment of a second agent and a threefold to fourfold increased risk of switch to a new agent compared with metformin, taking differences in baseline demographics and patient characteristics into account.
The results indicate superior glycemic durability with metformin compared with SU or meglitinide in real life.
Introduction
Early treatment with oral hypoglycemic agents (OHAs) is advocated in patients with type 2 diabetes mellitus (T2DM) in order to attain near-normal glycemia and reduce the risk of long-term complications.1 T2DM is characterized by several physiological disturbances, including progressive loss of β-cell function and insulin sensitivity.2 Owing to the progressive nature of T2DM,3 treatment intensifications such as adding a second glucose-lowering agent or switching to a more potent agent is often required to maintain acceptable glycated hemoglobin (HbA1c) levels over time.4 5 This gradual decline in effectiveness of OHAs over time has been called secondary drug failure or monotherapy failure.6 7 A Diabetes Outcome Progression Trial (ADOPT) compared the incidence of monotherapy failure in drug naïve patients with T2DM randomized to rosiglitazone, metformin, or glyburide.7 Monotherapy failure was defined as deterioration of glycemic control despite maximum dose of the initiated agent and was used as a measure of glycemic durability. The glycemic durability differed significantly between the agents, with cumulative incidences of monotherapy failure, at 5 years of follow-up, of 15% with rosiglitazone, 21% with metformin, and 34% with glyburide.
To evaluate the glycemic durability of different OHAs in real life, clinical trial data need to be complemented with analyses of observational data. Several observational studies have evaluated the persistence of different OHAs.8–11 The concept of persistence has similarities with durability and is typically defined as the frequency of patients continuing an initiated medication over time.12 Most studies on persistence of OHAs have not specified non-persistence as being due to treatment discontinuation, switch to a new agent, or add on of a second agent. As a result, we cannot be sure that non-persistence was due to loss of glycemic control in these studies. Rather, non-persistence was likely due to a variety of different causes. However, one study of 6729 patients with T2DM treated with metformin monotherapy or sulfonylurea (SU) monotherapy analyzed the time to progression to combination oral therapy or switch in oral therapy,13 which are clinical events that are driven by loss of glycemic control. The results showed a greater risk of progression to combination therapy or switch in therapy associated with SU as compared with metformin. Even though information about important covariates such as diabetes duration, HbA1c levels, and body mass index (BMI) were missing, the results indicated a greater risk of monotherapy failure with SU compared with metformin in real life.
This nationwide observational study was initiated to evaluate the durability of monotherapy with the available classes of OHAs in patients with T2DM in real life. The time to discontinuation of continuous use with the initial OHA, switch to a new agent, and add-on treatment of a second agent was analyzed separately and as a composite end point and was used as a measure of durability.
Methods
This is a nationwide cohort study based on information linked from five Swedish national registers: the National Diabetes Register (NDR), the Prescribed Drug Register, the Cause of Death Register, the Hospital Discharge Register, all held by the National Board of Health and Welfare (NBHW), and the longitudinal integration database for health insurance and labor market studies (LISA), administered by Statistics Sweden. All included patients have agreed by informed consent to be registered before inclusion.
Databases
Since the establishment in 1996, the NDR has been working with systematic quality improvement, as well as research and development in the field of diabetes mellitus.14 The NDR is a nationwide register representing hospital outpatient clinics and primary healthcare clinics from all geographical areas of Sweden. The register includes information about risk factors, complications, and medications. Physicians and nurses in hospital outpatient clinics and primary healthcare clinics report to the NDR at least annually via the internet or via direct transfer of data from medical record databases. The Prescribed Drug Register has full coverage of all filled prescriptions at Swedish pharmacies.15 The Cause of Death Register contains information about causes of mortality and death dates;16 the Hospital Discharge Register comprises information about diagnoses, performed procedures, and length of stay for inpatient care from 1987 onward;17 and the LISA contains information about the highest educational level for all Swedish residents.
Study population
Drug naïve patients with T2DM, who were registered in the NDR between July 1, 2005 and December 31, 2011, were eligible for inclusion in the study cohort. Patients had to be initiated on OHA monotherapy with their first filled prescription (index date) between July 1, 2006 and December 31, 2010 to be included in the study. Further inclusion criteria were age 18–85, BMI ≥18.5, and continuous use with the initiated OHA in monotherapy for at least 12 months. The epidemiological definition of T2DM used in the present study and in several previous publications from the NDR was: treatment with diet only, OHA only, or onset age of diabetes ≥40 years and treatment with insulin only or combined with OHA.4 18 19 To identify drug naïve patients, we excluded patients who had got a prescription of any glucose-lowering agent filled between July 1, 2005 and June 30, 2006. In Sweden, one prescription generally corresponds to 3 months of continuous use. Patients were considered continuous users if they filled at least three prescriptions during the first treatment year. The number of patients who initiated newer OHAs such as DPP-4 inhibitors, SGLT-2 inhibitors, glitazones, and α-glucosidase inhibitors was very small. Therefore, this study was restricted to patients initiating metformin, SU, and meglitinide, which were the most commonly used OHAs. Altogether, 69 667 patients met the inclusion criteria and were available for the study (figure 1). Patients with complete records on all baseline variables (n=17 309) were selected and used to elaborate four groups based on 1:5 matching by propensity score values for balancing of baseline covariates: SU (n=717) versus metformin (n=3585), and meglitinide (n=218) versus metformin (n=1090).
Figure 1.
