Abstract
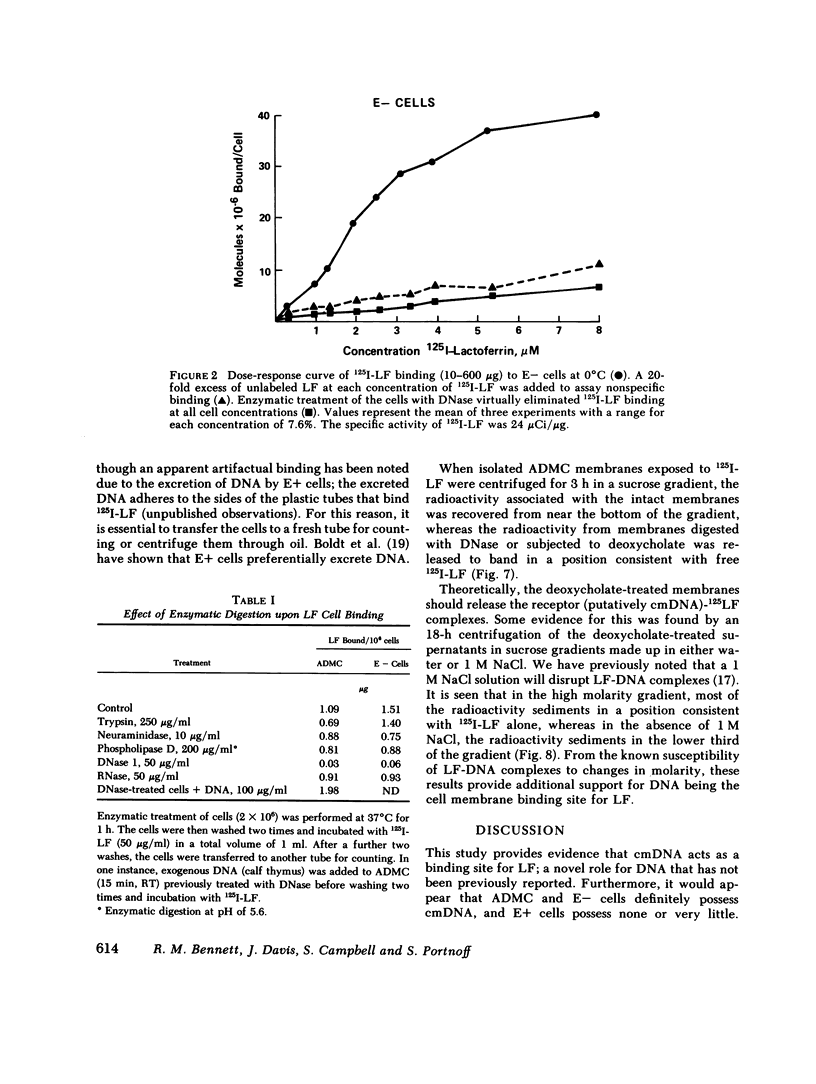
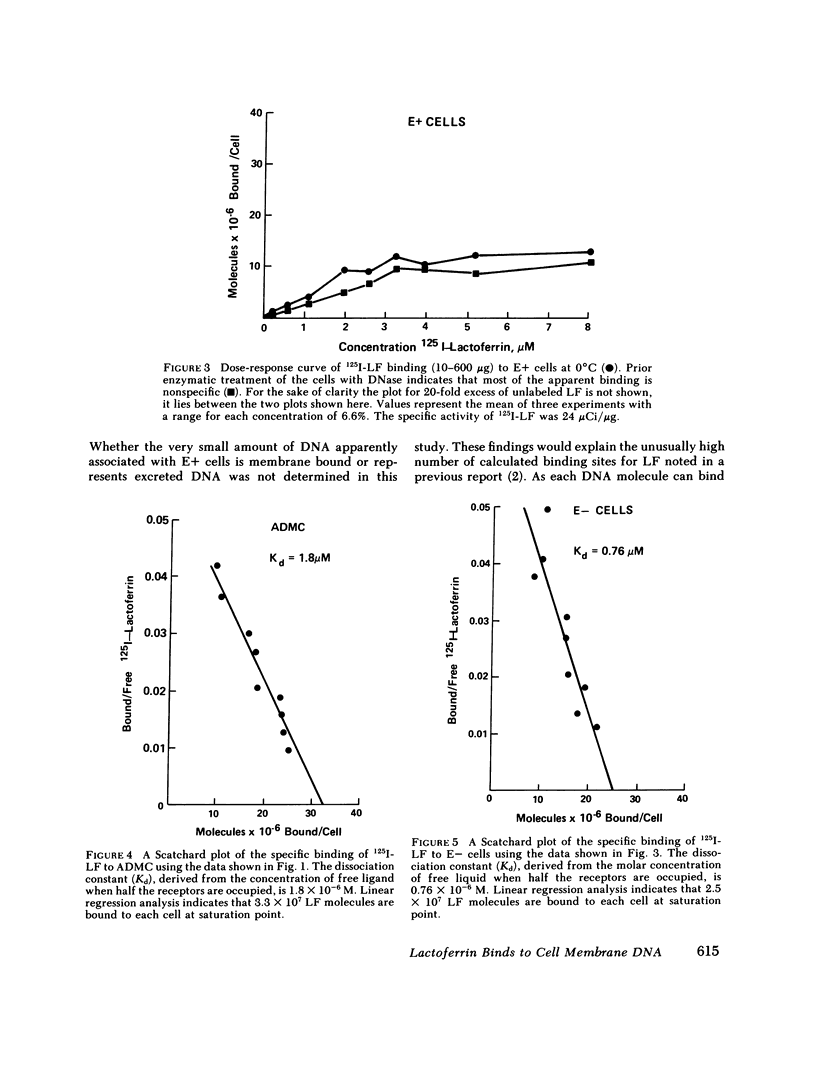
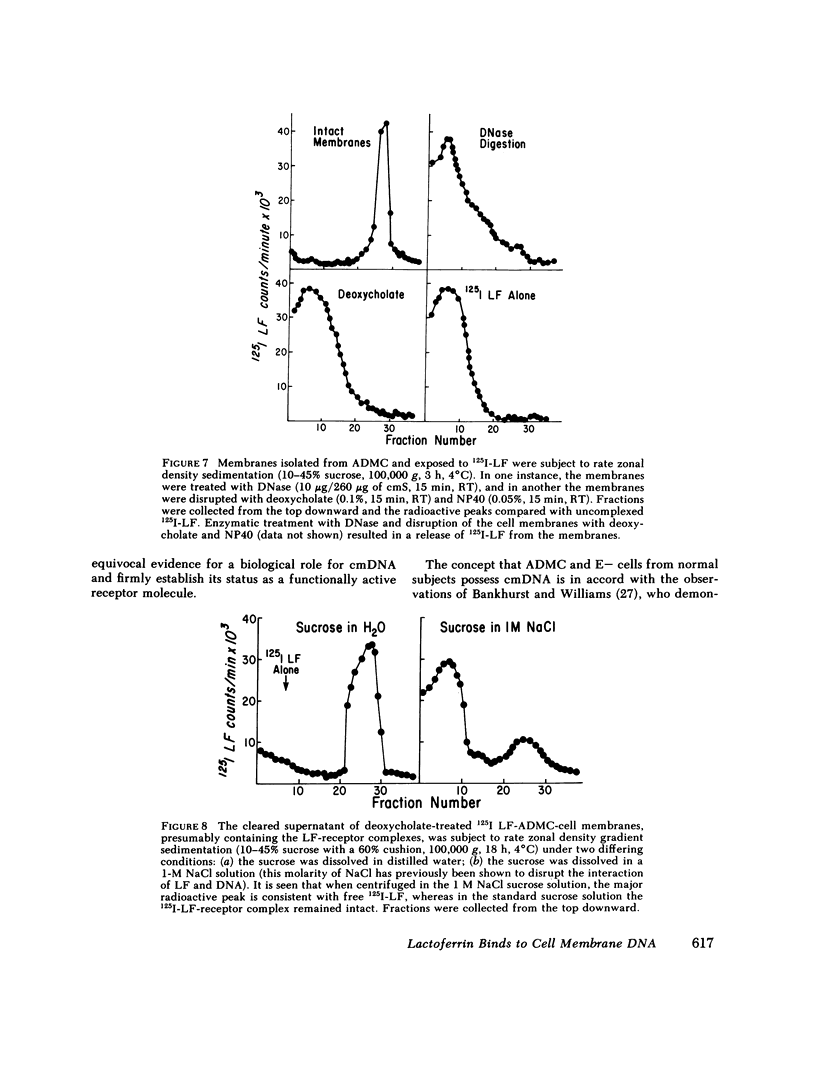
The binding of human 125I-labeled lactoferrin (LF) to a population of adherent mononuclear cells (ADMC) and nonrosetting lymphocytes (E-) was abolished by prior treatment of the cells with deoxyribonuclease (DNase), but not ribonuclease (RNase). When DNase-treated ADMC were incubated with exogenous DNA, the binding of 125I-LF was restored. Enzymatic digestion with other enzymes, trypsin, phospholipase D, and neuraminidase, did not significantly influence 125I-LF binding. Saturable binding of LF at 0 degrees C was demonstrated for both E- and ADMC, with equilibrium dissociated constants of 0.76 x 10(-6) M and 1.8 x 10(-6) M, respectively. E- cells bound 2.5 x 10(7) and ADMC bound 3.3 x 10(7) molecules of Lf at saturation. Cell membranes were isolated from ADMC, E- and E+ and reacted with 125I-labeled LF; significant binding was only seen with ADMC and E-. Prior treatment of the membranes with DNase abolished the binding. Immunofluorescence studies indicated that a population of ADMC and E-, but not E+, exhibited a peripheral staining pattern for LF. Prior treatment of ADMC and E- with DNase abolished the surface immunofluorescence. This study provides evidence that cell membrane DNA acts as a binding site for exogenous LF. This is a novel role for DNA that has not been previously reported. Furthermore, it points to a basic difference between E+ cells vs. ADMC and E- cells in respect to their possession of cell surface DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker P., Stroun M., Maurice P. A. Spontaneous release of DNA by human blood lymphocytes as shown in an in vitro system. Cancer Res. 1975 Sep;35(9):2375–2382. [PubMed] [Google Scholar]
- Bagby G. C., Jr, Bennett R. M. Feedback regulation of granulopoiesis: polymerization of lactoferrin abrogates its ability to inhibit CSA production. Blood. 1982 Jul;60(1):108–112. [PubMed] [Google Scholar]
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Williams R. C., Jr Identification of DNA-binding lymphocytes in patients with systemic lupus erythematosus. J Clin Invest. 1975 Dec;56(6):1378–1385. doi: 10.1172/JCI108218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. M., Bagby G. C., Davis J. Calcium-dependent polymerization of lactoferrin. Biochem Biophys Res Commun. 1981 Jul 16;101(1):88–95. doi: 10.1016/s0006-291x(81)80014-9. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Davis J. Lactoferrin binding to human peripheral blood cells: an interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J Immunol. 1981 Sep;127(3):1211–1216. [PubMed] [Google Scholar]
- Bennett R. M., Davis J. Lactoferrin interacts with deoxyribonucleic acid: a preferential reactivity with double-stranded DNA and dissociation of DNA-anti-DNA complexes. J Lab Clin Med. 1982 Jan;99(1):127–138. [PubMed] [Google Scholar]
