Abstract
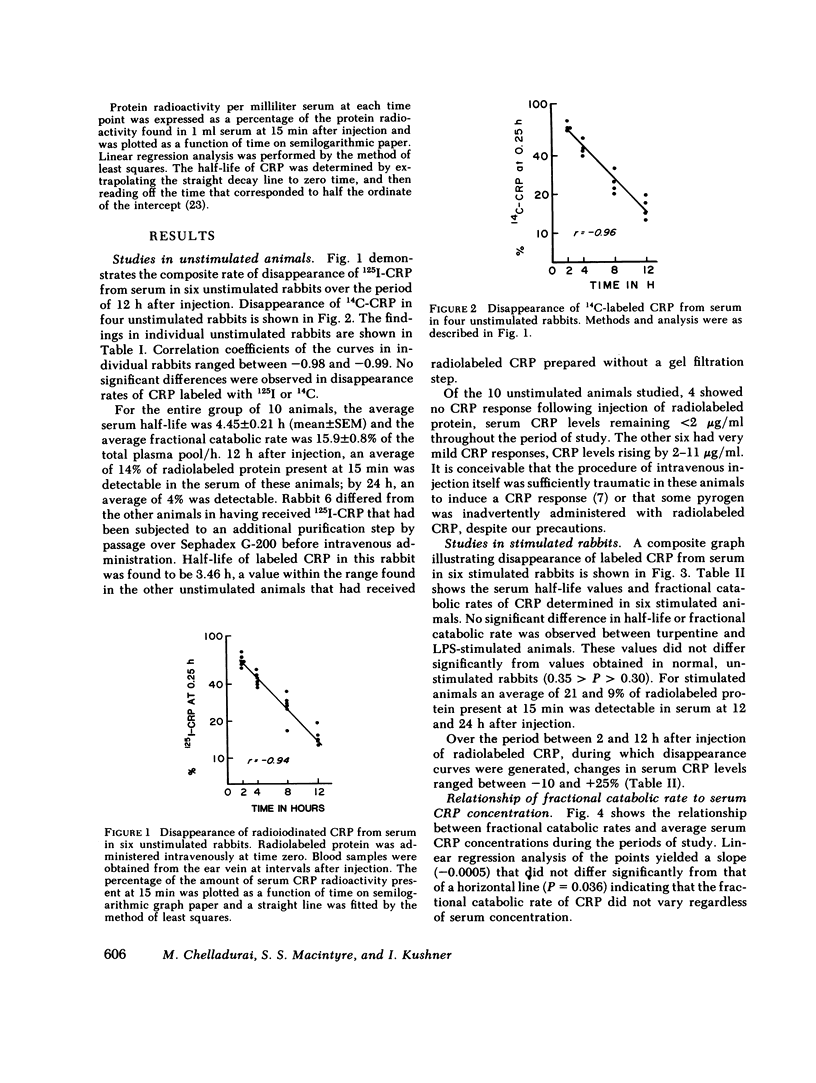
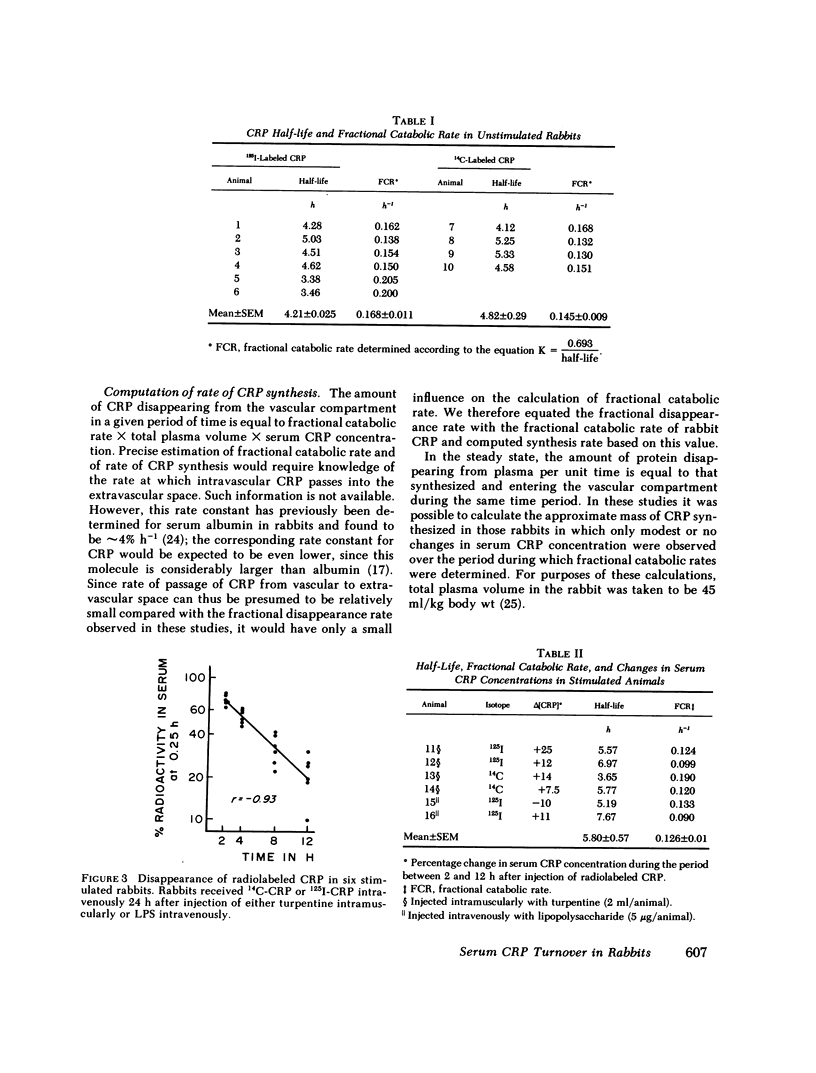
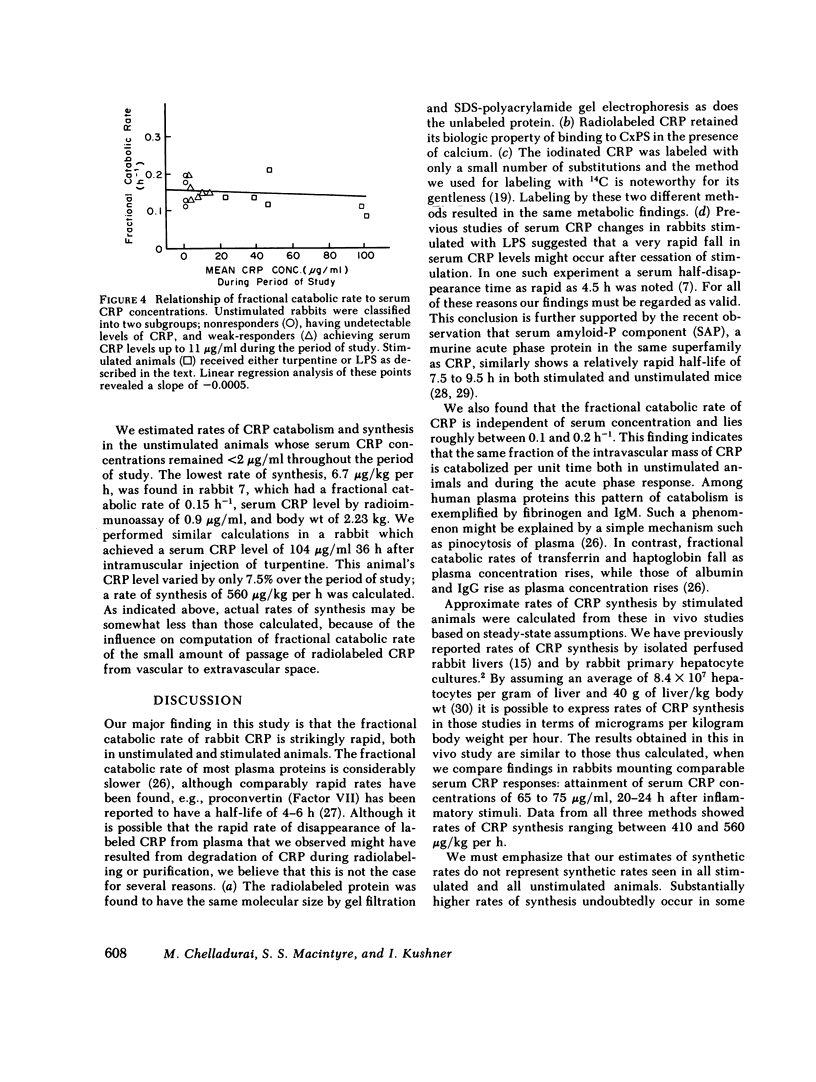
We determined the plasma half-life of the acute phase protein C-reactive protein (CRP) both in normal rabbits and in rabbits that had received inflammatory stimuli. Rabbit CRP was purified from acute phase serum by Cx-polysaccharide affinity chromatography, radiolabeled, and rendered pyrogen-free. Six unstimulated rabbits were injected intravenously with 1251-CRP prepared by the lactoperoxidase method and four were injected with CRP labeled by methylation using [14C]formaldehyde. Blood samples were obtained at 0.25 h and at intervals thereafter. Plasma half-life of CRP was calculated from the data generated during the first 12 h, by which time an average of 86% of labeled protein had disappeared from the blood stream. The mean half-life for CRP was 4.45±0.2 h, with no significant difference (0.40 < P < 0.45) between 1251- and 14C-labeled CRP. In six animals stimulated with either endotoxin or turpentine 24 h before injection of labeled CRP, a mean half-life of 5.8±0.6 h was found, not significantly different (0.30 < P < 0.35) from unstimulated rabbits. We equated fractional catabolic rate to fractional disappearance rate, since the rate constant for passage of CRP from vascular to extravascular compartment can be assumed to be relatively small compared to the observed fractional disappearance rate. Fractional catabolic rate was independent of serum CRP concentration; average fractional catabolic rate in all 16 animals was 14±0.8% h-1 of the plasma pool. We were able to estimate rate of CRP synthesis, based on steady-state assumptions of pool sizes in those rabbits whose serum CRP levels did not change substantially during the period of study. Values as low as 6.7 μg/kg per h in the unstimulated animals and as high as 560 μg/kg per h in the stimulated animals were found.