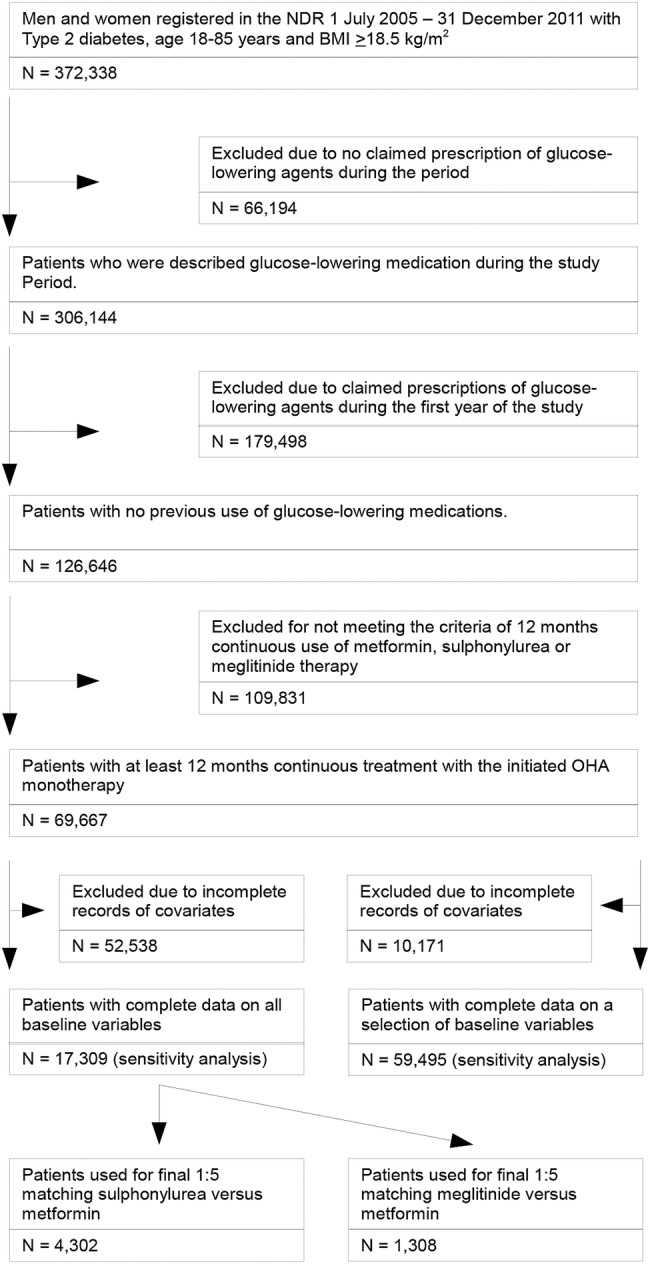
Enrollment of patients. (1) Age, sex, diabetes duration, index year, HbA1c, BMI, eGFR, previous cardiovascular disease, congestive heart failure and atrial fibrillation, educational level, use of psychiatric medications, and multidose drug dispensing. (2) Age, sex, diabetes duration, index year, previous cardiovascular disease, congestive heart failure and atrial fibrillation, educational level, use of psychiatric medications, and multidose drug dispensing. NDR, National Diabetes Register; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c: glycated hemoglobin.
Owing to the strict time period for collection of baseline variables (18 months before index date), a large portion of patients did not have information on BMI (65%), HbA1c (61%), and estimated glomerular filtration rate (eGFR; 64%). Therefore, we did sensitivity analyses on a group of patients with complete data on all baseline variables except BMI, HbA1c, and eGFR (n=59 495). Furthermore, sensitivity analyses were also performed on the 17 309 patients selected with complete records on all baseline variables.
Baseline variables
Baseline variables were type of OHA initiated, index year, age, diabetes duration, gender, HbA1c, eGFR, weight, BMI, educational level, medication with psychiatric agents, use of multidose drug dispensing, and history of cardiovascular disease (CVD), congestive heart failure (CHF) and atrial fibrillation (AF; table 1). History of CVD, CHF, and AF was defined as history of at least one event of CVD, CHF, or AF, respectively, anytime between the year of 1997 and the index date. CVD was defined as coronary heart disease (international classification of diseases, 10th revision (ICD-10) codes I20-I25, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)), stroke (ICD-10 codes I61, I63, I64), or peripheral vascular disease (ICD-10 codes I70.2, I73.1, I73.9, I79.2, E10.5, E11.5, E14.5). CHF was defined as ICD-10 code I50, and AF as ICD-10 code I48. Medication with psychiatric agents was defined as at least one filled prescription between July 2005 and the index date. BMI was calculated as weight in kilograms divided by height in meters squared. Educational level was presented as a value between 1 and 3. A value of 1 corresponded to elementary school, 2 to upper secondary school, and 3 to higher education. Laboratory analyses of HbA1c were carried out at local laboratories. HbA1c analyses are quality assured nationwide by regular calibration with the high-performance liquid chromatography Mono-S method. eGFR was calculated using the Modification of Diet in Renal Disease equation.20
Table 1.
