Abstract
Netrins are secreted proteins that regulate axon guidance and neuronal migration. DCC is a well-established Netrin-1 receptor mediating attractive responses. We provide evidence that its close relative neogenin is also a functional Netrin-1 receptor that acts with DCC to mediate guidance in vivo. We determined the structures of a functional Netrin-1 region, alone and in complexes with neogenin or DCC. Netrin-1 has a rigid elongated structure containing two receptor-binding sites at opposite ends through which it brings together receptor molecules. The ligand/receptor complexes reveal two distinct architectures: a 2:2 heterotetramer and a continuous ligand/receptor assembly. The differences result from different lengths of the linker connecting receptor domains FN4 and FN5, which differs among DCC and neogenin splice variants, providing a basis for diverse signaling outcomes.
Netrins, acting as both attractants and repellents, regulate neuronal migration, axon guidance and synaptogenesis (1–4). In non-neural tissues, netrins have a variety of functions, including promoting cell adhesion and invasion, leukocyte migration, angiogenesis, and cell survival (5). Netrins contain an N-terminal laminin domain (LN, a.k.a. domain VI), followed by three cysteine-rich laminin-type epidermal growth factor (EGF) –like modules (LE1, LE2, and LE3, a.k.a. domain V), and a small positively charged C-terminal domain (LC). In mammals, the secreted Netrins -1, -3 and -4 are only distantly related to the GPI-anchored G netrins (6, 7).
Netrin actions are mediated by distinct receptors (4). DCC mediates attractive responses to Netrin-1, whereas Unc5 proteins, alone or with DCC, are required for its repulsive effects (4, 8). The ectodomain of DCC is composed of four immunoglobulin-like (Ig) domains and six fibronectin type III (FNIII) domains. The DCC FNIII repeats mediate interactions with Netrin-1 through its LN-LE (1–3) region (9–11). That region, when added as an Fc-fusion protein, is sufficient to mimic the axon outgrowth activity of full length Netrin-1 (12). Neogenin is structurally similar to DCC and binds Netrin-1 and -3 (12, 13), but also binds the structurally distinct Repulsive Guidance Molecule (RGM) (14, 15). Knockdown analysis in zebrafish supports a role for neogenin in mediating axonal attraction to netrin (16), but this role has not been established in mammals, where it has mostly been studied as an adhesive factor (17) and a putative guidance receptor for RGM (15).
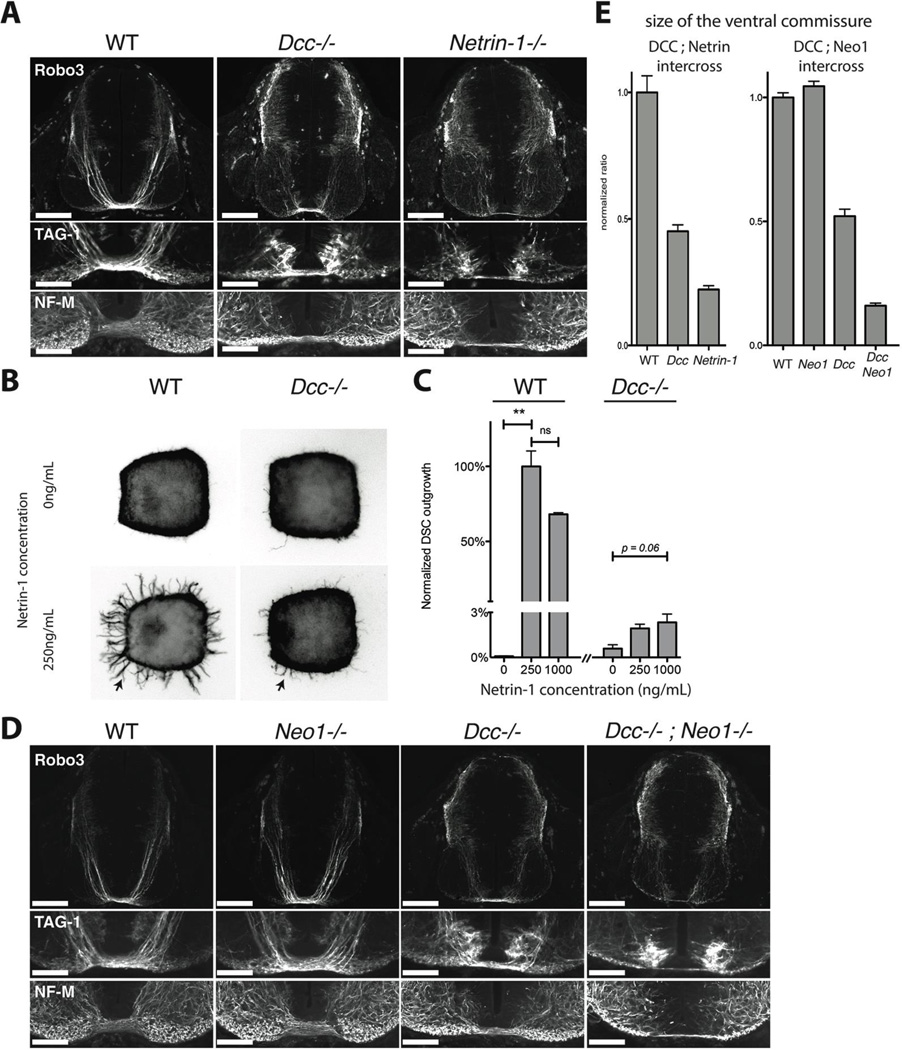