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON H. C., McCARTY M. The occurrence in the rabbit of an acute phase protein analogous to human C reactive protein. J Exp Med. 1951 Jan;93(1):25–36. doi: 10.1084/jem.93.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEEKEN W. L., VOLWILER W., GOLDSWORTHY P. D., GARBY L. E., REYNOLDS W. E., STOGSDILL R., STEMLER R. S. Studies of I-131-albumin catabolism and distribution in normal young male adults. J Clin Invest. 1962 Jun;41:1312–1333. doi: 10.1172/JCI104594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz M. L., de Beer F. C., Feinstein A., Munn E. A., Milstein C. P., Fletcher T. C., March J. F., Taylor J., Bruton C., Clamp J. R. Phylogenetic aspects of C-reactive protein and related proteins. Ann N Y Acad Sci. 1982;389:49–75. doi: 10.1111/j.1749-6632.1982.tb22125.x. [DOI] [PubMed] [Google Scholar]
- GOTSCHLICH E., STETSON C. A., Jr Immunologic cross-reactions among mammalian acute phase proteins. J Exp Med. 1960 Apr 1;111:441–451. doi: 10.1084/jem.111.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Mold C., Siegel J., Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345–372. [PubMed] [Google Scholar]
- Hurlimann J., Thorbecke G. J., Hochwald G. M. The liver as the site of C-reactive protein formation. J Exp Med. 1966 Feb 1;123(2):365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Kushner I., Broder M. L., Karp D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J Clin Invest. 1978 Feb;61(2):235–242. doi: 10.1172/JCI108932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I., Edgington T. S., Trimble C., Liem H. H., Muller-Eberhard U. Plasma hemopexin homeostasis during the acute phase response. J Lab Clin Med. 1972 Jul;80(1):18–25. [PubMed] [Google Scholar]
