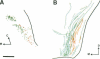Abstract
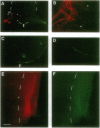
Retinal ganglion cell axons must make a decision at the embryonic optic chiasm to grow into the appropriate optic tract. To gain insight into the cues that play a role in sorting out the crossed from the uncrossed optic axons, we investigated the sequence of their initial ingrowth in rhesus monkey embryos. Two carbocyanine dyes, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate and 4-(4-dihexadecylaminostyryl)-N-methylpyridinium iodide, were placed, respectively, into the left and right retinas to identify the course of uncrossed and crossed retinal axons through the optic chiasm and tract. Our results show that at embryonic day 36 the most advanced retinal projections are uncrossed. At this age the leading crossed axons are just reaching the chiasmatic midline, whereas the uncrossed fibers have already entered the optic tract. This indicates that the pathfinding of these pioneer uncrossed fibers does not require the presence of retinal axons from the opposite eye. At subsequent stages of development (embryonic days 40 and 42) there is a clear partial segregation of the uncrossed and crossed retinal axons within the optic tract: the uncrossed-component course is in the deeper portion of the optic tract, whereas the crossed component lies in a more superficial region. Thus, the spatial organization of retinal axons within the primordial optic tract reflects the sequential addition of the uncrossed and crossed retinal fibers. The orderly and sequential ingrowth of these pioneer retinal axons indicates that specific chiasmatic cues are expressed early in development and that such pioneer fibers may serve as guides for the later-arriving retinal fibers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. M. Pioneer neurones in an insect embryo. Nature. 1976 Mar 4;260(5546):54–56. doi: 10.1038/260054a0. [DOI] [PubMed] [Google Scholar]
- Bentley D., Keshishian H. Pathfinding by peripheral pioneer neurons in grasshoppers. Science. 1982 Dec 10;218(4577):1082–1088. doi: 10.1126/science.218.4577.1082. [DOI] [PubMed] [Google Scholar]
- Chalupa L. M., Lia B. The nasotemporal division of retinal ganglion cells with crossed and uncrossed projections in the fetal rhesus monkey. J Neurosci. 1991 Jan;11(1):191–202. doi: 10.1523/JNEUROSCI.11-01-00191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello R. J., Guillery R. W. The early development of retinal ganglion cells with uncrossed axons in the mouse: retinal position and axonal course. Development. 1990 Mar;108(3):515–523. doi: 10.1242/dev.108.3.515. [DOI] [PubMed] [Google Scholar]
- Godement P., Salaün J., Mason C. A. Retinal axon pathfinding in the optic chiasm: divergence of crossed and uncrossed fibers. Neuron. 1990 Aug;5(2):173–186. doi: 10.1016/0896-6273(90)90307-2. [DOI] [PubMed] [Google Scholar]
- Godement P., Salaün J., Métin C. Fate of uncrossed retinal projections following early or late prenatal monocular enucleation in the mouse. J Comp Neurol. 1987 Jan 1;255(1):97–109. doi: 10.1002/cne.902550108. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Guillery R. W., Hickey T. L., Kaas J. H., Felleman D. J., Debruyn E. J., Sparks D. L. Abnormal central visual pathways in the brain of an albino green monkey (Cercopithecus aethiops). J Comp Neurol. 1984 Jun 20;226(2):165–183. doi: 10.1002/cne.902260203. [DOI] [PubMed] [Google Scholar]
- Ho R. K., Goodman C. S. Peripheral pathways are pioneered by an array of central and peripheral neurones in grasshopper embryos. Nature. 1982 Jun 3;297(5865):404–406. doi: 10.1038/297404a0. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Chalupa L. M. Ontogenetic change in the distribution of callosal projection neurons in the postcentral gyrus of the fetal rhesus monkey. J Comp Neurol. 1986 Feb 15;244(3):331–348. doi: 10.1002/cne.902440306. [DOI] [PubMed] [Google Scholar]
