Abstract
Early stage colorectal neuroendocrine carcinoma is rare. A small colon tumour was found in a 56-year-old man during diagnostic colonoscopy performed after a positive faecal occult blood test, and he was referred for treatment. A slightly reddish superficial elevated lesion with a shallow depression 10 mm in size was found in the transverse colon. Magnifying narrow-band imaging revealed disrupted irregular microvessels and the absence of a surface pattern in the depressed area. En bloc endoscopic mucosal resection (EMR) of the tumour was undertaken. The tumour was positive for chromogranin A and synaptophysin, and had a mitotic rate of >20/10 high-power fields and a Ki-67 proliferative index of >50%; it was diagnosed as a neuroendocrine carcinoma. The tumour minimally invaded the submucosa (300 μm) without lymphovascular involvement. The patient was followed up carefully, and at 1 year after EMR, no recurrence was found using colonoscopy and CT scans.
Background
Neuroendocrine carcinoma (NEC) is a high-grade malignant neoplasm with a predominantly neuroendocrine differentiation. Most patients with NEC in the colon and rectum are diagnosed at an advanced stage, especially with metastatic disease.1 2 Thus, early stage colon NEC is extremely rare.
NEC accounts for only 0.6% of colorectal carcinoma;3 nevertheless, colon NEC needs to be clinically and biologically detected because it is highly aggressive and has a poor prognosis. Currently, the WHO classifies neuroendocrine neoplasms (NEN) into neuroendocrine tumour (NET) and NEC according to the histological finding and grade (G) of tumour cell proliferation (mitotic count and Ki-67 index).4 NEC consists of tumour cells expressing neuroendocrine markers, and showing marked cellular atypia and high proliferative activity. The prevalence of colorectal NEC among colorectal NENs is 2.3%.5 NEC displays more pronounced malignant behaviour (poorer differentiation, greater lymphovascular invasion and a more advanced stage at diagnosis) than NET.2 The 5-year overall survival rate for colorectal NEC is worse than that for colorectal NET (G1, 78%; G2, 40%; G3, 31%).2
The characteristics of early stage colon NEC have not yet been fully explored. We report a case of early stage colon NEC that was removed en bloc using endoscopic mucosal resection (EMR).
Case presentation
A 56-year-old man presented to a local hospital as a result of a positive faecal occult blood test (FOBT). Diagnostic colonoscopy revealed a small tumour in the transverse colon. The patient was referred to our endoscopy unit for treatment. He did not present with flushing, diarrhoea or abdominal cramping. There was no remarkable history and no familial history of colorectal cancer. Blood tests were normal except for mild elevation of liver transaminase levels. Serum carcinoembryonic antigen, cytokeratin 19 fragment and α-fetoprotein were within normal limits. A contrast-enhanced CT scan revealed no lymph node metastasis or distant metastasis.
Colonoscopy detected a slightly reddish superficial elevated lesion (1 cm in size) with a shallow depression in the transverse colon (figure 1A). Magnifying narrow-band imaging (NBI) showed disrupted irregular microvessels and the absence of a surface pattern in the depressed area (figure 1B). Using magnifying chromoendoscopy with crystal violet, an irregular pit pattern was seen in a small region of the depressed area, whereas pits were not visible in the other areas because of the presence of thick mucus. The lesion was well elevated after submucosal saline injection and subsequently EMR was performed using a 13 mm snare (Captivator, Boston Scientific Japan, Tokyo, Japan). En bloc resection was achieved without adverse events.
Figure 1.
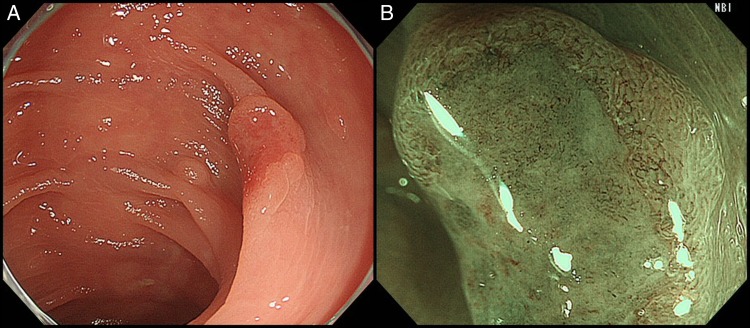
Colonoscopic findings. (A) A 1 cm slightly reddish superficial elevated lesion with a shallow depression in the transverse colon. (B) Disrupted irregular microvessels and the absence of a surface pattern were observed under magnifying narrow-band imaging.
