Abstract
We present a case of a 66-year-old woman with decompensated alcoholic liver cirrhosis and poorly controlled non-insulin-dependent diabetes mellitus who was admitted with a 1 day history of altered mental status, high-grade fevers, worsening jaundice and generalised malaise with subsequent development of hypotension requiring intensive care. She was diagnosed with severe babesiosis with high-grade parasitaemia. She was also found to have Lyme disease coinfection. Despite aggressive therapeutic measures including appropriate antibiotics and multiple exchange blood transfusions, she developed septic shock and fulminant multiple organ failure with eventual demise. In this article, we highlight multiple tick-borne illnesses in a vulnerable host, in this case an elderly patient with liver cirrhosis, as risk factors for severe morbidity and potentially fatal outcomes.
Background
Deer tick-borne illness (DTBI) including babesiosis, human granulocytic anaplasmosis (HGA), human monocytic ehrlichiosis (HME) and Lyme disease are endemic in the New England and Midwestern regions of the USA. Coinfections are observed frequently and are due to disease transmission by common tick vectors. The clinical spectrum of DTBIs varies widely, ranging from asymptomatic disease to overwhelming sepsis. Vulnerable immune compromised patients, including the elderly and those with liver cirrhosis, with babesiosis or HGA suffer worse outcomes. There are limited data on the morbidity and mortality implications of multiple TBIs occurring concurrently in the same individual. The importance of screening for and prompt recognition of tick-borne coinfections, and poorer prognosis when multiple TBIs occur in susceptible individuals, are emphasised in this presentation.
Case presentation
A 66-year-old woman presented to the emergency room with a reported 1 day history of fevers, altered mental status, generalised malaise, jaundice and acute on chronic lower back pain. Her medical history was significant for alcoholic liver cirrhosis complicated by non-bleeding oesophageal varices, ascites that was responsive to diuretic therapy, portosystemic encephalopathy, hypertension, non-insulin-dependent diabetes mellitus, last known haemoglobin A1c was 9.0% 2 months prior to presentation, depression, lumbar spine stenosis and transitional cell carcinoma of the bladder, which was in remission following treatment with Bacille Calmette-Guerin (BCG) and transurethral resection.
Her home medications included escitalopram, nadolol, spironolactone, furosemide and lantus insulin. No recent changes to her medication regimen were reported. She had no known medication allergies. There was no history of recent travel; she owned no pets. A history of tick bite(s) could not be reliably elicited.
On initial evaluation, her vital signs were: temperature of 103°F, heart rate of 89 bpm, blood pressure of 108/51 mm Hg, respiratory rate of 16 cycles per minute and oxygen saturation of 92% on room air. Within 10 h of admission, she became obtunded and subsequently developed hypotension necessitating intensive medical care. On examination, she had prominent scleral icterus. Pupils were equally round and reactive to light bilaterally. Oral examination revealed fair dentition with no oral lesions, and dry buccal mucosa. Cardiovascular examination revealed regular heart rate and rhythm with no murmurs, rubs or gallops. Lungs were clear on auscultation. Abdomen was distended with non-tender hepatosplenomegaly but with no shifting dullness, and normoactive bowel sounds. Central nervous system evaluation was limited by the patient's mental status, however, she had no nuchal rigidity and moved all extremities to painful stimuli. There was a generalised petechial rash and pitting ankle oedema on both distal lower extremities. No peripheral lymphadenopathy was detected.
