Abstract
Introduction
Promotion of healthy pregnancies has gained high priority in the Netherlands because of the relative unfavourable perinatal outcomes. In response, a nationwide study Healthy Pregnancy 4 All (HP4ALL) has been initiated. One of the substudies within HP4ALL focuses on preconception care (PCC). PCC is an opportunity to detect and eliminate risk factors before conception to optimise health before organogenesis and placentation. The main objectives of the PCC substudy are (1) to assess the effectiveness of a recruitment strategy for the PCC health services and (2) to assess the effectiveness of individual PCC consultations.
Methods/analysis
Prospective cohort study in neighbourhoods of 14 municipalities with perinatal mortality and morbidity rates exceeding the nation's average. The theoretical framework of the PCC substudy is based on Andersen's model of healthcare utilisation (a model that evaluates the utilisation of healthcare services from a sociological perspective). Women aged 18 up to and including 41 years are targeted for utilisation of the PCC health service by a four armed recruitment strategy. The PCC health service consists of an individual PCC consultation consisting of (1) initial risk assessment and risk management and (2) a follow-up consultation to assess adherence to the management plan. The primary outcomes regarding the effectiveness of consultations is behavioural change regarding folic acid supplementation, smoking cessation, cessation of alcohol consumption and illicit substance use. The primary outcome regarding the effectiveness of the recruitment strategy is the number of women successfully recruited and the outreach in terms of which population is reached in comparison to the approached population. Data collection consists of registration in the database of women that enrol for a visit to the individual PCC consultations (women successfully recruited), and preconsultation and postconsultation measurements among the included study population (by questionnaires, anthropometric measurements and biomarkers). Sample size calculation resulted in a sample size of n=839 women.
Ethics and dissemination
Approval for this study has been obtained from the Medical Ethical Committee of the Erasmus Medical Center of Rotterdam (MEC 2012-425). Results will be published and presented at international conferences.
Keywords: PRIMARY CARE, PREVENTIVE MEDICINE, PUBLIC HEALTH
Strengths and limitations of this study.
This study is rolled out in a ‘real time setting’ in collaboration with municipalities, public health services and curative care. Strategy is described thoroughly and evaluation aims to assess factors that influence implementation so that the programme’s method can be adopted and adapted to fit other local settings.
It is acknowledged to be a true challenge to reach prospective parents in the preconception period. In this study, a predefined strategy with active and passive recruitment components is employed to promote uptake of preconception care (PCC) consultations. Its effectiveness is seen as one of the primary study outcomes.
For the assessment of the effectiveness of PCC consultations—this study relies on biomarkers rather than on self-reported outcomes only in the assessment of the effectiveness of PCC consultations. To date, this has only been carried out in a PCC study regarding single outcomes (folic acid or smoking).
Background
The Healthy Pregnancy 4 All (HP4ALL) study was initiated because of the relatively high perinatal mortality rate of 10 per 1000 births, ranking the Netherlands at an unfavourable position in Europe.1 A huge concern was the inequality in the perinatal mortality rate within the country: deprived neighbourhoods were found to have an up to four times higher perinatal mortality rate than average.2 Societal, professional and political debates about how to improve perinatal health in the Netherlands dominated the policy agenda. One of the results was the launch of the HP4ALL study—commissioned by the Dutch Ministry of Health and Welfare—in May 2011.3 4
The objective of the HP4ALL study is to develop evidence-based strategies to improve perinatal health by interventions in the preconception or the antenatal period, which reduce adverse pregnancy outcomes. Accordingly, HP4ALL is divided into two substudies: the Preconception Care study (PCC)—a prospective cohort study—and the antenatal Rotterdam Risk Assessment (R4U) study—a cluster randomised controlled trial. This paper concentrates on the PCC substudy.
The rationale of PCC and its delivery approaches
The rationale of PCC originates in the growing recognition that the embryonic development and placentation phase is critical for the outcome of the pregnancy.5 PCC is a set of interventions that aims to identify and modify biomedical, behavioural and social risks to a woman’s health or pregnancy outcome through prevention and management.6
The preconception period can be seen as the earliest link between maternal and newborn health. Therefore, it has been recognised as a pivot point which can be utilised to improve perinatal health.7–9 In the Netherlands, 85% of the perinatal mortality cases were preceded by small for gestational age (SGA), premature birth and congenital anomalies10 11 In theory, many risk factors for these problems are present and potentially detectable and treatable/manageable before conception.5 7 By the time a women enters antenatal care, a large part of organogenesis has taken place. PCC should therefore be regarded as a necessary additional component to the obstetric care system in the improvement of perinatal outcomes.
Organisation of PCC
PCC can be organised in three forms: (1) collective PCC consisting of interventions targeted at the general public (eg, with group education or national campaigns); (2) general individual PCC consisting of individual consultations among couples contemplating pregnancy among the general public; (3) specialist individual PCC consisting of individual consultations among couples contemplating pregnancy with complex risk factors.12 These forms can be integrated in different approaches for delivery, dependent on the health system (eg, primary care, hospital based, PCC clinic) and the targeted audience (eg, high-risk population or general public).13
Individual PCC is a unique opportunity for professionally led PCC, which addresses general risk factors and personal risk factors after systematic screening. Furthermore, individual PCC is an opportunity to deliver PCC in a responsive fashion—so that besides the systematic standard risk factor screening the individual patient’s needs or preferences can be met.14 Thus, Individual PCC consultations are seen as the form of PCC to implement in the PCC substudy.