We revisited the role of neogenin in netrin attraction while studying commissural axon attraction to a Netrin-1 source at the spinal cord midline. Defects in this guidance in human leads to neurological syndromes, some of which result from mutations in DCC (18–20). Prior analysis suggested that DCC mediates the entire attractive effect of Netrin-1 because the phenotype observed with a commissural axon marker, antibody 4D7 to TAG-1, appeared stronger in Dcc mutant than in Netrin-1 mutant embryos (21). As new markers became available (22), we re-evaluated embryos mutant for Dcc or Netrin-1. Commissural projections develop between embryonic days 10.5 (E10.5) and E12.5, when spinal cord shape changes rapidly. To minimize artifacts from variation in embryo size and stage across litters, we compared size-matched embryos that were littermates from intercrosses of compound heterozygous animals. Using an antibody to Robo3 (22), in E11.5 Dcc mutant embryos we observed only a 55% reduction in width of the ventral commissure compared to wild-type littermates, less than in Netrin-1−/− embryos, which had a 78% reduction (Fig 1A,E). The same was seen with a new antibody to TAG-1 (Fig 1A) and with antibodies to the axonal markers Neurofilament-M (Fig 1A). The difference with the prior study appears to result from 4D7 giving weaker labeling of commissural axons that is also influenced by Dcc expression (Fig S1). Thus, the guidance phenotype is actually less severe than in Dcc than in Netrin-1 mutants, suggesting an additional Netrin-1 receptor(s) contributes to residual attraction in Dcc−/− embryos.
Fig. 1.
A) Cross sections of E11 wild-type, Dcc−/− and Netrin-1−/− littermate mouse embryos at the level of brachial spinal ganglia, stained for TAG-1, Robo3 and Neurofilament, Medium Chain (NF-M). Lower panels show details of the ventral commissural axon bundle. Dcc mutants have a reduced ventral commissure, but a large number of axons still cross. Netrin-1−/− embryos have a much-reduced ventral commissure. B) Axon outgrowth (arrows) in E11 wild type or Dcc−/− littermate mouse dorsal spinal cord explants cultured in 3D collagen gels with increasing concentrations of Netrin-1 and stained for Tuj1. C) Quantification of the response of axon outgrowth from wild type and Dcc−/− dorsal spinal cord explants to increasing Netrin-1 concentrations, normalized to wild type at 250ng/mL of Netrin-1. Dcc−/− mutants show a residual response to Netrin-1 application (arrow). D) Cross sections of E11 wild-type, Dcc−/−, Neo1−/− and Dcc−/−; Neo1−/− littermate mouse embryos at the level of brachial spinal ganglia, stained for TAG-1, Robo3 and Neurofilament (NF-M). Lower panels show details of the motor column and the ventral commissural axon bundle. The Dcc−/−; Neo1−/− double mutant has a much reduced ventral commissure and numerous axons in the motor column. E) Ratio of the commissural axon bundle size to the dorso-ventral spinal cord length of wild-type, Dcc−/− and Netrin1−/− embryos, normalized to wild types (left graph). Ratio of commissural axon bundle size to the dorso-ventral spinal cord length of Dcc−/−, Neo1−/− and Dcc−/−; Neo1−/− embryos normalized to wild type (right graph).The quantification shows the mean and SEM of 5 sections taken in brachial spinal cord in littermates, and is representative of 3 litters.
Scale bars are 200µm (Robo3) and 100µm (TAG-1 and NF-M).