- Kushner I., Feldmann G. Control of the acute phase response. Demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J Exp Med. 1978 Aug 1;148(2):466–477. doi: 10.1084/jem.148.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I., Ribich W. N., Blair J. B. Control of the acute-phase response. C-reactive protein synthesis by isolated perfused rabbit livers. J Lab Clin Med. 1980 Dec;96(6):1037–1045. [PubMed] [Google Scholar]
- Kushner I., Somerville J. A. Estimation of the molecular size of C-reactive protein and CX-reactive protein in serum. Biochim Biophys Acta. 1970 Apr 28;207(1):105–114. doi: 10.1016/0005-2795(70)90140-6. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Robey F. A., Wang C. M. Structural studies on C-reactive protein. Ann N Y Acad Sci. 1982;389:151–162. doi: 10.1111/j.1749-6632.1982.tb22133.x. [DOI] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Oliveira E. B., Gotschlich C., Liu T. Y. Primary structure of human C-reactive protein. J Biol Chem. 1979 Jan 25;254(2):489–502. [PubMed] [Google Scholar]
- Oliveira E. B., Gotschlich E. C., Liu T. Y. Comparative studies on the binding properties of human and rabbit C-reactive proteins. J Immunol. 1980 Mar;124(3):1396–1402. [PubMed] [Google Scholar]
- Osmand A. P., Friedenson B., Gewurz H., Painter R. H., Hofmann T., Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc Natl Acad Sci U S A. 1977 Feb;74(2):739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L., de Beer F. C., Dyck R. F., Holford S., Breathnach S. M., Black M. M., Tribe C. R., Evans