Recruitment for PCC
The concept of visiting a healthcare professional for PCC is not common in the Netherlands as well as in many other countries. First, the uptake of PCC requires that a pregnancy is planned. An explanation for the low uptake of PCC can be sought in the unfamiliarity of women or couples with the availability and potential benefits of the health service.15–17 Women or couples could assume that they are healthy and that it is not necessary to discuss their pregnancy wish with a professional. Women who are aware of risks might believe that nothing can be done to influence the course of a pregnancy in the future. Besides beliefs, actual barriers to attend PCC could also play a role. For instance, a woman's or couples’ willingness to disclose their pregnancy wish to a professional is a known barrier.17 Simply delivering PCC to women or couples on request does not seem to be sufficient to provide PCC at a scale to improve perinatal health. An active recruitment approach is necessary. Different active recruitment approaches are described in the literature. Wallace et al18 describe that the opportunistic approach of women during daily care is utilised most often by primary caregivers. However, despite the fact that couples in the general public are known to have at least more than one risk factor,19 many women/couples of reproductive age do not request a PCC consultation from their general practitioner (GP). An opportunistic approach is not feasible for midwives as they rarely see non-pregnant women. One trial employs the approach of inviting women through invitational letters.20 These approaches, however, require efforts from caregivers in an already stressed system; and the effectiveness is unknown. An evidence-based strategy is necessary to create an outreach for individual PCC consultations in order to improve perinatal health on a larger scale.
The main objective of this study is to implement and evaluate a local recruitment strategy for individual PCC and to promote and evaluate (health) behaviour change by delivery of PCC consultations in primary care. This paper provides an overview of the design and methodology of the HP4ALL PCC study.
Methods/design
Key attributes of the study
The PCC study is designed as a community-based study with a high-risk approach in a primary care setting with tools to improve the uniformity of the health message.
This section describes the key concepts of the study:
The high-risk approach: The Ministry of Health commissioned the HP4ALL study to target high-risk populations. After ranking the municipalities with the highest perinatal mortality and morbidity in the country, 14 municipalities were selected as candidates for participation (selection described elsewhere).4 The key approach of HP4ALL is to roll out the interventions in high-risk neighbourhoods—meaning neighbourhoods with rates of adverse perinatal outcomes above the average of the selected municipalities. It is presumed that women in these neighbourhoods can benefit the most from interventions in the HP4ALL study. Prevalence of risky lifestyle behaviours in the preconception phase is not exactly known as there is no surveillance among women contemplating pregnancy and risky behaviours tend to be under-reported during pregnancy. With regard to folic acid supplementation, a recent Dutch study conducted among a multiethnic population reported that 40% of pregnant women had used folic acid supplements before conception.21 A different study reported that of 7106 pregnant women, 8% smoked in the first trimester only and 17% continued smoking throughout pregnancy.22 In total, 35—50% of women are estimated to continue their alcohol consumption during pregnancy.23 The HP4ALL study will provide more information regarding risk behaviours before and in pregnancy in these high-risk neighbourhoods.
The community-based approach: A community-based approach has advantages as it (1) reaches populations which are hard to reach by ensuring trust: collaboration with local health authorities in a community-based approach provides the opportunity that the target population is approached by professionals they know and mostly trust; (2) promotes collaboration and local support as it draws together the sectors necessary for optimal outreach.24
A primary care approach: GPs and midwives practising in the high-risk neighbourhoods are recruited to deliver individual PCC consultations. They were selected because they can deliver the intervention in a responsive fashion, because they are familiar with the target population and by arranging them to provide the PCC consultations, accessibility of PCC is ensured.
Tools for the consistency of health messages: PCC for the general population necessitates a thorough risk factor screening. Therefore, GPs and midwives are facilitated with tools and training to ensure consistency, which is important as different health messages reduce the effectiveness of interventions.14
Theoretical framework of the HP4ALL PCC substudy
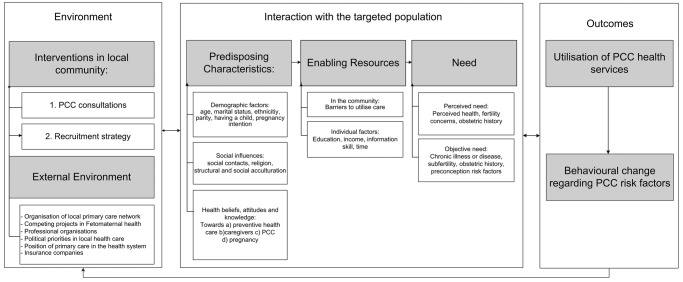
The delivery of PCC in the PCC substudy is based on Andersen's model of healthcare Utilisation.25 Andersen defines the utilisation of a health service and other personal health behaviours as an outcome. These outcomes are a function of the predisposition (to utilise the healthcare service) and enabling or impeding factors (to utilise the healthcare service) and perceived and objective need (to utilise the healthcare service). This model has been used to understand utilisation of PCC services and other healthcare services (oral health services, mental health services, primary care).26 27 Figure 1 should be read from left to right: The PCC substudy intervenes in the environment in order to target the population to achieve the outcomes on the right.
The environment: The study entails two organisational changes in the environment: First, individual PCC consultations are made available. Second, a strategy to recruit eligible women to utilise the PCC services is employed (see figure 2).
- The population: The study aims to target women of reproductive age (defined as 18 up to and including 41 years) living in municipalities with high perinatal mortality and morbidity rates with a specified recruitment strategy. Women will, however, decide individually, within their own context, whether they will use the healthcare service and/or change their health behaviours. We hypothesize this decision to be a function of:
- Predisposing factors: In accordance with Andersen's model of healthcare utilisation, we contemplate that a woman's decision to utilise PCC will depend on a function of her health beliefs and attitude towards the preconception health service and a healthy pregnancy, social influences and demographic factors.