To test this, we examined whether Dcc mutant commissural axons retain a response to Netrin-1 in vitro. We cultured dorsal spinal cord explants from E11 wild type and Dcc−/− embryos (Fig 1B,C). In control explants, Netrin-1 application induced robust axonal outgrowth that peaked at 250ng/mL. The peak response was reduced significantly (by ~97%) when explants from Dcc−/− embryos were used, confirming Dcc’s central role as a Netrin-1 receptor, but a dose-dependent response of Dcc mutant axons was still consistently observed (Fig. 1B, C). To determine which receptor mediates the residual Netrin-1 response, we screened known and putative Netrin-1 receptors by in situ hybridization and immuno-histochemistry in E11.5 spinal cord. We observed neogenin immunoreactivity on commissural axons (23), which was lost in neogenin (Neo1) mutant spinal cords (Fig. S2A), suggesting that neogenin might collaborate with Dcc in guiding these axons. Consistent with this, whereas commissural axon trajectories in transverse sections from Neo1−/− embryos were apparently normal (Fig. 1D), removing neogenin as well as Dcc in Dcc−/−;Neo1−/− double mutants resulted in an 84% reduction in ventral commissure size, i.e. greater than Dcc−/− but comparable to Netrin-1−/− embryos (Fig 1D, E). Moreover, we observed abnormal Robo3+ commissural axons in the motor column of Netrin-1−/− embryos; fewer are seen in Dcc−/− single mutants, but a comparable number was seen in Dcc−/−;Neo1−/− double mutants (Fig. S2B–D). Although the Neo1−/− and Netrin-1−/− alleles are severely hypomorphic rather than complete null alleles (Fig S2A for Neo1−/−), the Dcc allele is a null allele, so our finding that commissural axon guidance defects in Dcc−/−;Neo1−/− embryos are greater than in Dcc−/− mutants but comparable to those in Netrin-1−/− mutants are consistent with the model that neogenin is a functional Netrin-1 receptor that acts in concert with Dcc to direct commissural axons to the midline netrin source.
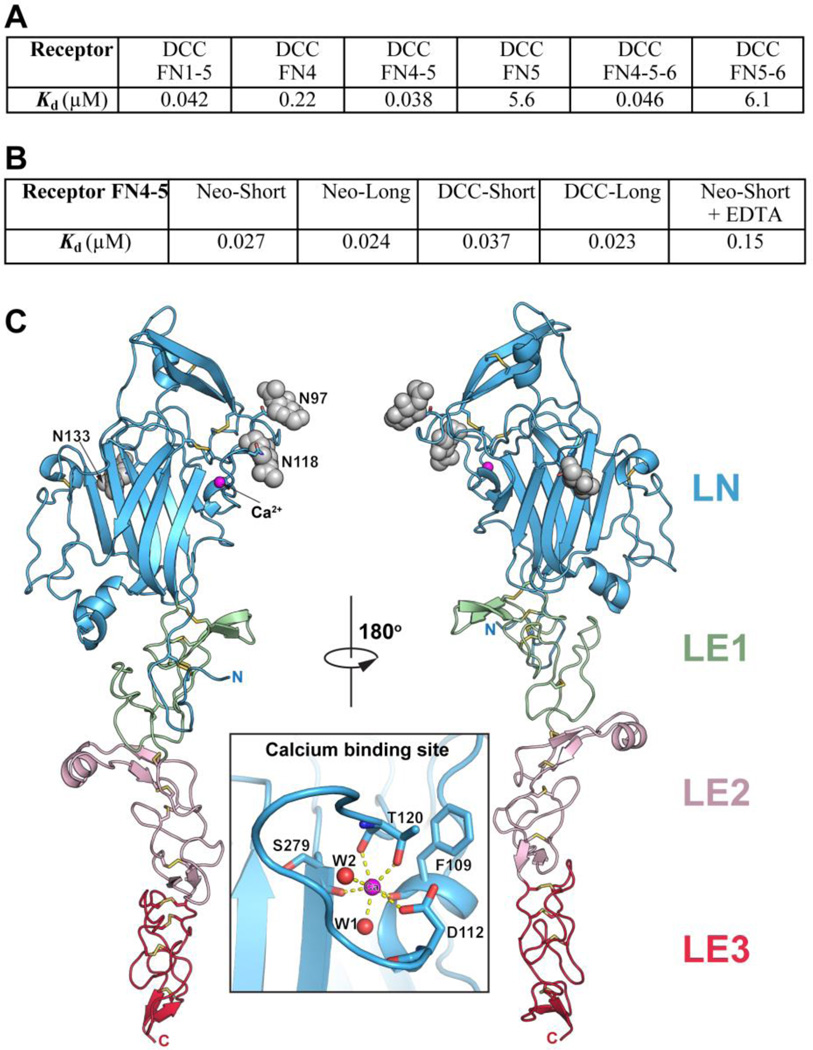
To study how neogenin and DCC function as Netrin-1 receptors, we investigated the structural basis of the Netrin-1/neogenin and Netrin-1/DCC interactions. There are conflicting reports regarding which DCC FNIII domains mediate interactions with the Netrin-1 LN-LE (1–3) region (9–11), so we conducted biolayer interferometry binding studies (Methods) to clarify this. Our results (Fig. 2A) show that domains FN4 and FN5 both interact with this ligand and that they account for the full in vitro binding affinity. Accordingly, in our structural studies we used a netrin construct that contains the LN and LE1-3 domains and neogenin/DCC constructs containing FN4 and FN5. We did not include the C-terminal, positively charged netrin domain (LC, a.k.a. C345C, suggested to bind heparan sulfate (24)), because it is attached via a flexible linker and not required for receptor binding (9–11), and because a Netrin-1-Fc-fusion construct lacking this domain induces similar axon outgrowth in vitro as full-length Netrin-1 (12). Splice variants (isoforms) of both neogenin and DCC with different length of the FN4–FN5 linker have been reported in most species. Both shorter and longer isoforms bind Netrin-1 with high affinity (Fig. 2B). For our structural studies we used the shorter isoforms (25).
