Abstract
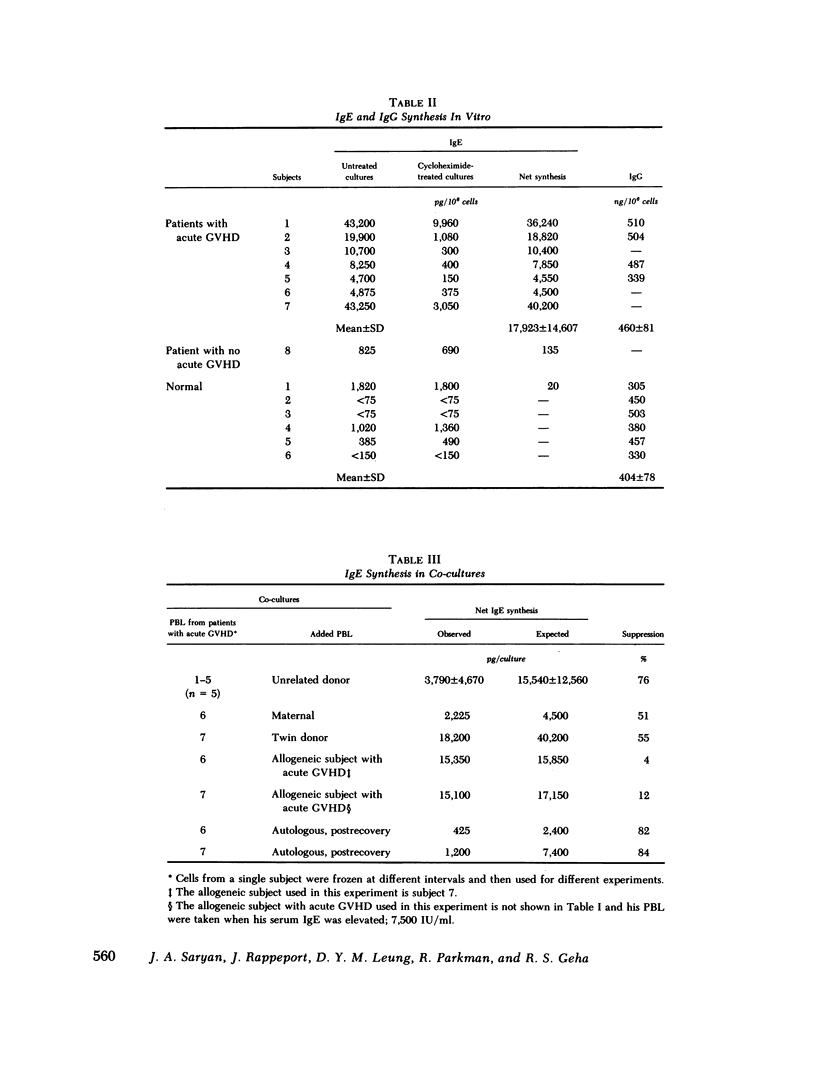
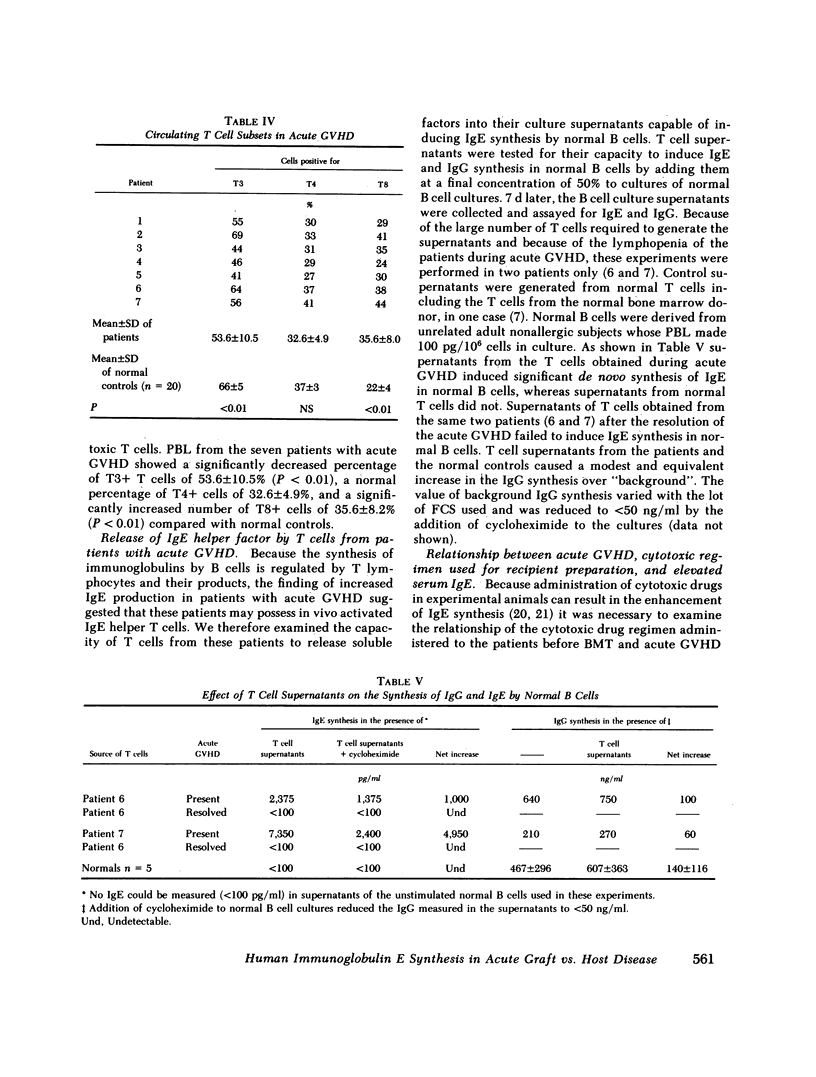
Immunoglobulin (Ig) E synthesis was studied in vitro in eight patients who had received transplants of allogeneic bone marrow. Seven of these patients developed acute graft vs. host disease (GVHD) and elevated serum IgE levels, whereas the eighth did not. In vitro synthesis of IgE, but not of IgG, was elevated in cultures of lymphocytes obtained during acute GVHD (17,923 +/- 14,607 pg/10(6) cells) but not in cultures of lymphocytes obtained after resolution of the acute GVHD when the serum IgE had returned to normal (106 +/- 31 pg/10(6) cells). In contrast, lymphocytes from the patient with no acute GVHD, like normal lymphocytes, failed to synthesize IgE in vitro. The increased in vitro IgE synthesis in acute GVHD was suppressed by normal allogeneic lymphocytes and by autologous lymphocytes obtained after the resolution of the acute GVHD, but not by allogeneic lymphocytes obtained from patients undergoing acute GVHD. The deficiency in functional IgE-specific suppressor cells in acute GVHD occurred in the face of normal or increased percentages of circulating T8+ cells, which in normal subjects contain the IgE-specific suppressor cells. In two patients studied, there was evidence of activated IgE-specific, circulating helper T cells. T cells from these two patients, but not normal T cells, secreted spontaneously upon culture in vitro a factor that induced IgE, but not IgG, synthesis by normal B cells. Finally, a survey of 21 bone marrow transplant recipients revealed that acute GVHD was a necessary requirement for the development of elevated serum IgE levels in recipients of bone marrow transplants. These results suggest that acute GVHD is accompanied by an imbalance in IgE-specific immunoregulatory T cells consisting of activated helper T cells and deficient suppressor cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley R. H., Becker W. G. Abnormalities in the regulation of human IgE synthesis. Immunol Rev. 1978;41:288–314. doi: 10.1111/j.1600-065x.1978.tb01469.x. [DOI] [PubMed] [Google Scholar]
- Buckley R. H. Regulation of IgE synthesis: introduction. Fed Proc. 1981 Jun;40(8):2159–2161. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N., Fox D. A., Katz D. H. Hapten-specific IgE antibody responses in mice. VII. Conversion of IgE "non-responder" strains to IgE "responders" by elimination of suppressor T cell activity. J Immunol. 1977 Jan;118(1):48–54. [PubMed] [Google Scholar]
- Chiorazzi N., Fox D. A., Katz D. H. Hapten-specific IgE antibody responses in mice. VII. Conversion of IgE "non-responder" strains to IgE "responders" by elimination of suppressor T cell activity. J Immunol. 1977 Jan;118(1):48–54. [PubMed] [Google Scholar]