Investigations
Initial laboratory evaluation revealed a white cell count (WCC) of 8.4×103/µL with automated differential of 16% monocytes, 67% neutrophils and 3% basophils. A manual differential showed 18% band forms. The patient's haemoglobin was 12.5 g/dL and platelet count—78×103/µL. Her basic metabolic panel showed a blood glucose level of 230 mg/dL, creatinine—0.9 mg/dL, sodium—122 mmol/L, chloride—92 mmol/L and potassium—4.5 mmol/L. Her hepatic panel had a total protein of 6.0 g/dL, albumin—2.7 g/dL, alanine aminotransferase and aspartate aminotransferase were 27 and 72 units/L, respectively, total bilirubin was elevated at 7.01 mg/dL (normal <1.2 mg/dL) and the direct fraction was 1.93 mg/dL (normal <0.2 mg/dL). Alkaline phosphatase was normal at 79 units/L. Ammonia was mildly elevated at 37 µmol/L, international normalised ratio was 1.5. She had a peripheral blood smear that showed intracorpuscular parasites consistent with Babesia. Babesia quantitation was estimated at 9.4%. BinaxNOW rapid diagnostic test for malaria was negative. Urine analysis showed trace glucose, small blood, no protein, 0–1 red blood cells (RBCs) and white blood cells on microscopy. A chest X-ray was normal, CT scan of the head showed an old left frontal lobe infarct but no acute changes. Ultrasound of the right upper quadrant of the abdomen showed a cirrhotic liver with no ascites. Multiple blood cultures eventually returned negative. A Lyme C6 peptide antibody testing was positive at 5.40 (cut-off for positive result: 0.9), confirmatory western blot showed IgG reactivity. A dedicated blood smear examination for Ehrlichia and Anaplasma did not reveal morulae in leucocytes.
Treatment
In the intensive care unit, the patient was initially managed with standard early goal-directed therapy protocol for sepsis and eventually required pressors. She was placed on antibiotics: intravenous vancomycin 1.5 g dosed every 36 h and intravenous piperacillin/tazobactam 3.375 g dosed 6-hourly; she was later placed briefly on ampicillin 2 g intravenous every 4 h and ceftriaxone 2 g intravenous dosed 12-hourly empirically for possible meningitis when her mental status deteriorated. Once the peripheral blood smear results confirmed presence of Babesia on day 2 of her hospital stay, she was started on oral atovaquone 750 mg every 12 h, oral azithromycin 250 mg daily and intravenous doxycycline 100 mg twice daily. On the same day, following evaluation by the infectious diseases consult service, the antibiotic regimen was changed to intravenous quinine sulphate 648 mg dosed every 8 h and intravenous clindamycin 600 mg dosed 8-hourly given her severe clinical presentation with high degree of parasitaemia. She began receiving daily exchange blood transfusions concurrently.
Outcome and follow-up
The patient continued to deteriorate clinically despite appropriate medical therapy for her TBIs and a decline in her Babesia parasitaemia from 9.2% to 2.6% on repeat blood smear examination by day 3 of admission. She progressed to multi-organ failure including development of refractory hypothermia, acute kidney injury and hypoxic respiratory failure requiring mechanical ventilation and worsening lactic acidosis. She passed away 4 days into her hospital stay.
Discussion
DTBI are prevalent in the Northeastern and Mid-Western regions of the US and occur predominantly during the months of April to October with peak incidence in the summer when tick vectors are most active. The most common tick-borne illness (TBI) in the US is Lyme disease, with 27 203 confirmed and 9104 probable human cases in 2013.1 The deer tick (Ixodes scapularis), the principal vector of Lyme disease, also transmits Babesia microti (babesiosis), Anaplasma phagocytophilum, the causative agent of HGA and Powassan virus. Another hard-bodied tick, the lone star tick (Amblyomma americanum) is the primary vector for Ehrlichia species, which causes HME, and also transits tularaemia and southern tick associated rash illness (STARI).1–4
Tick surveillance in the USA show rates of dual infection among I. scapularis species ranged from 1.0 to 28.2% and varied by region but no triple infected ticks were found.5 On the other hand, in a survey of confirmed human DTBI for the presence of coinfections, 71/192 individuals (37%) had dual infections that were predominantly Lyme disease and babesiosis, and 4/192 (2%) had triple infections with Lyme disease, babesiosis and HGA.6 Human TBI coinfections may be due to a single tick bite with multiple organism-carrying ticks or multiple bites from single disease-carrying ticks.