Baseline data for patients with T2DM using 1:5 matching with propensity score for each comparison
| Metformin versus SU |
Metformin versus meglitinide |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | SU | Mean difference | Standardized difference | p Value | Metformin | Meglitinide | Mean difference | Standardized difference | p Value | |
| Number | 3585 | 717 | 1090 | 218 | ||||||
| Propensity score | 0.106±0.081 | 0.105±0.079 | 0.001 | 0.01 | 0.8 | 0.0431±0.041 | 0.0439±0.041 | −0.0008 | −0.02 | 0.8 |
| Age | 71.02±8.9 | 71.01±10.4 | 0.01 | 0.01 | 0.9 | 71.5±8.9 | 71.4±10.4 | 0.1 | 0.01 | 0.9 |
| Diabetes duration | 4.4±4.1 | 4.6±3.8 | −0.2 | 0.03 | 0.4 | 5.0±5.1 | 4.9±4.5 | 0.1 | 0.03 | 0.7 |
| HbA1c | 56.0±12.2 | 55.8±10.2 | 0.2 | 0.02 | 0.7 | 55.9±11.9 | 56.2±12.2 | −0.3 | −0.02 | 0.8 |
| BMI | 27.9±3.5 | 27.9±4.3 | 0.0 | 0.01 | 0.9 | 27.5±3.8 | 27.3±4.7 | 0.2 | 0.04 | 0.5 |
| eGFR | 77.6±17.4 | 78.0±23.6 | −0.4 | −0.02 | 0.6 | 76.9±18.0 | 77.5±23.6 | −0.6 | 0.03 | 0.7 |
| Male gender | 59.8 | 59.3 | 0.5 | −0.01 | 0.8 | 68.3 | 68.4 | −0.1 | 0.01 | 0.9 |
| Previous CVD | 15.9 | 19.4 | 3.5 | −0.09 | 0.03 | 16.5 | 15.1 | 1.4 | 0.04 | 0.6 |
| Previous CHF | 4.3 | 4.7 | −0.4 | −0.02 | 0.6 | 6.1 | 6.4 | −0.3 | −0.02 | 0.8 |
| Atrial fibrillation | 7.7 | 7.3 | 0.4 | 0.02 | 0.7 | 9.3 | 11.0 | −1.7 | −0.06 | 0.4 |
| Education score 1–3 | 1.72±0.72 | 1.75±0.73 | −0.03 | −0.04 | 0.3 | 1.72±0.72 | 1.71±0.77 | 0.01 | 0.01 | 0.7 |
| Psychiatric drugs | 20.5 | 22.0 | −1.5 | −0.04 | 0.4 | 19.8 | 22.9 | −3.1 | −0.08 | 0.3 |
| Multidose | 1.1 | 1.8 | −0.7 | −0.06 | 0.11 | 1.0 | 0.5 | 0.5 | 0.06 | 0.4 |
| Start year, mean | 2008.2±1.3 | 2008.2±1.3 | 0.0 | 0.02 | 0.6 | 2007.8±1.3 | 2007.8±1.2 | 0.0 | −0.021 | 0.9 |
Data given as means±SD or frequencies (%).
Standardized difference: for continuous variables (means and SDs estimated as customary): (mean A—mean B)/sqrt ((SD_A2+SD_B2)/2; for categorical variables: (freq A—freq B)/sqrt ((freq A * [1-freq A])+(freq B * [1-freq B]))/2.
BMI, body mass index; CHF, congestive heart failure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; multidose: multidose drug dispensing; SU, sulfonylurea; T2DM, type 2 diabetes mellitus.
Follow-up and definition of end points
All patients were followed from the index date until the occurrence of an end point event, death or otherwise until the end of the study period in December 31, 2011. The primary end point was monotherapy failure with the initiated OHA. This was defined as either: discontinuation of continuous use with the initial OHA or switch to a new agent or add-on treatment of a second agent. Secondary end points were the separate components of monotherapy failure: discontinuation of continuous use with the initial OHA, switch to a new agent, and add on of a second agent. Discontinuation was defined as a gap of >180 days between two prescription fills. Switch was defined as discontinuation of the initial OHA while initiating a new glucose-lowering agent within 180 days following the last filled prescription of the initial OHA. Add on was defined as initiation of a second glucose-lowering agent in addition to continued treatment with the initial OHA.
Statistical methods
Baseline characteristics are presented with means and SDs for continuous variables and frequencies for categorical variables in the cohort used for the main analyses of matched cohorts (table 1) and for the sensitivity analyses (online supplementary table S1). Crude significance levels for differences in baseline characteristics for patients on SU or meglitinide compared with patients on metformin were calculated using student t test or χ2 test. Propensity scores, defined as the conditional probability of being treated with either SU or meglitinide given covariates, were used to balance baseline characteristics between the groups. In the cohort used for the main analyses, a 1:5 SU to metformin and meglitinide to metformin propensity score matching was performed (table 1). The matching procedure was performed using a greedy algorithm, which makes the closest matches first and second closest matches next, in a hierarchical sequence until no more matches could be made.21 The ability of the propensity scores to balance measured variables between the matched groups was evaluated by calculation of p values using student t test or χ2 test, and also by computing standardized differences between matched groups.22 Standardized differences of less than 0.1–0.2 were considered to indicate good balance. Separate propensity scores were estimated for the comparison of SU versus metformin and meglitinide versus metformin in each patient using logistic regression.23 In these two propensity score models, the dependent variable was defined as initiation of SU and initiation of meglitinide, respectively. In both propensity score models, the independent variables were as follows: age, sex, diabetes duration, index year, HbA1c, BMI, eGFR, previous CVD, CHF and AF, educational level, use of psychiatric medications, and multidose drug dispensing.