Fig. 2.
A) Binding of Netrin-1 (LN-LE1-LE2-LE3) to different DCC constructs documenting that the receptor FN4–FN5 region is necessary and sufficient for netrin binding. Kd, dissociation constant (in µM). B) Binding of Netrin-1 (LN-LE1-LE2-LE3) to the FN4–FN5 region of the different neogenin and DCC isoforms (see Fig. S4). To evaluate the role of the netrin-bound Ca++, 10 mM EDTA was added in one of the measurements. C) Structure of unbound Netrin-1. The individual netrin domains are labeled and colored in blue (LN), green (LE1), pink (LE2) and red (LE3). The glycosylation moieties at the three glycosylated Asn residues are shown as grey spheres. The N- and C- termini are labeled. The insert is a close-up view of the calcium-binding sire in the LN domain. The calcium ion is drawn in magenta and two bound water molecules – in red.
The structure of the Netrin-1 LN-LE(1–3) region was determined at 2.8 Å resolution (Table S1, Figs. S3–S7) revealing an elongated molecule with the same flower-like shape as laminin and Netrin-G (Fig. 2C). The LN domain forms the head, and LE(1–3) the stalk. The disulfide bond network throughout the molecule and the short linkers between the individual netrin domains result in a rigid molecular architecture with little inter-domain flexibility. The globular LN domain has the canonical laminin LN fold, including a conserved Ca++ binding site. The LE region contains three EGF repeats and its structure is similar to those of Laminin-α5 and –γ1 (26, 27), and Netrin G (6, 7), although the latter lacks the third EGF repeat (LE3).
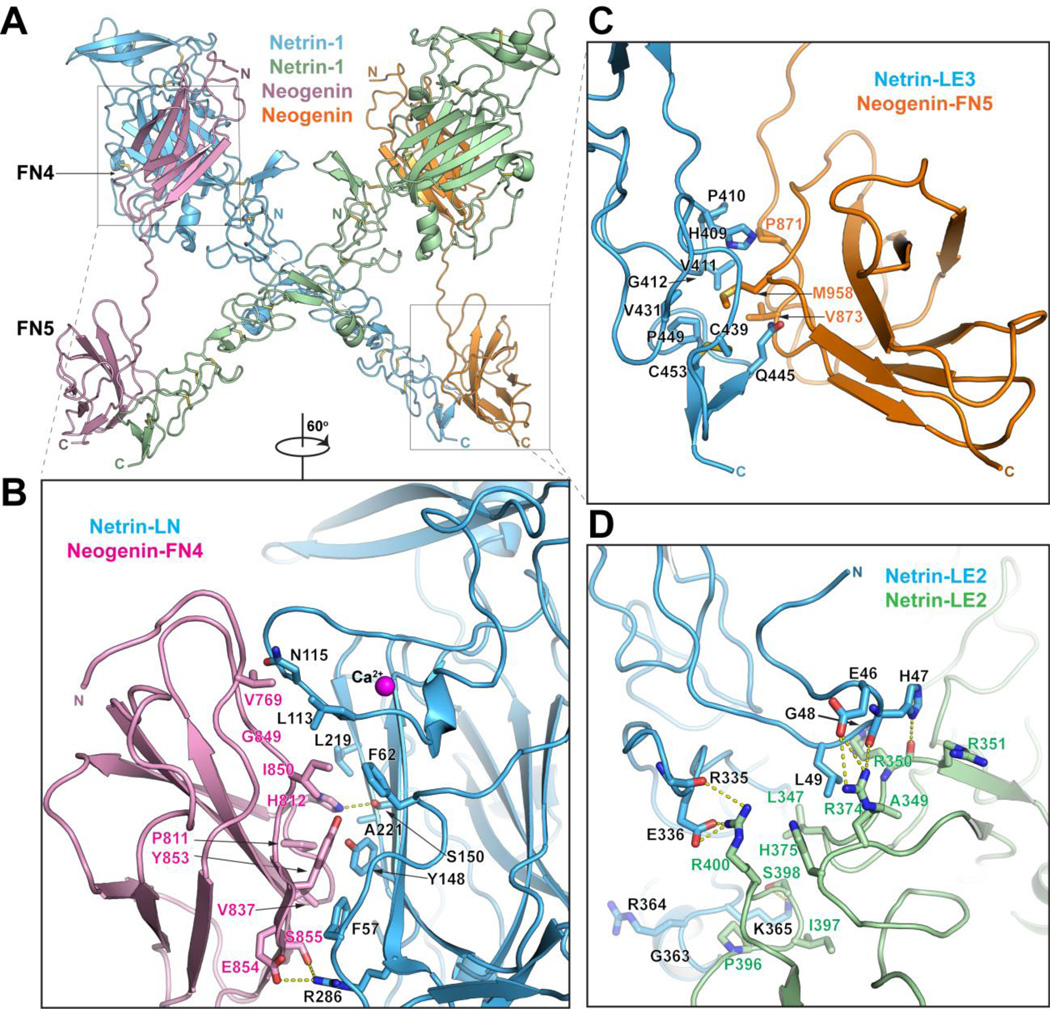
The structure of the netrin/neogenin complex (Fig. 3A) was determined at 3.2 Å resolution and reveals a 2:2 heterotetramer, consistent with its gel-filtration elution profile. At the heart of the complex are two netrin molecules forming a ‘head-to-head’ X-shaped dimer, interacting via an extensive LE2/LE2 interface. This dimer brings together two neogenin molecules, with receptor molecules arranged parallel to each other and their C-termini facing the same direction, presumably towards the neuronal membrane. The two receptor-binding regions are located about 90 Å apart on the two ends of the rigid netrin structure, but the distance between netrin-binding surfaces of neogenin FN4 and FN5 domains cannot exceed 55 Å, so the two receptor-binding sites on netrin must interact with two different receptor molecules. Netrin does not undergo any significant conformational changes upon receptor binding and the bound and unbound netrin structures could be superimposed with RMSD of 0.9Å over 353 Cα atoms.