- Fiser P. M., Buckley R. H. Human IgE biosynthesis in vitro: studies with atopic and normal blood mononuclear cells and subpopulations. J Immunol. 1979 Oct;123(4):1788–1794. [PubMed] [Google Scholar]
- Geha R. S., Rappaport J. M., Twarog F. J., Parkman R., Rosen F. S. Increased serum immunoglobulin E levels following allogeneic bone marrow transplantation. J Allergy Clin Immunol. 1980 Jul;66(1):78–81. doi: 10.1016/0091-6749(80)90142-6. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Reinherz E., Leung D., McKee K. T., Jr, Schlossman S., Rosen F. S. Deficiency of suppressor T cells in the hyperimmunoglobulin E syndrome. J Clin Invest. 1981 Sep;68(3):783–791. doi: 10.1172/JCI110315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Adachi T. Generation of specific helper cells and suppressor cells in vitro for the IgE and IgG antibody responses. J Immunol. 1976 Jul;117(1):40–47. [PubMed] [Google Scholar]
- Ishizaka K., Sandberg K. Formation of IgE binding factors by human T lymphocytes. J Immunol. 1981 May;126(5):1692–1696. [PubMed] [Google Scholar]
- Ishizaka K., Suemura M., Yodoi J., Hirashima M. Regulation of IgE response by IgE binding factors. Fed Proc. 1981 Jun;40(8):2162–2166. [PubMed] [Google Scholar]
- Katz D. H. Control of IgE antibody production by suppressor substances. J Allergy Clin Immunol. 1978 Jul;62(1):44–55. doi: 10.1016/0091-6749(78)90072-6. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Newburger P. E., Benacerraf B. Hapten-specific IgE antibody responses in mice. IV. Evidence for distinctive sensitivities of IgE and IgG B lymphocytes to the regulatory influence of T cells. J Immunol. 1974 Sep;113(3):974–983. [PubMed] [Google Scholar]
- Kishimoto T., Hirai Y., Suemura M., Yamamura Y. Regulation of antibody response in different immunoglobulin classes. I. Selective suppression of anti-DNP IgE antibody response by preadministration of DNP-coupled mycobacterium. J Immunol. 1976 Aug;117(2):396–404. [PubMed] [Google Scholar]
- Leung D. Y., Rhodes A. R., Geha R. S. Enumeration of T cell subsets in atopic dermatitis using monoclonal antibodies. J Allergy Clin Immunol. 1981 Jun;67(6):450–455. doi: 10.1016/0091-6749(81)90098-1. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Parkman R., Rappeport J., Rosen F. S., Schlossman S. F. Aberrations of suppressor T cells in human graft-versus-host disease. N Engl J Med. 1979 May 10;300(19):1061–1068. doi: 10.1056/NEJM197905103001901. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Reinisch C. L., Andrew S. L., Schlossman S. F. Suppressor cell regulation of immune response to tumors: abrogation by adult thymectomy. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2989–2992. doi: 10.1073/pnas.74.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringden O., Witherspoon R., Storb R., Ekelund E., Thomas E. D. B cell function in human marrow transplant recipients assessed by direct and indirect hemolysis-in-gel assays. J Immunol. 1979 Dec;123(6):2729–2734. [PubMed] [Google Scholar]