Cumulative failure of all end points by treatment group in patients included in the main analyses of the matched cohorts (figure 2A–D and online supplementary figure S1) was estimated with the Kaplan-Meier estimator. Significances of the differences between curves were assessed using the log-rank test. Cox proportional hazards regression models were used to estimate HRs with 95% CIs for the primary and secondary end points comparing SU versus metformin and meglitinide versus metformin in the propensity score-matched cohort (table 2).
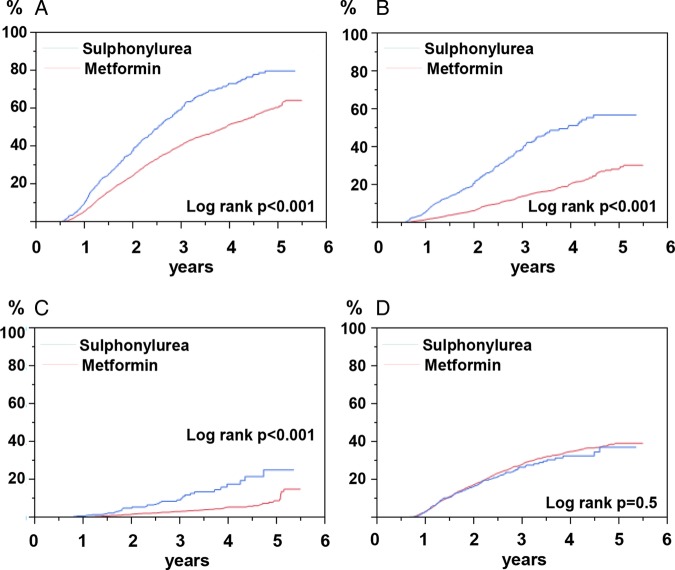
Figure 2.
Cumulative incidence of monotherapy failure in matched samples of sulfonylurea (n=717) versus metformin (n=3585), when followed for up to 5.5 years. All patients were on continuous treatment for at least 12 months. (A) All failure; (B) add on; (C) switch; (D) discontinuation. All graphs with log rank test p<0.001, except D with log-rank test non-significant.
Table 2.
HRs for monotherapy failures with sulfonylurea (SU) versus metformin, and with meglitinide versus metformin, using 1:5 matching with propensity score for each comparison
| Monotherapy failure | Events | HR | ||
|---|---|---|---|---|
| Drugs | N (%) | (95% CI) | p Value | |
| Overall | SU | 717/400 (55.8) | 1.74 (1.56 to 1.94) | <0.001 |
| Metformin | 3585/1415 (39.5) | 1.0 | ||
| Add on | SU | 717/214 (29.9) | 3.14 (2.66 to 3.69) | <0.001 |
| Metformin | 3585/429 (12.0) | 1.0 | ||
| Switch | SU | 717/50 (7.0) | 2.81 (2.01 to 3.92) | <0.001 |
| Metformin | 3585/114 (3.2) | 1.0 | ||
| Add on+switch | SU | 717/264 (36.8) | 3.06 (2.65 to 3.56) | <0.001 |
| Metformin | 3585/543 (15.2) | 1.0 | ||
| Discontinuation | SU | 717/136 (19.0) | 0.94 (0.79 to 1.13) | 0.5 |
| Metformin | 3585/872 (24.3) | 1.0 | ||
| Overall | Meglitinide | 218/136 (62.4) | 1.66 (1.37 to 2.00) | <0.001 |
| Metformin | 1090/503 (46.2) | 1.0 | ||
| Add on | Meglitinide | 218/65 (29.8) | 2.52 (1.89 to 3.37) | <0.001 |
| Metformin | 1090/163 (15.0) | 1.0 | ||
| Switch | Meglitinide | 218/23 (10.6) | 3.78 (2.25 to 6.32) | <0.001 |
| Metformin | 1090/40 (3.7) | 1.0 | ||
| Add on+switch | Meglitinide | 218/88 (40.4) | 2.76 (2.15 to 3.55) | <0.001 |
| Metformin | 1090/203 (18.6) | 1.0 | ||
| Discontinuation | Meglitinide | 218/48 (22.0) | 0.95 (0.70 to 1.29) | 0.7 |
| Metformin | 1090/300 (27.5) | 1.0 |
In additional Cox proportional hazards regression models (online supplementary table S2), adjustments were made by covariate adjustment (model 1) and by stratification with quintiles of the propensity scores (model 2). Model 1 included all covariates that were included as independent variables in the propensity score, as well as change in eGFR during follow-up. We also estimated adjusted HRs for the primary and secondary end points comparing SU versus metformin and meglitinide versus metformin in patients with missing data on several covariates (BMI, HbA1c, and eGFR were missing data; n=59 495), see online supplementary table S3. In this larger cohort, adjustments were made only by the available covariates (age, sex, diabetes duration, previous CVD, CHF and AF, educational level, use of psychiatric medications, and multidose drug dispensing).
The proportional hazards assumption at Cox proportional hazards regression was confirmed by simultaneously introducing all covariates as time dependent. Unmeasured confounders may affect the results if they are unrelated to or not fully accounted for by measured covariates. Therefore, we performed sensitivity analyses by quantifying the effects of a hypothetical unmeasured binary confounder with an HR for all end points of 2.0, given the different distributions of the confounder in the comparison of SU versus metformin (online supplementary table S4) and meglitinide versus metformin (online supplementary table S5).24
Unadjusted changes in HbA1c, eGFR, and weight during follow-up were analyzed using pairwise measurements (table 3). Changes were calculated as the last available value minus the index value and were presented as the absolute number of units with 95% CIs as well as percentages. Daily doses were calculated as the total amount of medication (mg) prescribed, divided by the number of days between the first prescription and end of last prescription.