Symptoms of babesiosis range from asymptomatic disease or mild febrile illness to septic shock with mortality rates ranging from 5 to 30%.7 HGA usually results in mild illness resembling a ‘flu-like syndrome’, but severe cases with a toxic shock-like syndrome may occur in the elderly or individuals with immune compromising conditions.8 Lyme disease typically manifests as a rash at the site of inoculation of skin by a tick and can disseminate to cause extracutaneous syndromes including arthritis, carditis, meningitis and cranial neuropathies.9
Our patient met definite clinical and laboratory diagnostic criteria for multiple tick-borne infections. Her blood smears demonstrated Babesia (figure 1) including some showing classic ‘Maltese cross’ formation as well as confirmatory serological evidence of Lyme disease. The US Centers for Disease Control and Prevention (CDC) recommends a two-step testing approach for the diagnosis of active Lyme disease, which requires confirmation of a positive enzyme immunoassay test (eg, C6 peptide antibody) with IgM and IgG western blot if the patient is less than 30 days from symptom onset or only an IgG western blot for individuals with symptoms greater than 30 days.10 Our patient's Lyme test results were compatible with infection acquired over 30 days. She had blood smears that were negative for morulae in leucocytes as part of a screen for other tick-borne coinfections, however, it is known that the sensitivity of blood smears is poor, as morulae are identified in acute disease caused by HME in only 1–20% of patients and 20–80% of patients with HGA.11 12 One week after her demise, results of tests sent during her initial evaluation including serologies for Ehrlichia chaffeensis IgG returned positive at 1:256 dilution and Anaplasma phagocytophilum IgG antibody was elevated at 1:160 dilution. Since the patient had confirmed Lyme disease and babesiosis, it is likely that she had anaplasmosis, which is carried by the same tick vector, and that the positive Ehrlichia serologies may represent a test cross reaction.13 As paired sera were not checked and no parasites were detected on blood smear, the significance of the tests remain unclear. However, the patient was on doxycycline, which has prompt and excellent activity against HME and HGA, and as such should have modified their impact on her treatment outcome.
Figure 1.
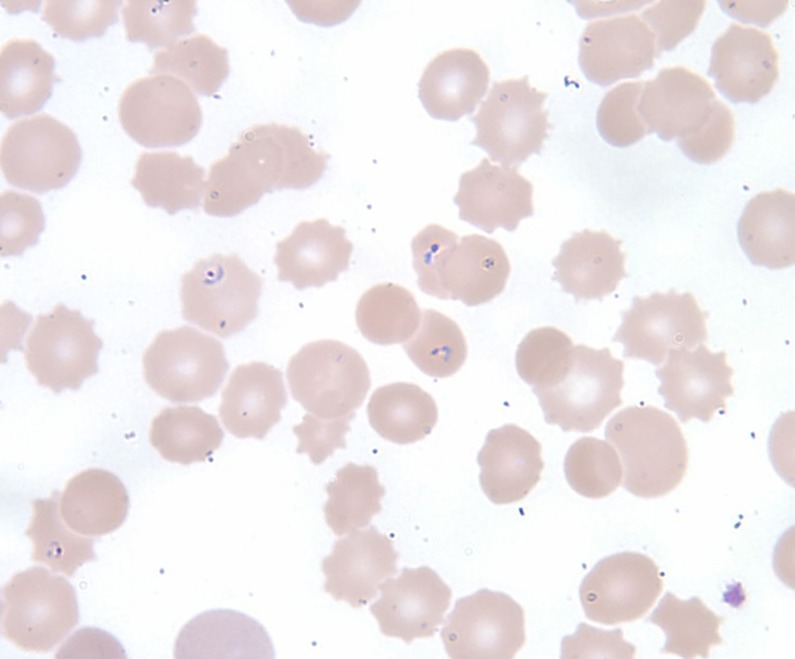
Peripheral blood film showing multiple intraerythrocytic ring forms of Babesia.