- Klose M., Bentley D. Transient pioneer neurons are essential for formation of an embryonic peripheral nerve. Science. 1989 Sep 1;245(4921):982–984. doi: 10.1126/science.2772651. [DOI] [PubMed] [Google Scholar]
- Kutsch W., Bentley D. Programmed death of peripheral pioneer neurons in the grasshopper embryo. Dev Biol. 1987 Oct;123(2):517–525. doi: 10.1016/0012-1606(87)90410-6. [DOI] [PubMed] [Google Scholar]
- La Vail M. M., Rapaport D. H., Rakic P. Cytogenesis in the monkey retina. J Comp Neurol. 1991 Jul 1;309(1):86–114. doi: 10.1002/cne.903090107. [DOI] [PubMed] [Google Scholar]
- McConnell S. K., Ghosh A., Shatz C. J. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989 Sep 1;245(4921):978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McKanna J. A. Optic chiasm and infundibular decussation sites in the developing rat diencephalon are defined by glial raphes expressing p35 (lipocortin 1, annexin I). Dev Dyn. 1992 Oct;195(2):75–86. doi: 10.1002/aja.1001950202. [DOI] [PubMed] [Google Scholar]
- Reese B. E., Cowey A. Fibre organization of the monkey's optic tract: II. Noncongruent representation of the two half-retinae. J Comp Neurol. 1990 May 15;295(3):401–412. doi: 10.1002/cne.902950305. [DOI] [PubMed] [Google Scholar]
- Reese B. E., Cowey A. Segregation of functionally distinct axons in the monkey's optic tract. Nature. 1988 Jan 28;331(6154):350–351. doi: 10.1038/331350a0. [DOI] [PubMed] [Google Scholar]
- Reese B. E. The distribution of axons according to diameter in the optic nerve and optic tract of the rat. Neuroscience. 1987 Sep;22(3):1015–1024. doi: 10.1016/0306-4522(87)92977-0. [DOI] [PubMed] [Google Scholar]
- Silver J. Studies on the factors that govern directionality of axonal growth in the embryonic optic nerve and at the chiasm of mice. J Comp Neurol. 1984 Feb 20;223(2):238–251. doi: 10.1002/cne.902230207. [DOI] [PubMed] [Google Scholar]
- Sretavan D. W., Reichardt L. F. Time-lapse video analysis of retinal ganglion cell axon pathfinding at the mammalian optic chiasm: growth cone guidance using intrinsic chiasm cues. Neuron. 1993 Apr;10(4):761–777. doi: 10.1016/0896-6273(93)90176-r. [DOI] [PubMed] [Google Scholar]
- Stone J., Leicester J., Sherman S. M. The naso-temporal division of the monkey's retina. J Comp Neurol. 1973 Aug;150(3):333–348. doi: 10.1002/cne.901500306. [DOI] [PubMed] [Google Scholar]
- Torrealba F., Guillery R. W., Eysel U., Polley E. H., Mason C. A. Studies of retinal representations within the cat's optic tract. J Comp Neurol. 1982 Nov 10;211(4):377–396. doi: 10.1002/cne.902110405. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Bastiani M. J., Lia B., Chalupa L. M. Growth cones, dying axons, and developmental fluctuations in the fiber population of the cat's optic nerve. J Comp Neurol. 1986 Apr 1;246(1):32–69. doi: 10.1002/cne.902460104. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Borodkin M., Rakic P. Growth cone distribution patterns in the optic nerve of fetal monkeys: implications for mechanisms of axon guidance. J Neurosci. 1991 Apr;11(4):1081–1094. doi: 10.1523/JNEUROSCI.11-04-01081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizenmann A., Thanos S., von Boxberg Y., Bonhoeffer F. Differential reaction of crossing and non-crossing rat retinal axons on cell membrane preparations from the chiasm midline: an in vitro study. Development. 1993 Feb;117(2):725–735. doi: 10.1242/dev.117.2.725. [DOI] [PubMed] [Google Scholar]