Table 3.
Description of changes in HbA1c, eGFR, and weight during the study period
| Metformin |
Sulfonylurea |
Meglitinide |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Continuous n=13 845 |
Add on n=2814 |
Switch n=572 |
Discontinuation n= 5166 |
Continuous n=684 |
Add on n=369 |
Switch n=125 |
Discontinuation n=293 |
Continuous n=107 |
Add on n=87 |
Switch n=32 |
Discontinuation n=63 |
|
| Index HbA1c, mean±SD | 54.8±11.7 | 59.9±15.1 | 56.8±11.5 | 54.2±11.0 | 56.0±10.2 | 59.4±14.2 | 56.9±9.7 | 55.9±11.0 | 55.8±12.0 | 58.8±13.8 | 57.6±11.0 | 52.9±7.9 |
| Last HbA1c, mean±SD | 50.2±8.1 | 61.4±12.8 | 56.7±12.2 | 50.0±8.1 | 50.9±8.2 | 59.5±12.0 | 57.6±15.3 | 50.5±10.2 | 50.5±10.0 | 59.9±10.7 | 57.5±13.4 | 48.1±6.5 |
| Delta* HbA1c, mean (95% CI) | −4.6; −4.8–−4.4 | 1.5; 0.9–2.1 | −0.08; −1.2–1.1 | −4.1; −4.4–−3.8 | −5.1; −5.9–−4.3 | 0.11; −1.4–1.7 | 0.73; −1.9–3.3 | −5.4; −6.7–−4.0 | −5.4; −7.9–−2.8 | 1.1; −1.8–4.1 | −0.1; −5.1–4.9 | −4.9; −6.8–−2.9 |
| Change† in HbA1c (%) | −8.4 | 2.5 | −0.14 | −7.6 | −9.1 | 0.19 | 1.3 | −9.7 | −9.7 | 1.9 | −0.17 | −9.3 |
| Continuous n=12 569 | Add on N=2456 |
Switch N=526 |
Discontinuation N=4464 |
Continuous N=604 |
Add on N=322 |
Switch N=101 |
Discontinuation N=251 |
Continuous N=96 |
Add on N=72 |
Switch N=28 |
Discontinuation N=56 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index eGFR, mean±SD | 84.8±19.9 | 87.8±20.9 | 78.5±21.1 | 84.4±20.5 | 70.3±22.2 | 80.7±22.2 | 74.0±24.7 | 72.9±23.8 | 74.8±23.3 | 80.7±25.2 | 78.2±25.9 | 76.9±23.1 |
| Last eGFR, mean±SD | 84.6±20.9 | 88.2±21.2 | 76.1±24.4 | 84.2±20.3 | 67.9±21.7 | 80.3 ±24.1 | 72.2±23.3 | 70.4±23.1 | 71.9±23.8 | 80.2±23.9 | 74.1±27.5 | 73.2±21.5 |
| Delta* eGFR, mean (95% CI) | −0.14; −0.4–0.1 | 0.38; −0.2–1.0 | −2.4; −3.7–−1.0 | −0.17; −0.6–0.2 | −2.4; −3.4–−1.4 | −0.41; −1.8–0.1 | −1.84; −4.0–0.3 | −2.5; −4.0–−1.0 | −2.9; −5.2–−0.6 | −0.57; −3.6–2.4 | −4.1; −8.1–0.0 | −3.6; −6.0–−1.3 |
| Change† in eGFR (%) | −0.17 | 0.43 | −3.1 | −0.20 | −3.4 | −0.51 | −2.5 | −3.4 | −3.9 | −0.71 | −5.2 | −4.7 |
| Continuous n=13 045 |
Add on N=2644 |
Switch N=525 |
Discontinuation N=4852 |
Continuous N=622 |
Add on N=342 |
Switch N=111 |
Discontinuation N=266 |
Continuous N=105 | Add on N=83 |
Switch N=30 |
Discontinuation N=58 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index weight, mean±SD | 90.1±17.2 | 94.2±17.9 | 90.3±17.3 | 90.0±17.5 | 77.9±14.3 | 77.6±13.7 | 77.0±16.4 | 76.6±13.1 | 75.9±14.9 | 83.7±17.0 | 80.7±16.6 | 81.1±15.3 |
| Last weight, mean±SD | 87.5±17.2 | 93.3±18.0 | 88.3±17.6 | 88.9±17.3 | 77.7±14.6 | 79.3±14.3 | 76.3±14.9 | 76.8±13.1 | 75.8±14.8 | 84.4±17.1 | 81.6±17.3 | 80.9±15.1 |
| Delta* weight, mean (95% CI) | −2.5; −2.6–−2.4 | −0.9; −1.1–−0.7 | −2.0; −2.4–−1.6 | −2.1; −2.2–−1.9 | −0.2; −0.5–0.2 | 1.7; 1.4–2.1 | −0.7; −2.7–1.2 | 0.3; −0.2–0.7 | −0.1; −1.0–0.8 | 0.7; 0.0–1.4 | 0.9; −0.7–2.4 | −0.3; −1.2–0.7 |
| Change† in weight (%) | −2.8 | −0.9 | −2.2 | −2.3 | −0.2 | 2.2 | −1.0 | 0.4 | −0.1 | 0.8 | 1.1 | −0.3 |
Changes were calculated using pairwise measurements.