Fig. 3.
Structure of the Netrin-1/neogenin complex. A) Structure of the 2:2 Netrin-1/neogenin complex. The netrin molecules are in green and blue and the neogenin – in orange and magenta. The N- and C- termini of the molecules are labeled. B) Close-up view of the netrin-LN/neogenin-FN4 interface (Interface-1). Interacting residues and the netrin-bound calcium are labeled. C) Close-up view of the netrin-LE3/neogenin-FN5 interface (Interface-2). Interacting residues are labeled. D) Close-up view of the netrin-LE2/ netrin-LE2 interface (Interface-3). Interacting residues are labeled.
The neogenin FN4 and FN5 domains share the canonical FNIII folding topology (Fig. S7) and are arranged linearly, with the linker between them in a fully extended conformation. This linker region would be flexible in the absence of bound ligand.
The netrin/neogenin 2:2 complex (Fig. 3) contains 5 protein-protein interfaces that fall in 3 categories:Interface-1, between neogenin-FN4 and netrin-LN, buries ~680 Å2 in each interacting domain and is dominated by van der Waals interactions between two largely hydrophobic surfaces. In addition, there are several peripheral hydrogen bonds and a salt bridge (Fig. 3B and Figs. S3, S4, S8). The LN Ca++ binding site is immediately adjacent to interface-1, and bound Ca++ would be required to maintain its proper conformation. Indeed, EDTA reduces the netrin/receptor binding affinities (Fig. 2B).
Interface-2, between neogenin-FN5 and netrin-LE3, buries ~610 Å2 in each interacting domain and contains a mix of hydrophobic and polar contacts, including hydrogen bonds involving main-chain carbonyls and nitrogens (Fig. 3C and Figs. S3, S4, S8). This interface is centered around Met985 of neogenin, the hydrophobic side chain of which is buried in a netrin surface hydrophobic pocket. Interface-1 is slightly larger and more hydrophobic than interface-2, consistent with our binding affinity measurements (Fig. 2A).