D. J., Feinstein A. Biology of serum amyloid P component. Ann N Y Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Fletcher T. C., Richardson N., Munn E. A., Feinstein A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978 May 11;273(5658):168–170. doi: 10.1038/273168a0. [DOI] [PubMed] [Google Scholar]
- REEVE E. B., ROBERTS J. E. The kinetics of the distribution and breakdown of 1131-albumin in the rabbit. Observations on several mathematical descriptions. J Gen Physiol. 1959 Nov;43:415–444. doi: 10.1085/jgp.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSCHILD M. A., ORATZ M., WIMER E., SCHREIBER S. S. Studies on albumin synthesis: the effects of dextran and cortisone on albumin metabolism in rabbits studied with albumin-I-131. J Clin Invest. 1961 Mar;40:545–554. doi: 10.1172/JCI104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine B., de Beer F. C., Pepys M. B. Solid phase radioimmunoassays for human C-reactive protein. Clin Chim Acta. 1981 Nov 25;117(1):13–23. doi: 10.1016/0009-8981(81)90005-x. [DOI] [PubMed] [Google Scholar]
- Skinner M., Sipe J. D., Yood R. A., Shirahama T., Cohen A. S. Characterization of P-component (AP) isolated from amyloidotic tissue: half-life studies of human and murine AP. Ann N Y Acad Sci. 1982;389:190–198. doi: 10.1111/j.1749-6632.1982.tb22137.x. [DOI] [PubMed] [Google Scholar]
- Volanakis J. E., Narkates A. J. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers and complement. J Immunol. 1981 May;126(5):1820–1825. [PubMed] [Google Scholar]
- Yen-Watson B., Kushner I. Rabbit CRP response to endotoxin administration: dose-response relationship and kinetics. Proc Soc Exp Biol Med. 1974 Sep;146(4):1132–1136. doi: 10.3181/00379727-146-38260. [DOI] [PubMed] [Google Scholar]