- Enabling factors: The targeted woman can be stimulated to visit (or be impeded from visiting) the PCC health service by community factors (eg, a good infrastructure to attend a PCC consultation) or by individual factors (eg, speaking a different language than the PCC care provider can be a barrier to attending the PCC consultations).
- Need: The targeted woman needs to feel a need to utilise the service. There are two kinds of need: (1) an objective need (the service is necessary in terms of medical risks) and (2) a perceived need (the service is perceived as necessary by the woman herself). Perceived need can be related to the objective need, but this is not necessarily the case. Factors from the literature or those which we hypothesised to influence the objective and/or perceived need for a PCC consultation are mentioned in figure 1. Need, as a resultant of a perceived need and objective need, is thought to be influenced by predisposing characteristics (eg, knowledge regarding risks, social network to point out the relevance of PCC for the individual).
The outcomes: The primary outcomes of the PCC substudy are reduction of preconception risks by the (1) utilisation of PCC health services and (2) behavioural change regarding preconception risk behaviours. Reduction of preconception risk factors is thought to subsequently reduce (the risk for) perinatal morbidity and mortality.
Figure 1.
The Framework for the Healthy Pregnancy 4 All—Preconception Care substudy.
Figure 2.

The recruitment strategy of the Healthy Pregnancy 4 All—Preconception Care substudy.
The intervention
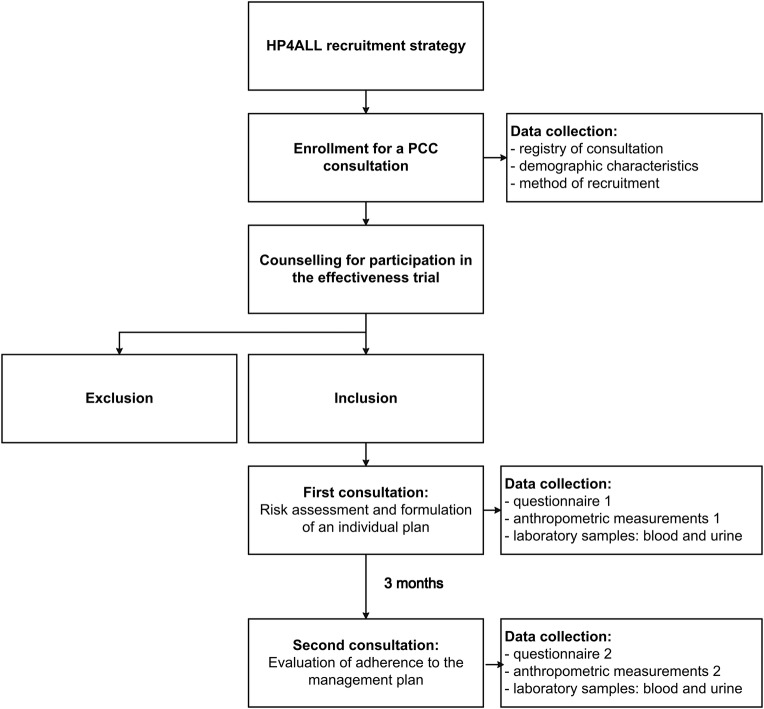
As displayed in figure 3, the PCC consultation consists of two visits to a participating GP or midwife in the course of 3 months. During the first consultation, systematic risk assessment is performed and a tailored management plan provided to address risk factors. Caregivers evaluate whether goals are reached or whether additional measures are necessary. Consultations are supported and archived with PCC tools (see box 1).28 29 The tool for risk assessment (Zwangerwijzer in Dutch) is a validated tool.30 When women participate in the cohort study as study participants, extra data are collected to assess the effectiveness of the care provided (see section on data collection).
Figure 3.
Flow chart for enrolment and intervention and data collection.
Box 1. Tools for delivery of preconception care in the Healthy Pregnancy 4 All—Preconception Care substudy.
Box 1 Tools for delivery of PCC in the Healthy Pregnancy 4 All—PCC substudy
Standardised risk assessment instruments improve delivery of PCC, unify risk assessment and facilitate documentation needs. The Healthy Pregnancy 4 All programme uses two tools:
The ZwangerWijzer Tool:
ZwangerWijzer is a self-administered questionnaire for couples designed to be filled in prior to consultation.
The questionnaire is freely accessible on the internet29 or on paper. The web-based survey has additional features: (1) additional information is provided when a risk factor is present (2) a list of risk factors is generated, listing what should be discussed during the PCC consultation. This list can be emailed to the PCC caregiver.
The questionnaire is adopted from The Preconception Health Assessment Form and a Family History Survey. It covers the following risk domains and risk factors:
| Background | Lifestyle | Medical history | Obstetric and gynaecological history | Family | Work |
|---|---|---|---|---|---|
| Maternal age BMI Ethnicity |
Exposure to radiation Smoking Alcohol Drugs Eating disorders Nutrition Folic acid supplement use Vitamin A Toxoplasmosis Listeria |
STDs HIV Rubella vaccination (Chronic) illness Prescribed medication Over-the-counter drugs |
Prior pregnancies Pregnancy complications Uterine anomalies Prior gynaecological surgeries |
Family history consanguinity |
Chemical exposure Other exposure Infectious agents Shifts/irregular hours Physically demanding work Stress |
Zwangerwijzer is a validated tool.30
The Preconceptiewijzer Tool:
Preconceptiewijzer is a web-based PCC archive system complementary to the ZwangerWijzer questionnaire.28 Providers can create a PCC file for their patients in which the questionnaire can be archived and the consultation(s) can be documented. The tool provides an overview sheet in which present risk factors (identified in ZwangerWijzer) are linked to digital patient information leaflets and protocols for the caregiver about these risk factors. The latter is a measure to improve the uniformity of health messages and interventions. Preconceptiewijzer is available online; providers have an own account which is secured for own use. This account and technical support is freely available.