- Romagnani S., Del Prete G. F., Maggi E., Troncone R., Giudizi G. M., Almerigogna F., Ricci M. In vitro production of IgE by human peripheral blood mononuclear cells. II. Cells involved in the spontaneous IgE production in atopic patients. Clin Exp Immunol. 1980 Dec;42(3):579–588. [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Morrow C., Stevens R. H. Subpopulations of circulating B cells and regulatory T cells involved in in vitro immunoglobulin E production in atopic patients with elevted serum immunoglobulin E. J Clin Invest. 1980 Jun;65(6):1457–1468. doi: 10.1172/JCI109810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T. Regulation of reaginic antibody formation in animals. Prog Allergy. 1975;19:122–194. [PubMed] [Google Scholar]
- Thomas E., Storb R., Clift R. A., Fefer A., Johnson F. L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975 Apr 17;292(16):832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- Tjio A. H., Hull W. M., Gleich G. J. Production of human immunoglobulin E antibody in vitro. J Immunol. 1979 May;122(5):2131–2133. [PubMed] [Google Scholar]
- Watanabe N., Kojima S., Ovary Z. Suppression of IgE antibody production in SJL mice. I. Nonspecific suppressor T cells. J Exp Med. 1976 Apr 1;143(4):833–845. doi: 10.1084/jem.143.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Hirashima M., Ishizaka K. Regulatory role of IgE-binding factors from rat T lymphocytes. II. Glycoprotein nature and source of IgE-potentiating factor. J Immunol. 1980 Oct;125(4):1436–1441. [PubMed] [Google Scholar]
- Zuraw B. L., Nonaka M., O'Hair C., Katz D. H. Human IgE antibody synthesis in vitro: stimulation of IgE responses by pokeweed mitogen and selective inhibition of such responses by human suppressive factor of allergy (SFA). J Immunol. 1981 Sep;127(3):1169–1177. [PubMed] [Google Scholar]
- de Bruin H. G., Astaldi A., Leupers T., van de Griend R. J., Dooren L. J., Schellekens P. T., Tanke H. J., Roos M., Vossen J. M. T lymphocyte characteristics in bone marrow-transplanted patients. II. Analysis with monoclonal antibodies. J Immunol. 1981 Jul;127(1):244–251. [PubMed] [Google Scholar]
- ten Napel C. H., The T. H. Acute cytomegalovirus infection and the host immune response. II. Relationship of suppressed in vitro lymphocyte reactivity to bacterial recall antigens and mitogens with the development of cytomegalovirus-induced lymphocyte reactivity. Clin Exp Immunol. 1980 Feb;39(2):272–278. [PMC free article] [PubMed] [Google Scholar]