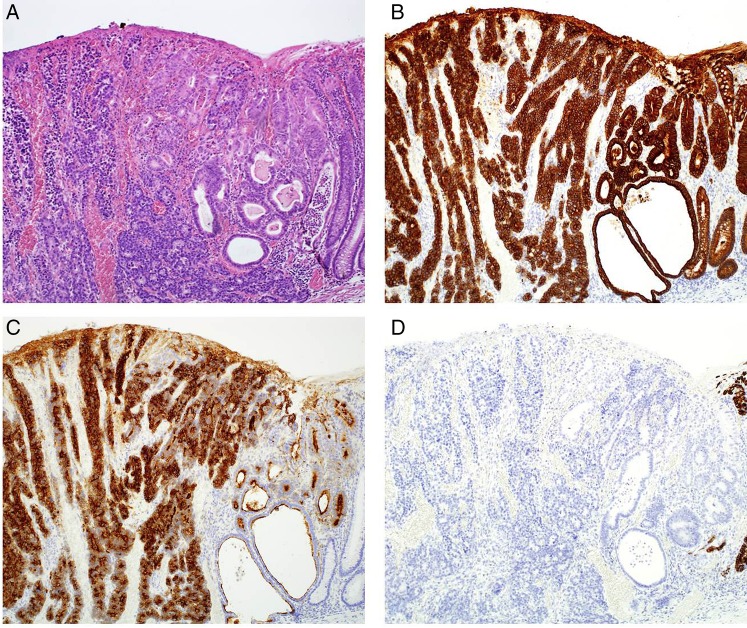
Macroscopically, the tumour had the appearance of an 11×5 mm superficial elevated lesion with a shallow depression (figure 2). The specimen was serially sectioned at 2 mm intervals. On histological examination, the tumour was found to consist of cancer cells that showed trabecular architecture and solid cancer cell nests (figure 3A, B), and a small adenocarcinoma component at its periphery (figure 4A). Immunohistochemical staining revealed that the main tumour was positive for neuroendocrine markers, namely chromogranin A (figure 5A), synaptophysin (figure 5B) and CD56. Both of the tumour components showed similar immunohistochemical characteristics. They were positive for cytokeratin AE1/AE3 (figure 4B) and CD10 (figure 4C), and negative for MUC2 (figure 4D), MUC5AC, MUC6 and Alcian blue. There were no chromogranin A-stained cell nests in the non-neoplastic mucosa. The tumour showed a high mitotic rate of >20/10 high-power fields and a Ki-67 proliferative index of >50%. According to the WHO 2010 classification, we diagnosed the tumour as NEC. The tumour minimally invaded the submucosa (300 μm) without lymphovascular invasion.
Figure 2.
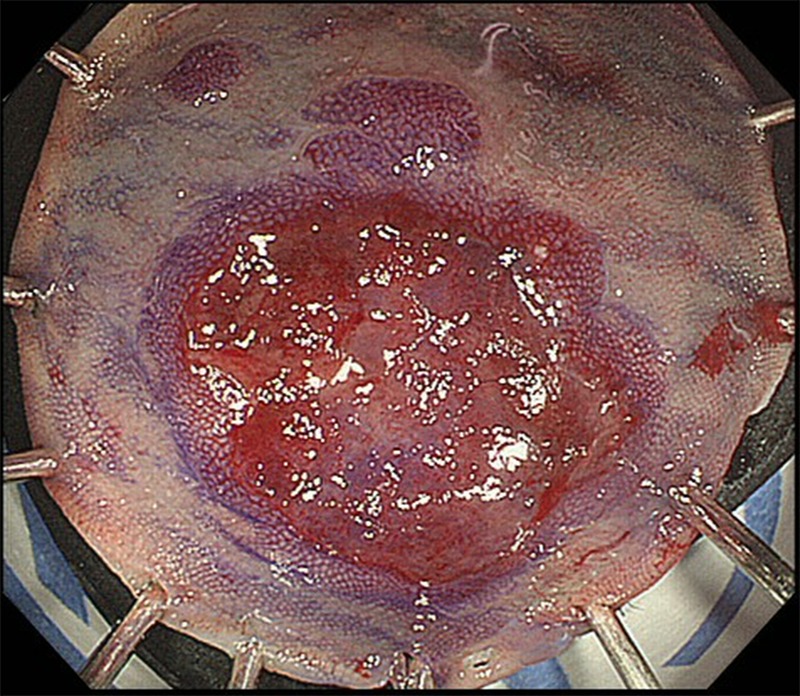
Macroscopic view of specimen obtained using endoscopic mucosal resection.