BMI, body mass index; PCC, preconception care; STD, sexually transmitted diseases.
Study population
All women aged 18 up to and including 41 years (the adult reproductive lifespan) who make an appointment at the PCC health service are enrolled (registered in the database) to assess the effectiveness of the recruitment strategy. The additional criteria for inclusion in the cohort study to assess the effectiveness of PCC are: (1) a pregnancy wish (regardless of in which phase) and (2) voluntary participation. Exclusion criteria are: (1) no permission to be encountered about participation in the study (2) non-response to approach for inclusion (3) not speaking one of the following languages (Dutch, English, Turkish, Polish or Arabic) (4) cancellation/no-show at the appointment.
Recruitment and enrolment
The recruitment strategy consists of four components (see figure 2):
An invitational letter from the municipal public health service;
An invitational letter from participating general practices to their own patients;
Youth Health Care Physicians or nurses inform parents who visit the routine check-ups at the youth healthcare centre for their child with an information leaflet containing the names of the participating practices;
Referral by a preconception health educator after PCC education sessions. (A preconception health educator is a person from the peer group (the local community) who has completed certified training in health communication skills/preconception health). All female applicants for the PCC healthcare services are registered. These women are sent an information letter followed by a telephone call for individual counselling about participation in the study by the research team. Participants who agreed to take part in the study signed an informed consent form. Women receive the same PCC regardless of participation in the study; participation requires the participant to partake in data collection (questionnaires and laboratory tests) parallel to the care she receives.
Outcomes
The primary outcome regarding the effectiveness of the individual PCC consultations is behavioural change. Behavioural change is assessed for folic acid supplementation, smoking, alcohol consumption and illicit substance use. These four preconception health behaviours were chosen as primary outcomes due to their prevalence and their impact on the fetus and modifiability.31–36 Differences in these behaviours are assessed by premeasurement and postmeasurement by questionnaires (self-reported changes) and biomarkers (biochemical assessment of behavioural change). Biomarkers are used, as it is known that self-reported outcomes can show socially desirable answers. For example, in case of the use of folic acid supplements, a Dutch study found an over-report of 22% for self-reported folic acid supplement use.21
The primary outcome regarding the effectiveness of the recruitment strategy is the utilisation of the PCC services of the programme. This is measured quantitatively by the number of women who utilised the PCC programme (women successfully recruited) in relation to the number of women approached by the recruitment strategy. Second, the effectiveness of the recruitment strategy is assessed in terms of the outreach: by assessing characteristics of women reached. This includes basic demographics of women who were successfully recruited and identification of predisposing factors, need and enabling factors according to Andersen's model of healthcare utilisation among women included in the study. Data regarding the target group (all women aged 18 up to and including 41 years in the geographically targeted area) are obtained from municipal administrative records.
Outcomes, measures and data sources are presented in table 1.
Table 1.
Outcome assessment listed per study aim
| Research aim | Outcome measure | Data source |
|---|---|---|
| Effectiveness of individual PCC consultations | ||
| Primary outcomes | ||
| Folic acid suppletion | Self-reported folic acid use Biomarker (erythrocyte folate) confirmed folic acid suppletion |
Questionnaire and blood analysis at first consultation and 3 months after first consultation |
| Smoking | Self-reported smoking cessation Biomarker (serum cotinine) confirmed smoking cessation |
Questionnaire and blood analysis at first consultation and 3 months after first consultation |
| Alcohol | Self-reported cessation of alcohol consumption Self-reported reduction of alcohol consumption Biomarker (serum %CDT; urinary EtG or PeTH) confirmed reduction or cessation of alcohol consumption |
Questionnaire and blood/urine analysis at first consultation and 3 months after first consultation |
| Illicit substance use | Self-reported cessation of illicit substance use Biomarker (drug assessment in urine) confirmed cessation of illicit substance use |
Questionnaire and urine analysis at first consultation and 3 months after first consultation |
| Effectiveness of recruitment strategy | ||
| Primary outcomes | ||
| Characteristics of the cohort measured by Andersen's model | Characteristics of women who utilised the PCC health service according to the framework of the substudy (figure 1) | Questionnaire at first consultation |
| Outreach of the municipal letter | Proportion of women successfully recruited through the letter from the municipality in relation to the number of women approached with a letter from the municipal health service/municipality Characteristics of women successfully recruited after receiving the letter from the municipality in relation to characteristics of women residing in the selected neighbourhood(s) |
Data on women successfully recruited (the Gemstracker database) and data from women included in the study (questionnaire 1). (Anonymous) Municipal administrative records provide characteristics of the target population: all women aged 18–42 residing in the high-risk neighbourhood |
| Outreach of the GP letter | Proportion of women successfully recruited in relation to the number of women approached by a letter from their general practice. Characteristics of these women in relation to characteristics of women residing in the selected neighbourhood(s) |
Data on women successfully recruited (the Gemstracker database) and data from women included in the study (questionnaire 1). (Anonymous) register of women who were sent a letter by general practices. (Anonymous) Municipal administrative records provide characteristics of the target population: all women aged 18–42 residing in the high-risk neighbourhood |
| Outreach of the Preconception health educators | Proportion of women successfully recruited after being approached about the service during a peer health education session. Characteristics of these women in relation to characteristics of women residing in the selected neighbourhood(s) |
Data on women successfully recruited (the Gemstracker database) and data from women included in the study (questionnaire 1). Questionnaires of participants of preconception health education sessions (Anonymous) Municipal administrative records provide characteristics of the target population: all women aged 18–42 residing in the high-risk neighbourhood |
| Outreach of the Child Welfare service | Proportion of women successfully recruited after being approached during a visit to the Child Welfare service Characteristics of these women in relation to characteristics of women residing in the selected neighbourhood(s) |
Data on women successfully recruited (Gemstracker database) and data from women included in the study (questionnaire 1). (Anonymous) Municipal administrative records provide characteristics of the target population: all women aged 18–42 residing in the high-risk neighbourhood |
CDT, carbohydrate deficient transferrin; EtG, ethylgluconeride; PCC, preconception care; PeTH, phosphatidylethanol.