Interface-3 is the netrin dimerization interface in the netrin/neogenin 2:2 complex, burying ~1020 Å2 in each interacting molecule. Interestingly, LE2 is longer than the other netrin LE domains, containing an extra strand-helix-strand motif, which provides most of the dimerization contacts. This netrin region is conserved between the canonical netrins (Netrin 1–5), but is very different in the G netrins, suggesting that the latter might not support this netrin-dimer architecture. Interface-3 is two-fold symmetric, although it is not on a crystallographic symmetry axis, as the crystal asymmetric unit contains the full 2:2 heterotetramer. Unlike interface-1 and -2, the vast majority of the interface-3 residues are polar, forming several hydrogen bonds, and four salt bridges (Fig. 3D and Figs. S3, S8).
The netrin LN-LE region is positively charged (pI ~8.5), as is the neogenin/DCC FN4–FN5 region (pI ~9.2). The main positively charged surfaces on netrin (on its LE2 domain) and receptors (on FN5) are exposed to solvent in the complex, making them potentially available for interactions with negatively charged entities like proteoglycans (28, 29).
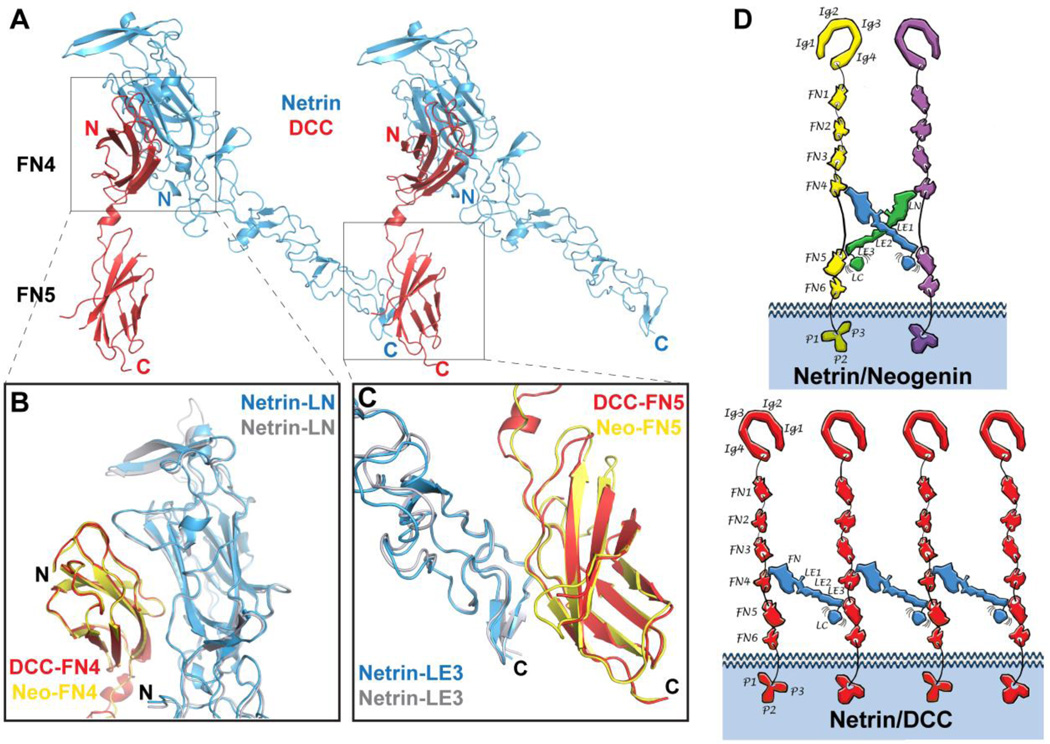
The structure of the Netrin-1/DCC complex (Fig. 4A) was determined at 2.9 Å resolution and shows a different overall architecture than the Netrin-1/neogenin structure, namely a continuous -DCC-netrin-DCC-netrin-DCC- assembly. Each netrin molecule still interacts, via its two receptor-binding sites on the LN and LE3 domains, with two different DCC molecules. At the same time, each DCC receptor interacts with two netrin molecules via its two distinct netrin-binding sites on FN4 and FN5, but these two netrins are shared with two other DCC neighbors (Fig. 4D). The reason the netrin/neogenin complex architecture cannot be replicated in the netrin/DCC complex is that the FN4–FN5 linker is slightly shorter in DCC than in neogenin (Fig. S4) and the DCC linker also forms a short α helix. Formation of the 2:2 Netrin-1/neogenin signaling complex around the X-shaped netrin dimer requires full extension of neogenin FN4–FN5 linkers, but the DCC FN4–FN5 linker is not long enough to accommodate this architecture. As in the netrin/neogenin complex, the DCC receptor molecules are parallel to each other and their C-termini face the same direction.