Figure 3.
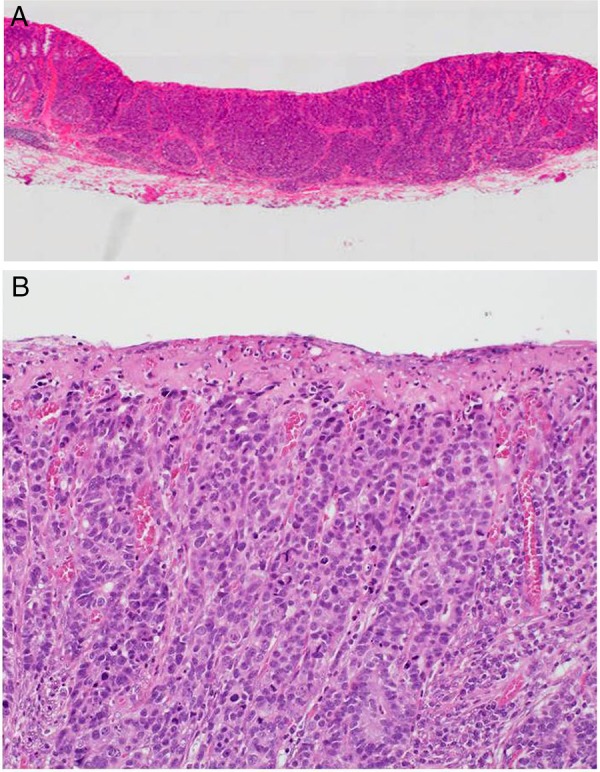
H&E-stained tissue sections. (A) The tumour has minimally invaded the submucosa (magnification ×5). (B) The cancer cells exhibit trabecular architecture (magnification ×50).
Figure 4.
Histological findings regarding the tumour. Two distinct components were observed. (A) H&E-stained tissue section. Neuroendocrine carcinoma and adenocarcinoma components can be seen. (B) Tissue section stained for cytokeratin AE1/AE3. Both components were positively stained. (C) Tissue section stained for CD10. The cytoplasm of the neuroendocrine carcinoma component and the brush border of the adenocarcinoma component were positively stained. (D) Tissue section stained for MUC2. Both components were not stained (magnification ×50).
Figure 5.
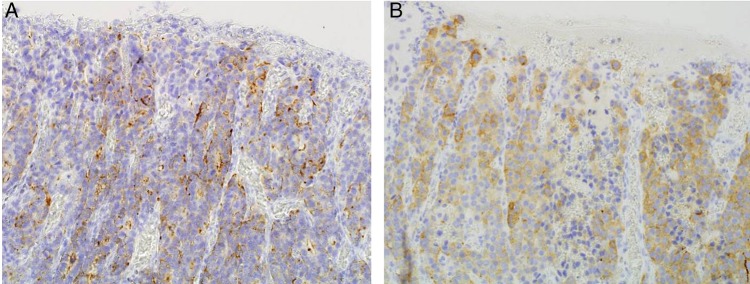
Tissue section stained for neuroendocrine markers. The neuroendocrine carcinoma component was (A) positive for chromogranin A and (B) positive for synaptophysin (magnification ×50).
Differential diagnosis
Colon adenocarcinoma
Colon NET
Treatment
Endoscopic mucosal resection.
Outcome and follow-up
One year after EMR, no recurrence was found using colonoscopy and CT scans. Careful follow-up has been continued by means of a biannual CT scan and annual colonoscopy.