Data collection and measurement
The process of data collection is illustrated in figure 3.
All women who make an appointment are registered in the Gemstracker (Generic Medical Survey Tracking system) database.37 The Gemstracker system first helps keep a log of all the consultations. Furthermore, the system assists in keeping a log of the inclusion process after which it organises data collection within a specific time track, by activating fields or questionnaires for respondents or PCC providers.
Data collection consists of a questionnaire (questionnaires 1 and 2, respectively), anthropometric measurements and laboratory tests, performed as a baseline measurement (before the first consultation) and follow-up measurement (around the second consultation).
Questionnaires: Questionnaire 1 is filled in prior to the first PCC visit. This questionnaire assesses the characteristics of the study participant and health behaviours regarding the primary outcomes and other preconception risk factors. Questionnaire 2 is filled in after 3 months to assess changes in health behaviour regarding preconception risk factors. The questionnaires are filled in on paper or via the internet. Participants were reminded up to two times to fill in the questionnaire. The questionnaires were available in Dutch, English, Arabic, Turkish and Polish.
Anthropometric measurements: PCC providers measure the following anthropometric measurements at both PCC visits: blood pressure (mm Hg), length (cm), weight (kg), hip and waist circumference (cm). These measurements were performed according to a predefined protocol.
Laboratory tests: Data from the questionnaires regarding the primary outcomes of behavioural change are verified with biomarkers. Folic acid supplementation is assessed by measuring red cell folate in the serum.31 Smoking cessation is assessed by serum cotinine levels.38 39 Applying biomarkers for alcohol consumption is challenging because diagnostic accuracy is generally moderate and diagnostic properties differ over different alcohol consumption quantities and patterns. Ideally, one would match a biomarker to the presumed alcohol consumption (quantitatively in time) of the study population. However, the prevalence of alcohol consumption is difficult to predict as there is a lack of consensus regarding the classification of alcohol consumption.40 Numbers in the general population and in cohorts of pregnant women vary.35 As there is no consensus regarding safety of alcohol consumption in the preconception phase, the recommendation is to not consume alcohol in the preconception phase. Thus, we will be interested in biomarkers for any level of alcohol consumption. To detect alcohol drinking in the heavy end of the spectrum, we chose to use carbohydrate deficient transferrin (CDT%).41 As a biomarker to assess the mild–moderate drinking spectrum, we will explore the availability of ethylgluconeride (EtG) or a serum test of phosphatidylethanol (PEth) for the mild–moderate drinking spectrum.42 Illicit substance use will be tested with conventional urinary drug tests (assessing amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, methadone, opioids (codeine, morphine, heroin, oxycodone, etc), phencyclidine, propoxyphene, synthetic cannabinoids). Providers are blinded to data from questionnaires and from the mentioned biomarkers.
‘In case of no-show at either consultation, PCC providers are encouraged to provide a new appointment for the consultation. If the woman does not attend the first PCC consultation, she will be included for the analysis of the recruitment strategy. However, she will be excluded from the PCC cohort. If a woman does not attend the second PCC consultation, she will be asked to complete her second questionnaire and where it is logistically opportune, she will be asked to undergo a second laboratory assessment. She will be included in the outcome assessment of the effectiveness trial in that case.
Data analysis and sample size calculation
Data analysis
Characteristics of the study population and preconception health behaviours at baseline will be described continuously (mean or median, SD or IQR) or descriptively (percentages and CIs).
Changes in preconception health behaviours will be analysed paired. Preconsultation and postconsultation differences will be expressed with mean differences and SDs in continuous variables (in case of normality) or with median and IQR (in case of skewed data). Student t tests (in case of normality) or Wilcoxon Matched Pairs Test (in case of skewed data) will be performed for hypothesis testing. Dichotomous variables will be tested with the exact binomial test. Significance testing will be performed two-sided with an α of 0.05. Regarding change in folic acid supplementation and smoking cessation, one-sided testing will be performed with an α of 0.025 (in line with the hypothesis used for sample size calculation).
Regarding the effectiveness of the recruitment strategy, utilisation of the PCC health service will be expressed in percentages in relation to the number of women approached by the recruitment strategy. Characteristics of women who visit the PCC health service will be assessed according to the framework for utilisation of the PCC service (see figure 1).
Sample size calculation
Sample size calculation was performed for the two most important primary outcomes: folic acid supplementation and smoking cessation.
Regarding folic acid supplementation, 839 women are needed in order to reject the null hypothesis (H0) that the PCC service will lead to a 20% increase of folic acid users in women who were not already using folic acid supplements at baseline (assumptions for this power calculation were (1) the smallest clinically relevant difference (‘Δ’) is a 20% increase of folic acid in non-users at baseline, (2) the proportion of women using folic acid at baseline is 30% (π0=30%),41 (3) a select dropout rate of 10%, (4) pairwise analysis of results, (5) a statistical significance level of α<0.025 (one-sided correction for multiple testing due to primary outcome measures) and (6) a power (1-β) of 0.80). Regarding smoking cessation, 687 women are needed to reject the null hypothesis (Ho) that the PCC service will lead to a <5% decrease in smoking cessation among women who smoked at baseline. (Assumptions for the power calculation were (1) the smallest clinically relevant difference (‘Δ’) is a 5% decrease in smoking compared to baseline, (2) the proportion of women smoking at baseline is 30% (π0=30%)42 43 (3) a random drop-out rate of 10%, (4) pairwise analysis of results, (5) a statistical significance level of α<0.025 (one-sided correction for multiple testing due to primary outcome measures) and (6) a power (1-β) of 0.80.)