Elderly patients as well as individuals with a history of alcoholism including those with liver cirrhosis are also recognised as specific risk groups for severe babesiosis.1 14 Severe disease also occurs in immunocompromised patients, including anatomically or functionally asplenic individuals, as the spleen plays a major role in the clearance of parasitised red blood cells. Similarly, poor clinical outcomes have been reported in older and immune compromised patients with HGA.8 Lyme disease very seldom results in fatalities in isolation. Since the patient continued to deteriorate clinically despite a decline in her Babesia parasitemia, we suspect that her concurrent tick-borne coinfections contributed to her demise. There are reports in published literature that multiple TBIs occurring concurrently in an individual are associated with higher morbidity than single infections.5 15
Doxycycline remains the drug of choice for HGA, HME and Lyme disease but lacks activity against Babesia. Combination therapy with oral azithromycin and atovaquone are the mainstay of therapy for mild to moderate babesiosis while intravenous quinine and oral or intravenous clindamycin are indicated for severe disease along with exchange transfusion for heavy parasitaemia.16 Outcomes are best when coinfections are recognised and appropriate treatment is initiated early.
Learning points.
The causative organisms of Lyme disease, babesiosis and human granulocytic anaplasmosis (HGA) are carried by the same tick vector and, therefore, can co-occur in a single individual.
Multiple tick-borne infections (TBI) occurring concurrently in the same individual are associated with increased morbidity and mortality, mainly in immunocompromised patients such as the elderly and patients with liver cirrhosis, predominantly those with decompensated liver disease.
It is important to screen for coinfections with any TBI, especially in patients with either HGA, Lyme disease or babesiosis, not only to guide appropriate management but also for its prognostic implications.
Footnotes
Contributors: SC and OO both provided direct clinical care to the patient and identified this case as worth sharing. Both authors were responsible for conception of the article, literature search, drafting and revision of the article as well as approval of the submitted draft.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.CDC. Reported cases of Lyme disease by state or locality, 2004–2013. Last updated on 27 August 2014 http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html (accessed 20 Oct 2014).
- 2.Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis 1995;20:1102–10. 10.1093/clinids/20.5.1102 [DOI] [PubMed] [Google Scholar]
- 3.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med 2010;30:261–92. 10.1016/j.cll.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner FE, Pinger RR, Vann CN et al. . Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J Med Entomol 2008;45:289–97. 10.1093/jmedent/45.2.289 [DOI] [PubMed] [Google Scholar]
- 5.Swanson SJ, Neitzel D, Reed KD et al. . Coinfections acquired from ixodes ticks. Clin Microbiol Rev 2006;19:708–27. 10.1128/CMR.00011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause PJ, McKay K, Thompson CA et al. . Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis 2002;34:1184–91. 10.1086/339813 [DOI] [PubMed] [Google Scholar]
- 7.Herwaldt BL, Springs FE, Roberts PP et al. . Babesiosis in Wisconsin: a potentially fatal disease. Am J Trop Med Hyg 1995;53:146–51. [DOI] [PubMed] [Google Scholar]
- 8.Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci 2006;1078:236–47. 10.1196/annals.1374.042 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro ED. Clinical practice. Lyme disease. N Engl J Med 2014;370:1724–31. 10.1056/NEJMcp1314325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBiasi RL. A concise critical analysis of serologic testing for the diagnosis of lyme disease. Curr Infect Dis Rep 2014;16:450 10.1007/s11908-014-0450-9 [DOI] [PubMed] [Google Scholar]
- 11.Chapman AS, Bakken JS, Folk SM et al. . Diagnosis and management of tickborne rickettsial diseases: rocky mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006;55:1–27. [PubMed] [Google Scholar]
- 12.Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2008;22:433–448, viii 10.1016/j.idc.2008.03.011 [DOI] [PubMed] [Google Scholar]
- 13.Unver A, Felek S, Paddock CD et al. . Western blot analysis of sera reactive to human monocytic ehrlichiosis and human granulocytic ehrlichiosis agents. J Clin Microbiol 2001;39:3982–6. 10.1128/JCM.39.11.3982-3986.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatcher JC, Greenberg PD, Antique J et al. . Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 2001;32:1117–25. 10.1086/319742 [DOI] [PubMed] [Google Scholar]
- 15.Belongia EA. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis 2002;2:265–73. 10.1089/153036602321653851 [DOI] [PubMed] [Google Scholar]
- 16.White DJ, Talarico J, Chang HG et al. . Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med 1998;158:2149–54. 10.1001/archinte.158.19.2149 [DOI] [PubMed] [Google Scholar]