*δ values were calculated by taking the last available value minus index value, and was calculated for HbA1c (mmol/mol), eGFR (mL/min/1.73 m2), and weight (kg).
†Change (%) during the study period was calculated by dividing the δ value by the index value and multiplying by 100.
eGFR, estimated glomerular filtration rate; HbA1c: glycated hemoglobin.
All statistical analyses were performed with the use of SAS, V.9.3 (SAS Institute) except failure plots, which were produced in JMP V.11.0. A two-sided p value <0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 shows baseline data for patients included in the SU (n=717) to metformin (n=3585) and meglitinide (n=218) to metformin (n=1090) propensity score-matched cohort. The propensity score matching achieved good balance with non-significance in baseline characteristics between the groups, and with standardized differences less than 0.1. In all treatment groups, mean age was about 71 years, mean diabetes duration about 5 years, mean HbA1c about 56 mmol/mol, and mean BMI about 28 kg/m2. Online supplementary table S1 shows unadjusted baseline characteristics for patients included in the sensitivity analyses (n=59 495). Mean daily doses were approximately 1300 mg for metformin, 3 mg for SU, and 3 mg for meglitinide.
Monotherapy failure
Figure 2A–D shows cumulative incidences of monotherapy failure and the separate components of monotherapy failure over time for patients in 1:5 matched groups of SU (n=717) versus metformin (n=3583). The cumulative incidence of monotherapy failure during the follow-up period was high in both treatment groups, although it was higher for SU than for metformin with add on and switch (p<0.001), but not for discontinuation (not significant). Online supplementary figure S1 shows the corresponding failure plots for patients in 1:5 matched groups of meglitinide (n=218) versus metformin (n=1090). These curves were similar to those in figure 2.
Table 2 shows HRs with 95% CIs for monotherapy failure with SU versus metformin, and with meglitinide versus metformin, in the propensity score-matched cohorts. SU and meglitinide, as compared with metformin, were associated with an increased risk of overall monotherapy failure (HR 1.74; 95% CI 1.56 to 1.94 and 1.66; 1.37 to 2.00 for SU and meglitinide, respectively). When looking at the separate components of monotherapy failure, the results were similar to those in the cumulative failure plots shown in figure 2 and online supplementary figure S1. SU and meglitinide were associated with an increased risk of add-on treatment of a second agent (HR 3.14 95% CI 2.66 to 3.69 and 2.52; 1.89 to 3.37 for SU and meglitinide, respectively) and of switch to a new agent (HR 2.81; 95% CI 2.01 to 3.92 and 3.78; 2.25 to 6.32 for SU and meglitinide, respectively). The risk of discontinuation did not differ significantly between the groups.
Online supplementary table S2 shows results from the sensitivity analyses of all patients with complete data (n=17 309), using covariate adjusted (model 1) and propensity score stratified (model 2) models. The results from these analyses were fairly similar to those obtained from the propensity score-matched main analyses in table 2. Online supplementary table S3 shows results from the sensitivity analyses consisting of patients with complete records only on a selection of baseline variables (data on BMI, HbA1c, and eGFR missing; n=59 495). HRs for monotherapy failure and its separate components in these less extensively adjusted analyses were also quite similar to those in the matched samples shown in table 2.
To invalidate our findings in table 2 concerning overall monotherapy failure, a binary confounder with an HR of 2.0 would have to be present in at least 60% (absolute) more SU users or at least 40% more meglitinide users than metformin users. To invalidate our findings in table 2 concerning add-on treatment of a second agent or switch to a new agent, a binary confounder with an HR for these outcomes of 2.0 would have to be present in over 80% more SU users or meglitinide users than metformin users (online supplementary tables S4–5).
Changes in HbA1c, weight, and eGFR
Patients who continued their initial OHA monotherapy throughout the study showed an 8.4%, 9.1%, and 9.7% decrease in HbA1c for those initiated on metformin, SU, and meglitinide, respectively (table 3). Patients who discontinued their initial OHA showed similar improvements in glycemia between baseline and the date of discontinuation, while patients who got add-on treatment of a second agent or switched to a new agent showed stable or increased HbA1c values between baseline and the date of treatment change. Patients initiating metformin showed a decrease in weight throughout the study, with the largest decrease (2.8%) seen in those continuing metformin monotherapy throughout the study. Patients initiating SU or meglitinide were generally stable in weight. Small decreases in eGFR were seen in all groups during the study period.
Discussion
This is a large nationwide observational study analyzing the durability of metformin, SU, and meglitinide in real life. We found that almost half of the patients got add on of a second agent, switched to a new agent, or discontinued the initial agent when followed for up to 5.5 years. SU and meglitinide, as compared with metformin, were associated with a significantly increased risk of overall monotherapy failure, taking differences in baseline demographics and patient characteristics into account. The increased risk of overall monotherapy failure associated with SU and meglitinide was driven by a twofold to fourfold increased risk of add on of a second agent and switch to a new agent, whereas the risk of discontinuation of the initial OHA did not differ significantly between the groups. Patients continuing their initial monotherapy throughout the study showed improved HbA1c levels of approximately 10% irrespective of the type of OHA used, whereas patients who switched to a new agent or got add on of a second agent showed stable or slightly increased HbA1c levels. The improved HbA1c levels among patients who continued their initial monotherapy throughout the study confirm that these patients represented responders to treatment. Somewhat more surprisingly, patients who discontinued their initial OHA showed similar improvement in glycemic control. This group most certainly represents a very heterogenic group of patients, with a range of different underlying causes explaining their treatment discontinuation. Patients who got add on of a second agent or switched to a new agent showed unchanged or slightly increased HbA1c-levels leading up to these events. This indicates that deterioration of glycemic control was the primary underlying cause of events of add on of a second agent or switch to a new agent, and that these end points can be used as a measure of glycemic durability.