Thus, the cohort study should comprise 839 women to meet the needs of both primary outcomes.
Organisation and time schedule
Municipalities were encountered for participation from June 2011 to November 2013. General practices and midwife practices were encountered for participation from November 2011 to July 2013. They were prepared to deliver PCC in the PCC substudy after a one-on-one training to deliver PCC according to the study protocol. They received a self-study e-learning course and study material about PCC in general and about risk factors. Practices were provided with information leaflets, posters and kits to hand out for the laboratory tests. Recruitment strategies were rolled out when practices were ready to receive participants for the study. Data collection is performed in close collaboration with the practices by means of the Gemstracker system. In order to promote the readiness of study participants to provide blood and urine samples and to ensure timely handling of the samples, all laboratory sampling and processing are performed at neighbourhood health centres or local laboratories in the participating municipalities. Finally, to reduce bias, the non-time critical laboratory tests are performed at one central laboratory (the trial laboratory of the Erasmus Medical Center of Rotterdam, the Netherlands). After each PCC consultation study, participants receive a preassembled laboratory kit from their PCC provider. This kit includes 1 mL freezer capsules, a urine container and an application form to process the material according to the standard operating procedure of our study. All local laboratories were accredited by CCKL (in Dutch: Coördinatie Commissie ter bevordering van de Kwaliteitsbeheersing op het gebied van Laboratoriumonderzoek in de Gezondheidszorg or the organisation that audits laboratories in the healthcare system in the Netherlands).44 Blood samples are collected in EDTA, SST or sodium fluoride vacutainers (in size and numbers as routine to the local laboratories). Local laboratories perform tests which have to be performed within 1 h (eg, glucose) or before refrigeration (haemoglobin, haematocrit, mean corpuscular volume, red blood cell count) directly after blood sampling. Two mL of whole blood is then pipetted and stored and the remainder is centrifuged (depending on local equipment: ±2000 g/10 min). Approximately 2 mL of plasma and 7 mL of serum is pipetted into 1 mL freezer capsules. Urine is centrifuged (depending on local equipment: ±2000 g/10 min) after which 4 mL is stored. Whole blood is stored at −20°C; plasma/serum fraction and urine at −70 or 80°C (depending on local equipment). All laboratories closely monitor their storing protocol and are able to provide a report of the storing conditions at request. At set times, all samples are distributed to the central trial laboratory. The first municipality started enrolment in February 2013 and the last municipality started inclusion in February 2014. Enrolment is ongoing until the time period: April 2014 or until the calculated sample size of included participants has been reached to meet current research goals. As the sample size has not been reached as yet, the inclusion period is currently planned until December 2014.
Ethical considerations
The HP4ALL PCC substudy has been approved by the Medical Ethical Committee of the Erasmus Medical Center of Rotterdam (MEC 2012-425). In line with regulations, an independent physician is available for consultation by the (eligible) study population.
Discussion
The Healthy Pregnancy 4 All—PCC substudy aims to provide evidence for comprehensive and systematic delivery of PCC to the general public that contemplates pregnancy and to identify effective ways to reach women to promote utilisation of the PCC health services. The study is rolled out in municipalities with disadvantaged perinatal health. Outcome measures of the study are the effectiveness of the employed recruitment strategy and the effectiveness of the PCC service in achieving behavioural change regarding preconception risk behaviours. In doing so, we acknowledge that merely providing PCC is insufficient and we aim to develop an integrated approach in which recruitment is combined with delivery of PCC—ultimately to improve perinatal healthcare by PCC.
Internationally, PCC is implemented in various ways and within different settings. This study will place PCC in a cross-domain perspective in the Netherlands for the first time as multidisciplinary collaborations are initiated among municipalities, public health services and the curative care setting. Although this can be a strength, it might also be challenging to motivate different partners with different points of departure in the health system. Prior to this programme, for instance, the role of public health in fetal-maternal healthcare was very limited. Furthermore, PCC has been brought under attention within general practice and midwifery. However, the experience seems to be that PCC is, at best, only delivered at a small scale within these echelons. By implementing PCC in these echelons in the context of the current study, more can be learnt about what is necessary to upscale delivery of PCC, if effective.
A limitation in our approach to improve perinatal health outcome with PCC is that we target the group that plans pregnancy, as this is a precondition for PCC. Although the Netherlands has a high planned pregnancy rate and excellent access to contraceptives,12 43 planned pregnancy rates and the access to contraceptive care could be lower within a population with lower socioeconomic status. Initially, we aim to optimise outcomes of planned pregnancies. If this is effective, it would be the time to assess where PCC and family planning could be integrated to increase further effectiveness.
A limitation in the assessment of the effectiveness of PCC consultations will be that the sampled population could be prone to a participation bias. First, since the eligible population will rely on the extent to which the recruitment strategy is able to recruit a study population that is representative of the community. Second, eligible high-risk women might be more difficult to include in the effectiveness study. However, we believe that we will have the data to explore the representativeness of the included population in relation to the population that did not want to participate in the cohort study and in relation to the targeted population in the community.
This is one of the first cohort studies in the Netherlands that assesses effectiveness of a PCC approach in a high-risk area in a general practitioner and midwifery setting. We have future aspirations to do further research within the context of this study.