Fig. 4.
Structure of the Netrin-1/DCC complex. A) Structure of the Netrin-1/DCC complex. Netrin-1 is colored in blue, and neogenin in yellow and magenta. B) Superimposition of the LN-FN4 interaction site (Interface-1) in the Netrin-1/DCC (blue/yellow) and Netrin-1/neogenin (grey/pink) complexes. C) Superimposition of the LE3-FN5 interaction site (Interface-2) in the Netrin-1/DCC (blue/magenta) and Netrin-1/neogenin (grey/orange) complexes. D) Schematic drawing comparing the distinct Netrin-1/neogenin and Netrin-1/DCC signaling assemblies. The individual netrin and receptor domains are labeled. Ig, immunoglobulin; FN, fibronectin; LN, laminin-like N-terminal; LE, laminin-like EGF; LC, laminin-like C-terminal. Top, schematic representation if the 2:2 Netrin-1/neogenin complex. The netrin molecules are colored in blue and green, and the neogenin – in yellow and magenta. Bottom, schematic representation if the continuous Netrin-1/DCC assembly. The netrin molecules are colored in blue, and the DCC – in red. Interestingly, in both Netrin-1/DCC and Netrin-1/neogenin assemblies, the positively-charged netrin LC domain would be positioned towards the negatively-charged plasma membrane, thus potentially further stabilizing the signaling complexes at the neuronal surface.
The individual DCC and neogenin FNIII domains share about 70% sequence identity and their structures are very similar, with RMSDs between equivalent Cα positions of 0.47Å for FN4 and 0.38Å for FN5. The Netrin-1 structure is also very similar in the complexes with its two receptors, with RMSDs between Cα positions of 0.73Å. The netrin/DCC interfaces are nearly identical to the netrin/neogenin interfaces (Fig. 4 and Fig. S4). Interface-1 is again formed between the netrin LN domain and the DCC FN4 domain, while interface-2 is formed between the netrin LE3 domain and the DCC FN5 domain. Panels B and C of Fig. 4 illustrate the similarly of the interacting LN-FN4 and LE3-FN5 domains in the two complexes.
Based on the structures reported here, we propose that netrin induces signaling by binding to and bringing together receptor molecules via its two binding sites, thus creating ligand/receptor signaling assemblies at the neuronal surface. One netrin molecule cannot form a bivalent complex with a single receptor molecule, even with the long isoforms, as the distance between the two receptor-binding sites on netrin is significantly larger than the distance between the two ligand-binding sites on receptors. Our structures illustrate the potential for netrins to cross-link different receptor types via distinct receptor-binding sites: e.g. DCC or neogenin on one end and Unc5 the other, or DCC on one end and neogenin the other. The netrin LC domain might further concentrate or cluster assemblies, e.g. via interactions with heparan sulfate, as the degree of axon outgrowth seen with the netrin LN-LE region linked to a dimeric Fc tag is similar to that with full length Netrin-1, and much greater than with the LN-LE region alone (12).
The differences in the two signaling architectures result from different lengths of the linker connecting the receptor FN4 and FN5 domains, which differs between the DCC and neogenin isoforms studied here. In most species with these molecules, two different isoforms for each receptor, short and long, arise from alternative splicing of the FN4–FN5 linker sequence (25) (Fig. S4). The two neogenin isoforms and the long DCC isoform all contain linkers long enough to support the 2:2 signaling-complex architecture, whereas the shorter DCC isoform does not allow this formation. Our binding experiments (Fig. 2B) suggest that the 2:2 assembly is energetically favored over the continuous assembly, presumably owing to the additional large interface between the two netrin molecules that it permits. The short neogenin and DCC isoforms are reported to predominate in many tissues (30) and could have distinct signaling properties mediated by distinct signaling-complex architectures (Fig. 4D). However, our results also highlight the need to evaluate which isoform of DCC is used in any given cell type, to determine the potential of distinct assemblies to elicit particular cellular responses to netrin.
Supplementary Material
Acknowledgments
We thank Momchil Kolev for technical support, Marina Himanen for illustrations, and Dr. Yehuda Goldgur for help with data collection and processing. X-ray diffraction studies were conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines, which are supported by a grant from the National Institute of General Medical Sciences (P41 GM103403) from the National Institutes of Health. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. The Netrin-1, Netrin-1/Neogenin, and Netrin-1/DCC structures have been deposited in the Protein Data Bank under codes 4PLM, 4PLN, and 4PLO, respectively.