Discussion
Recently, as a result of the increasing recognition of NEN and the widespread use of screening colonoscopy, the incidence of colorectal NET and NEC has increased significantly.5 However, around 50–70% of patients with colorectal NECs are diagnosed at an advanced stage with metastatic disease.1 2 Although most of the colorectal NET develops in the rectum, 40% of colorectal NEC is found on the right side of the colon.2 This may be the reason why NEC is diagnosed at a late stage; tumours on the right side of the colon present symptoms later than those on the left side of the colon. In our case, the lesion was detected at diagnostic colonoscopy carried out for positive-screening FOBT, and the tumour was about 1 cm in size, but had already invaded the submucosa. Therefore, early detection of colorectal NEC may still be difficult.
NBI contrasts the surface structure and vascular architecture of the superficial mucosa that corresponds to the histological finding using two narrow-banded short wavelength lights. The combination of NBI with magnifying endoscopy enables the differentiation of neoplasia from non-neoplasia and the prediction of the depth of invasion of the carcinoma.6 Recently, the NBI International Colorectal Endoscopic (NICE) classification was proposed to facilitate the diagnosis of colorectal tumours.6 Under magnifying NBI, adenoma or intramucosal cancer have brown vessels surrounding white marginal crypt epithelium, because subepithelial capillaries surround the orifice of tubular glands. Deep submucosal invasive cancer has an area of disrupted or missing vessels because of damage to the glandular structure. In our case, vessel detection was disrupted in magnifying NBI images, and it was consistent with a Type 3 NICE classification indicating deep submucosal cancer invasion. However, most of the tumour was confined to the mucosa, and it only minimally invaded the submucosa at its bottom end. The histological structure of the NEC differs from that of ordinary tubular adenoma or adenocarcinoma; thus, the NICE classification may not be applicable for the diagnosis of colon NEC.
According to the European Neuroendocrine Tumour Society consensus guidelines, colonic tumours <2 cm in size may be excised endoscopically, and in the case of incomplete resection or G3 proliferative activity, additional surgical resection is recommended.7 In fact, according to a series of Japanese case studies involving early stage colorectal NEC, most of the NEC tumours exhibited deep submucosal invasion and/or lymphovascular involvement, which carried the risk of lymph node metastasis (table 1).8–13 In our case, the tumour was small and only minimally (300 μm) invaded the submucosa, without lymphovascular involvement. We discussed the situation with the patient and he requested careful follow-up without additional surgery in accordance with the colorectal cancer treatment guidelines.14 Further case accumulation is required to establish the standard treatment for early colorectal NEC.
Table 1.
Reported Japanese cases of early stage colorectal NEC
| Number | Author | Age | Gender | Location | Type | Size (mm) | Depth | Adenoma or adenocarcinoma component | Treatment | ly | v | Reference number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Okada | 77 | M | Rectum | IIa+IIc | 23 | sm | + | Surgery | + | + | 8 |
| 2 | Kurokawa | 62 | M | Rectum | IIc+IIa | 2.5 | m | − | Surgery | + | − | 9 |
| 3 | Hosaka | 84 | M | Transverse colon | IIa | 12 | sm | + | Polypectomy | − | − | 10 |
| 4 | Suto | 57 | M | Rectum | Isp | 42 | sm | + | Surgery | + | + | 11 |
| 5 | Komatsu | 85 | F | Sigmoid colon | IIa+IIc | 17 | sm | + | Surgery | + | + | 12 |
| 6 | Ikebe | 56 | M | Rectum | Isp | 10 | sm | − | Polypectomy and additional surgery | + | − | 13 |
| 7 | Our case | 56 | M | Transverse colon | IIa+IIc | 11 | sm | + | EMR | − | − |
F, female; ly, lymphatic involvement; M, male; m, mucosa; NEC, neuroendocrine carcinoma; sm, submucosa; v, venous involvement.
NEC is frequently associated with an adjacent adenoma or adenocarcinoma. The Japanese case reports regarding early colorectal NEC showed that most of the colorectal NECs had an adenoma/adenocarcinoma component at an early stage (table 1).8–13 In gastric NEC, the disease predominantly arises from the endocrine precursor cell clones located in the preceding adenocarcinoma component, which subsequently transform into endocrine cell carcinoma.15 In our case, there was a small adenocarcinoma component on the periphery of the NEC, and it showed similar immunohistochemical features with the exception of neuroendocrine markers. This suggested that colorectal NEC might be derived from a preceding adenocarcinoma.