Footnotes
Acknowledgements: The authors would like to thank the following institutions/persons from the Erasmus Medical center: the Department of Trials and Research Coordination, Dr JC van der Woude (independent physician for the (eligible) study population), Department of Public Health (F Santegoets) for construction and continuous support in the use of the Gemstracker system, Preconceptiewijzer (S Horsman) for facilitation with accounts and Clinical Chemists (Dr R de Jonge). They would like to thank the Erfocentrum for collaboration in the use of tools. The authors would also like to thank the following parties for participating in the substudy: local project managers in participating HP4ALL municipalities (M Bunnik, M. van der Ham, M van der Biezen, G Franssen, B Zeelen, A de Graaf, R Stoffels, S Wigggers, G de Rijk, B Heusschen, S Badal, E Hochheimer, A Verhoeff), participating midwife practices and general practices, preconception health educators and participating Youth Health Care services. They thank all clinical chemists and local trial coordinators of participating laboratories (Erasmus MC, ATAL-medial, Atrium MC, SHO, Flevoziekenhuis, Certe, Labwest, MST, Saltro). The authors would also like to thank the advisory board of the HP4ALL study.
Contributors: SFvV, AAV, LCdJ-P, AJMW, EAPS and SD were involved in the conception and design of the study. SFvV drafted the manuscript. SFvV, AAV, LCdJ-P, AJMW, EAPS and SD revised the manuscripts critically for intellectual content and read and approved the final manuscript. All authors agree to be accountable for the contents and integrity of this manuscript.
Funding: Dutch Ministry of Health (grant number 318 804); National Organization for Health Research and Development.
Competing interests: None.
Ethics approval: Medical Ethical Committee of the Erasmus Medical Center of Rotterdam (MEC 2012-425).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.EURO-PERISTAT: PERISTAT II study. European perinatal health report. Better statistics for better health for pregnant women and their babies in 2004. EURO-PERISTAT project in collaboration with SCPE EaE ed.; 2008.
- 2.de Graaf JP, Ravelli AC, Wildschut HI et al. . [Perinatal outcomes in the four largest cities and in deprived neighbourhoods in The Netherlands] Perinatale uitkomsten in de vier grote steden en de prachtwijken in Nederland. Ned Tijdschr Geneeskd 2008;152:2734–40. [PubMed] [Google Scholar]
- 3.Denktas S, Waelput A, van Voorst SF et al. . Healthy Pregnancy 4 All—Preconceptiezorg en risicoselectie tijdens de zwangerschap. Tijdschrift voor Gezondheidswetenschappen (TSG) 2012;90:479–83. 10.1007/s12508-012-0163-0 [DOI] [Google Scholar]
- 4.Denktas¸ S, Poeran J, van Voorst SF et al. . Design and outline of the Healthy Pregnancy 4 All Study. BMC Pregnancy Childbirth 2014;14:253 10.1186/1471-2393-14-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macklon NS, Geer IA, Steegers EAP. Textbook of periconceptional medicine. London: Informa Healthcare, 2009. [Google Scholar]
- 6.Recommendations to Improve Preconception Health and Health Care—United States. http://origin.glb.cdc.gov/mmwR/preview/mmwrhtml/rr5506a1.htm
- 7.Jack BW, Culpepper L. Preconception care. Risk reduction and health promotion in preparation for pregnancy. JAMA 1990;264:1147–9. 10.1001/jama.1990.03450090083032 [DOI] [PubMed] [Google Scholar]
- 8.Atrash HK, Johnson K, Adams M et al. . Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J 2006;10:S3–11. 10.1007/s10995-006-0100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassi ZS, Majeed A, Rashid S et al. . The interconnections between maternal and newborn health—evidence and implications for policy. J Matern Fetal Neonatal Med 2013;26(Suppl 1):3–53. 10.3109/14767058.2013.784737 [DOI] [PubMed] [Google Scholar]
- 10.van der Kooy J, Poeran J, de Graaf JP et al. . Planned home compared with planned hospital births in the Netherlands: intrapartum and early neonatal death in low-risk pregnancies. Obstet Gynecol 2011;118:1037–46. 10.1097/AOG.0b013e3182319737 [DOI] [PubMed] [Google Scholar]
- 11.Bonsel GJ, Birnie E, Denktas¸ S et al. . Dutch report: Lijnen in de Perinatale Sterfte, Signalementstudie Zwangerschap en Geboorte 2010. Rotterdam: Erasmus MC, 2010. http://www.nvk.nl/Nieuws/Dossiers/Dossier Perinatalezorg.aspx. Retrieved Jan 5, 2011. [Google Scholar]
- 12.Netherlands tHCot. Preconception care: a good beginning [Preconceptiezorg, een goed begin] the Hague, the Netherlands, 2007. [Google Scholar]
- 13.van der Zee B, de Beaufort I, Temel S et al. . Preconception care: an essential preventive strategy to improve children's and women's health. J Public Health Policy 2011;32:367–79. 10.1057/jphp.2011.13 [DOI] [PubMed] [Google Scholar]
- 14.Shannon GD, Alberg C, Nacul L et al. . Preconception healthcare delivery at a population level: construction of Public Health Models of Preconception Care. Matern Child Health J 2014;18:1512–31. 10.1007/s10995-013-1393-8 [DOI] [PubMed] [Google Scholar]
- 15.van der Zee B, de Beaufort ID, Steegers EA et al. . Perceptions of preconception counselling among women planning a pregnancy: a qualitative study. Fam Pract 2013;30:341–6. 10.1093/fampra/cms074 [DOI] [PubMed] [Google Scholar]
- 16.Lia-Hoagberg B, Rode P, Skovholt CJ et al. . Barriers and motivators to prenatal care among low-income women. Soc Sci Med 1990;30:487–95. 10.1016/0277-9536(90)90351-R [DOI] [PubMed] [Google Scholar]