References and Notes
- 1.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 2.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 3.Forcet C, Stein E, Pays L, Corset V, Llambi F, Tessier-Lavigne M, Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 4.Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Advances in experimental medicine and biology. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- 5.Rajasekharan S, Kennedy TE. The netrin protein family. Genome biology. 2009;10:239. doi: 10.1186/gb-2009-10-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasch J, Harrison OJ, Ahlsen G, Liu Q, Shapiro L. Crystal structure of the ligand binding domain of netrin G2. Journal of molecular biology. 2011;414:723–734. doi: 10.1016/j.jmb.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiradake E, Coles CH, Perestenko PV, Harlos K, McIlhinney RA, Aricescu AR, Jones EY. Structural basis for cell surface patterning through NetrinG-NGL interactions. The EMBO journal. 2011;30:4479–4488. doi: 10.1038/emboj.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 9.Kruger RP, Lee J, Li W, Guan KL. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10826–10834. doi: 10.1523/JNEUROSCI.3715-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. The Journal of biological chemistry. 2003;278:32561–32568. doi: 10.1074/jbc.M302943200. [DOI] [PubMed] [Google Scholar]
- 11.Mille F, Llambi F, Guix C, Delloye-Bourgeois C, Guenebeaud C, Castro-Obregon S, Bredesen DE, Thibert C, Mehlen P. Interfering with multimerization of netrin-1 receptors triggers tumor cell death. Cell death and differentiation. 2009;16:1344–1351. doi: 10.1038/cdd.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Copeland NG, Gilbert DJ, Jenkins NA, Tessier-Lavigne M. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4938–4947. doi: 10.1523/JNEUROSCI.19-12-04938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell CH, Healey E, van Erp S, Bishop B, Tang C, Gilbert RJ, Aricescu AR, Pasterkamp RJ, Siebold C. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science. 2013;341:77–80. doi: 10.1126/science.1232322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller BK, Strittmatter SM. Neogenin mediates the action of repulsive guidance molecule. Nature cell biology. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- 16.Wilson NH, Key B. Neogenin interacts with RGMa and netrin-1 to guide axons within the embryonic vertebrate forebrain. Developmental biology. 2006;296:485–498. doi: 10.1016/j.ydbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Developmental cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 18.Engle EC. Human genetic disorders of axon guidance. Cold Spring Harbor perspectives in biology. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vulliemoz S, Raineteau O, Jabaudon D. Reaching beyond the midline: why are human brains cross wired? Lancet neurology. 2005;4:87–99. doi: 10.1016/S1474-4422(05)00990-7. [DOI] [PubMed] [Google Scholar]
- 20.Srour M, Riviere JB, Pham JM, Dube MP, Girard S, Morin S, Dion PA, Asselin G, Rochefort D, Hince P, Diab S, Sharafaddinzadeh N, Chouinard S, Theoret H, Charron F, Rouleau GA. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- 21.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 22.Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- 23.Palmesino E, Haddick PC, Tessier-Lavigne M, Kania A. Genetic analysis of DSCAM's role as a Netrin-1 receptor in vertebrates. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:411–416. doi: 10.1523/JNEUROSCI.3563-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappler J, Franken S, Junghans U, Hoffmann R, Linke T, Muller HW, Koch KW. Glycosaminoglycan-binding properties and secondary structure of the C-terminus of netrin-1. Biochemical and biophysical research communications. 2000;271:287–291. doi: 10.1006/bbrc.2000.2583. [DOI] [PubMed] [Google Scholar]
- 25.Shen H, Illges H, Reuter A, Stuermer CA. Cloning, expression, and alternative splicing of neogenin1 in zebrafish. Mechanisms of development. 2002;118:219–223. doi: 10.1016/s0925-4773(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 26.Hussain SA, Carafoli F, Hohenester E. Determinants of laminin polymerization revealed by the structure of the alpha5 chain amino-terminal region. EMBO reports. 2011;12:276–282. doi: 10.1038/embor.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carafoli F, Hussain SA, Hohenester E. Crystal structures of the network-forming short-arm tips of the laminin beta1 and gamma1 chains. PloS one. 2012;7:e42473. doi: 10.1371/journal.pone.0042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto Y, Irie F, Inatani M, Tessier-Lavigne M, Yamaguchi Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4342–4350. doi: 10.1523/JNEUROSCI.0700-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manitt C, Thompson KM, Kennedy TE. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. Journal of neuroscience research. 2004;77:690–700. doi: 10.1002/jnr.20199. [DOI] [PubMed] [Google Scholar]
- 31.Bae GU, Yang YJ, Jiang G, Hong M, Lee HJ, Tessier-Lavigne M, Kang JS, Krauss RS. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Molecular biology of the cell. 2009;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minor ZOaW. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 33.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. Journal of applied crystallography. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.