To the best of our knowledge, this is the first study reporting real-world data on the durability of meglitinide, an agent that contributes a therapeutic option in patients with contraindications for metformin or in those requiring a second agent to attain glycemic control.1 Our results, with highly significantly increased risks for add-on treatment of a second agent and switch to a new agent associated with SU, are in line with results from ADOPT. In ADOPT, initial monotherapy with metformin provided superior glycemic durability compared with glyburide,7 caused by a faster decline in β-cell function and insulin sensitivity in patients initiated on glyburide.25 In ADOPT, the Kaplan-Meier cumulative incidence of monotherapy failure (fasting plasma glucose >10 mmol/L) at 5 years was lower than in this study. This study and ADOPT differ in many respects, which could explain the difference in the cumulative incidence of monotherapy failure. First, this study is observational and represents the durability of different OHAs in real life. In contrast, ADOPT was a clinical trial, which reported the effects of protocol-driven treatments in a selected group of motivated patients who were followed closely with frequent visits. Second, the treatment guidelines available during the study period of this study recommended lower glycemic thresholds for treatment intensification than a fasting plasma glucose level >10 mmol/L,26 which was used in ADOPT. Finally, the patients included in this study were older and had a longer diabetes duration, suggesting that these patients had a more advanced disease than those included in ADOPT.
What about the results from other observational studies? A Canadian cohort study analyzed the risk of progression to combination oral therapy or a switch in oral therapy associated with metformin compared with SU.13 They found a modest decrease in risk of monotherapy failure associated with metformin compared with SU (HR 0.89; 95% CI 0.82 to 0.98). There were, however, important differences between this study and the Canadian study. Most importantly, we had information about essential covariates such as diabetes duration, HbA1c, and BMI, which were lacking in the Canadian study. Both HbA1c and BMI have been shown to be associated with progression from prediabetes to overt diabetes;27 28 diabetes duration is related to the remaining β-cell function. As a result, these variables are crucial covariates for estimating the glycemic durability of hypoglycemic agents. Another observational study of 12 697 veterans, including mainly male patients with T2DM, investigated the importance of study design choices in durability analyses. Interestingly, they found no significant difference in the durability (time to reach HbA1c >8%) between SU and metformin when extremely strict criteria for continuous treatment were used. However, when using criteria comparable to the ones used in this and other studies,9 11 SU was associated with a highly significantly increased risk of reaching HbA1c >8% (HR 1.45; 95% CI 1.29 to 1.63). The analyses were adjusted for important covariates, including diabetes duration, HbA1c, and BMI, but the follow-up time was short (approximately 1 year). ADOPT showed superior effects on β-cell function and glycemic control with glyburide compared with metformin or rosiglitazone during the first 6 months. After 6 months, patients on glyburide had a faster decline in β-cell function and loss of glycemic control, which resulted in significantly lower long-term glycemic durability with glyburide compared with metformin or rosiglitazone. Therefore, the risk of reaching HbA1c >8% during the first treatment year may not represent a good measure of long-term durability.
We were able to study smaller matched cohorts with well-balanced baseline covariates, as well as larger samples, of patients with T2DM for up to 5.5 years. Data were collected from the NDR database, which contains data from primary healthcare clinics and hospital outpatient clinics in all geographical areas of Sweden. The extensive information on patient characteristics and demographics enabled us to perform Cox proportional hazards regression analyses adjusted for many important covariates. Adjustments were made by several different statistical techniques and managed to balance baseline variables adequately between the groups with use of matching based on propensity scores. Despite extensive adjustments for relevant covariates, the possibility of residual confounding due to unknown and unmeasured covariates is a limitation in this study. However, the performed sensitivity analyses showed that a very strong confounder (HR 2.0) had to be present in more than 80% more SU or meglitinide users than metformin users to invalidate our findings concerning risk of add-on treatment of a second agent or switch to a new agent. To ensure complete data on all covariates, a large portion of patients had to be excluded due to missing data. However, owing to the large number of eligible patients, the study still had reasonable power to detect differences between the groups. Furthermore, sensitivity analyses on a larger group of patients (n=59 495) with missing data on some covariates showed similar results. The analyses in this study were based on filled prescriptions, which carry several limitations. Even though 12 months of continuous treatment was required to be included in the study, some treatment switches may have been due to late adverse effects and not loss of glycemic control. However, the results were similar when analyzing the risk of add-on treatment of a second agent, an event that should be less susceptible to misclassification due to adverse effects. There is an ongoing debate concerning criteria for continuous medication use in studies determining drug exposure using electronic data on filled prescriptions.8 We allowed gaps of maximum twice the day’s supply of an ordinary prescription, which was considered reasonable in a validation study of persistence and durability to diabetes medication.8 Similar criteria have been used in most previous studies in the field.9–11 Finally, the original objective of this study was to evaluate the durability of many different OHAs. Since few patients were initiated on newer OHAs, the analyses were restricted to metformin, meglitinide, and SU.