- 17.Delvoye P, Guillaume C, Collard S et al. . Preconception health promotion: analysis of means and constraints. Eur J Contracept Reprod Health Care 2009;14:307–16. 10.1080/13625180903056123 [DOI] [PubMed] [Google Scholar]
- 18.Wallace M, Hurwitz B. Preconception care: who needs it, who wants it, and how should it be provided? Br J Gen Pract 1998;48:963–6. [PMC free article] [PubMed] [Google Scholar]
- 19.van der Pal-de Bruin KM, le Cessie S, Elsinga J et al. . Pre-conception counselling in primary care: prevalence of risk factors among couples contemplating pregnancy. Paediatr Perinat Epidemiol 2008;22:280–7. 10.1111/j.1365-3016.2008.00930.x [DOI] [PubMed] [Google Scholar]
- 20.Elsinga J, van der Pal-de Bruin K et al. . Preconception counselling initiated by general practitioners in the Netherlands: reaching couples contemplating pregnancy [ISRCTN53942912]. BMC Fam Pract 2006;7:41 10.1186/1471-2296-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikkens JJ, van Eijsden M, Bonsel GJ et al. . Validation of self-reported folic acid use in a multiethnic population: results of the Amsterdam Born Children and their Development study. Public Health Nutr 2011;14:2022–8. 10.1017/S1368980011000012 [DOI] [PubMed] [Google Scholar]
- 22.Bakker R, Steegers EA, Mackenbach JP et al. . Maternal smoking and blood pressure in different trimesters of pregnancy: the Generation R study. J Hypertens 2010;28:2210–18. 10.1097/HJH.0b013e32833e2a3d [DOI] [PubMed] [Google Scholar]
- 23. Factsheet on Alcohol and Pregnancy [in Dutch: Factsheet alcohol en zwangerschap]. www.stap.nl.
- 24.Rabinowitz P. Chapter 18, Section 4: Using Community Sectors to Reach Targets and Agents of Change. (Development. KWGfCHa ed. pp. Chapter 18; 2014:Chapter 18.
- 25.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1–10. 10.2307/2137284 [DOI] [PubMed] [Google Scholar]
- 26.Babitsch B, Gohl D, von Lengerke T. Re-revisiting Andersen's Behavioral Model of Health Services Use: a systematic review of studies from 1998–2011. Psychosoc Med 2012;9:Doc11 doi: 10.3205/psm000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan VK, Amamoo MA, Anderson AD et al. . Barriers to women's participation in inter-conceptional care: a cross-sectional analysis. BMC Public Health 2012;12:93 10.1186/1471-2458-12-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Preconception consultation tool for preconception care providers [Preconceptiewijzer]. www.preconceptiewijzer.nl.
- 29. Preconception questionnaire [Zwangerwijzer]. http://www.zwangerwijzer.nl.
- 30.Landkroon AP, de Weerd S, van Vliet-Lachotzki E et al. . Validation of an internet questionnaire for risk assessment in preconception care. Public Health Genomics 2010;13:89–94. 10.1159/000228980 [DOI] [PubMed] [Google Scholar]
- 31.de Weerd S, Thomas CM, Cikot RJ et al. . Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol 2002;99:45–50. 10.1016/S0029-7844(01)01573-3 [DOI] [PubMed] [Google Scholar]
- 32.De-Regil LM, Fernandez-Gaxiola AC, Dowswell T et al. . Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2010:CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vardavas CI, Chatzi L, Patelarou E et al. . Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. Eur J Pediatr 2010;169:741–8. 10.1007/s00431-009-1107-9 [DOI] [PubMed] [Google Scholar]
- 34.Castles A, Adams EK, Melvin CL et al. . Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med 1999;16:208–15. 10.1016/S0749-3797(98)00089-0 [DOI] [PubMed] [Google Scholar]
- 35.Mullally A, Cleary BJ, Barry J et al. . Prevalence, predictors and perinatal outcomes of peri-conceptional alcohol exposure—retrospective cohort study in an urban obstetric population in Ireland. BMC Pregnancy Childbirth 2011;11:27 10.1186/1471-2393-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Gelder MM, Reefhuis J, Caton AR et al. , National Birth Defects Prevention S. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology 2009;20:60–6. 10.1097/EDE.0b013e31818e5930 [DOI] [PubMed] [Google Scholar]
- 37. Gemstracker. https://gemstracker.org/general-information/
- 38.Connor Gorber S, Schofield-Hurwitz S, Hardt J et al. . The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12–24. 10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
- 39.de Weerd S, Thomas CM, Kuster JE et al. . Variation of serum and urine cotinine in passive and active smokers and applicability in preconceptional smoking cessation counseling. Environ Res 2002;90:119–24. 10.1006/enrs.2002.4395 [DOI] [PubMed] [Google Scholar]
- 40.Greenfield TK, Kerr WC. Alcohol measurement methodology in epidemiology: recent advances and opportunities. Addiction 2008;103:1082–99. 10.1111/j.1360-0443.2008.02197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999;19:261–71. 10.1016/S0741-8329(99)00044-0 [DOI] [PubMed] [Google Scholar]
- 42.Tavakoli HR, Hull M, Michael Okasinski L. Review of current clinical biomarkers for the detection of alcohol dependence. Innov Clin Neurosci 2011;8:26–33. [PMC free article] [PubMed] [Google Scholar]
- 43.Barometer of womens’ access to contraceptive choice in 10 EU countries. (IPPFEN) TIPPFEN ed.; 2013.
- 44. Coördinatie Commissie ter bevordering van de Kwaliteitsbeheersing op het gebied van Laboratoriumonderzoek in de Gezondheidszorg CCKL. http://www.cckl.nl/index.php?pagina=72 (accessed 5 May 2014). [Google Scholar]