Learning points.
A case of colon neuroendocrine carcinoma (NEC) that exhibited minimal (300 μm) submucosal invasion without lymphovascular involvement was treated using endoscopic mucosal resection and followed up carefully.
The Narrow-band imaging International Colorectal Endoscopic (NICE) classification may not be adequate for the diagnosis of colorectal NEC because its histological structure is different from conventional tubular adenoma/adenocarcinoma.
Most colorectal NECs may arise from the preceding adenocarcinoma component and could show aggressive biological features.
Footnotes
Contributors: YY drafted the manuscript. NU critically revised the manuscript for important intellectual content and performed endoscopic therapy. YT described histological findings. RI gave final approval of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Smith JD, Reidy DL, Goodman KA et al. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol 2014;21:2956–62. 10.1245/s10434-014-3725-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JL, Yu CS, Kim M et al. Prognostic impact of diagnosing colorectal neuroendocrine carcinoma using the World Health Organization 2010 classification. Surgery 2014;155:650–8. 10.1016/j.surg.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 3.Bernick PE, Klimstra DS, Shia J et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 2004;47:163–9. 10.1007/s10350-003-0038-1 [DOI] [PubMed] [Google Scholar]
- 4.Bosman FT, Carneiro F, Hruban RH et al. WHO classification of tumors of the digestive system. 4th edn Lyon: IARC, 2010. [Google Scholar]
- 5.Ito T, Igarashi H, Nakamura K et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 2015;50:58–64. 10.1007/s00535-014-0934-2 [DOI] [PubMed] [Google Scholar]
- 6.Hewett DG, Kaltenbach T, Sano Y et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143:599–607. 10.1053/j.gastro.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Caplin M, Sundin A, Nillson O et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology 2012;95:88–97. 10.1159/000335594 [DOI] [PubMed] [Google Scholar]
- 8.Okada S, Fujioka M, Taguchi S et al. A case of neuroendocrine carcinoma of the rectum. Nihon Shokakibyo Gakkai Zasshi 2002;99:282–8. (in Japanese). [PubMed] [Google Scholar]
- 9.Kurokawa S, Imamura A, Muraoka S et al. Type IIc IIa (Superficial Depressed Type) intramucosal endocrine cell carcinoma of the rectum, report of a case. Stomach Intestine 2004;39:1547–54. (in Japanese with English abstract). [Google Scholar]
- 10.Hosaka S, Matsuzawa K, Maruyama K et al. Rapid four-month growth of an early-stage adenocarcinoma of the colon with neuroendocrine characteristics. Dig Dis Sci 2003;48:295–8. 10.1023/A:1021923325881 [DOI] [PubMed] [Google Scholar]
- 11.Suto T, Fujita T, Mitomo S et al. A long surviving case of coexistent endocrine cell carcinoma and well differentiated adenocarcinoma in tubulovillous adenoma of the rectum with lymph node involvement. Nihon Shokaki Geka Gakkai Zasshi 2013;46:143–50. (in Japanese with English abstract). 10.5833/jjgs.2012.0167 [DOI] [Google Scholar]
- 12.Komatsu H, Nagasaki T, Haseba M et al. A case of rapidly progressed endocrine cell carcinoma of the sigmoid colon. Nihon Rinsho Geka Gakkai Zasshi 2010;71:995–9. (in Japanese with English abstract). 10.3919/jjsa.71.995 [DOI] [Google Scholar]
- 13.Ikebe T, Mayumi K, Nishioka T et al. A case of endocrine cell carcinoma (submucosal depressed) in the rectum resected but indicating an extremely poor prognosis. Nihon Gekakei Rengo Gakkaishi 2011;36:829–34. (in Japanese with English abstract). 10.4030/jjcs.36.829 [DOI] [Google Scholar]
- 14.Watanabe T, Itabashi M, Shimada Y et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1–29. 10.1007/s10147-011-0315-2 [DOI] [PubMed] [Google Scholar]
- 15.Domori K, Nishikura K, Ajioka Y et al. Mucin phenotype expression of gastric neuroendocrine neoplasms: analysis of histopathology and carcinogenesis. Gastric Cancer 2014;17:263–72. 10.1007/s10120-013-0281-7 [DOI] [PubMed] [Google Scholar]