In conclusion, in this nationwide observational study of drug naïve patients with T2DM, the majority discontinued treatment, switched to a new agent, or got add-on treatment of a second agent when followed for up to 5.5 years. SU and meglitinide, as compared with metformin, were associated with a considerably increased risk of switch to a new agent and add on of a second agent, which remained during the entire follow-up period. These results strengthen the current evidence of a superior durability with metformin compared with SU in real life. The results from this study suggest that this also applies when comparing metformin with meglitinide.
Acknowledgments
The authors thank all regional NDR coordinators, contributing nurses, physicians, and patients. The patient organization Swedish Diabetes Association, and the Swedish Society of Diabetology support the NDR.
Footnotes
Contributors: All authors contributed to the conception and design of the study. MM, AMS, JC, and NE contributed to the acquisition of data and performed the statistical analyses. NE, KAS, BZ, BE, and SG contributed to the interpretation of data. NE contributed to the drafting of the article. NE, KAS, JC, BZ, BE, and SG contributed to the critical revision of the article for important intellectual content.
Funding: The Swedish Association of Local Authorities and Regions funds the NDR.
Competing interests: None.
Ethics approval: The Regional Ethics Review Board at the University of Gothenburg.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–79. 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–83. 10.1016/S0140-6736(13)62154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32(Suppl 2):S151–6. 10.2337/dc09-S301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekstrom N, Miftaraj M, Svensson AM et al. Glucose-lowering treatment and clinical results in 163 121 patients with type 2 diabetes: an observational study from the Swedish national diabetes register. Diabetes Obes Metab 2012;14:717–26. 10.1111/j.1463-1326.2012.01591.x [DOI] [PubMed] [Google Scholar]
- 5.Turner RC, Cull CA, Frighi V et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999;281:2005–12. 10.1001/jama.281.21.2005 [DOI] [PubMed] [Google Scholar]
- 6.Groop LC, Pelkonen R, Koskimies S et al. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care 1986;9:129–33. 10.2337/diacare.9.2.129 [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE, Haffner SM, Heise MA et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–43. 10.1056/NEJMoa066224 [DOI] [PubMed] [Google Scholar]
- 8.Greevy RA Jr., Huizinga MM, Roumie CL et al. Comparisons of persistence and durability among three oral antidiabetic therapies using electronic prescription-fill data: the impact of adherence requirements and stockpiling. Clin Pharmacol Ther 2011;90:813–19. 10.1038/clpt.2011.228 [DOI] [PubMed] [Google Scholar]
- 9.Gregoire JP, Sirois C, Blanc G et al. Persistence patterns with oral antidiabetes drug treatment in newly treated patients—a population-based study. Value Health 2010;13:820–8. 10.1111/j.1524-4733.2010.00761.x [DOI] [PubMed] [Google Scholar]
- 10.Jermendy G, Wittmann I, Nagy L et al. Persistence of initial oral antidiabetic treatment in patients with type 2 diabetes mellitus. Med Sci Monit 2012;18:Cr72–7. 10.12659/MSM.882459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rathmann W, Kostev K, Gruenberger JB et al. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab 2013;15:55–61. 10.1111/j.1463-1326.2012.01674.x [DOI] [PubMed] [Google Scholar]
- 12.Andrade SE, Kahler KH, Frech F et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565–74; discussion 75–7 10.1002/pds.1230 [DOI] [PubMed] [Google Scholar]
- 13.Eurich DT, Simpson SH, Majumdar SR et al. Secondary failure rates associated with metformin and sulfonylurea therapy for type 2 diabetes mellitus. Pharmacotherapy 2005;25:810–16. 10.1592/phco.2005.25.6.810 [DOI] [PubMed] [Google Scholar]
- 14.Gudbjornsdottir S, Cederholm J, Nilsson PM et al. The National Diabetes Register in Sweden: an implementation of the St. Vincent Declaration for Quality Improvement in Diabetes Care. Diabetes Care 2003;26:1270–6. 10.2337/diacare.26.4.1270 [DOI] [PubMed] [Google Scholar]
- 15.Wettermark B, Hammar N, Fored CM et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 16.Merlo J, Lindblad U, Pessah-Rasmussen H et al. Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two Swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol 2000;16:235–43. 10.1023/A:1007634722658 [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstrom N, Cederholm J, Zethelius B et al. Aspirin treatment and risk of first incident cardiovascular diseases in patients with type 2 diabetes: an observational study from the Swedish National Diabetes Register. BMJ Open 2013;3:e002688 10.1136/bmjopen-2013-002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekstrom N, Schioler L, Svensson AM et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2012;2:e001076 10.1136/bmjopen-2012-001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 21.Parsons LS. Performing a 1:N case-control match on propensity score. Proceedings of the 29th SAS Users Group International, Montreal, Canada 2004. [Google Scholar]
- 22.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734–53. 10.1002/sim.2580 [DOI] [PubMed] [Google Scholar]
- 23.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948–63. 10.2307/2533848 [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Lachin JM, Zinman B et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60:1552–60. 10.2337/db10-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009;32(Suppl 1):S13–61. 10.2337/dc09-S013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Mello VD, Lindstrom J, Eriksson J et al. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: the Finnish Diabetes Prevention Study. Diabetes Care 2012;35:211–17. 10.2337/dc11-1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Defronzo RA, Tripathy D, Schwenke DC et al. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care 2013;36:3607–12. 10.2337/dc13-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]