- Bennett R. M., Mohla C. A solid-phase radioimmunoassay for the measurement of lactoferrin in human plasma: variations with age, sex, and disease. J Lab Clin Med. 1976 Jul;88(1):156–166. [PubMed] [Google Scholar]
- Boldt D. H., MacDermott R. P., Speckart S. F., Nash G. S. Excretion of DNA by purified human lymphocyte subpopulations. J Immunol. 1977 Apr;118(4):1495–1498. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E. Lactoferrin acts on Ia-like antigen-positive subpopulations of human monocytes to inhibit production of colony stimulatory activity in vitro. J Clin Invest. 1979 Dec;64(6):1717–1720. doi: 10.1172/JCI109635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- DIXON T. F., PURDOM M. Serum 5-nucleotidase. J Clin Pathol. 1954 Nov;7(4):341–343. doi: 10.1136/jcp.7.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelhorst C. W., Cramer K., Rogers J. C. Selective release of excreted DNA sequences from phytohemagglutinin-stimulated human peripheral blood lymphocytes. Effects of trypsin and divalent cations. J Clin Invest. 1978 May;61(5):1204–1217. doi: 10.1172/JCI109036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Mendes N. F., Tolnai M. E., Silveira N. P., Gilbertsen R. B., Metzgar R. S. Technical aspects of the rosette tests used to detect human complement receptor (B) and sheep erythrocyte-binding (T) lymphocytes. J Immunol. 1973 Sep;111(3):860–867. [PubMed] [Google Scholar]
- Moyer M. P. The association of DNA and RNA with membranes. Int Rev Cytol. 1979;61:1–61. doi: 10.1016/s0074-7696(08)61994-4. [DOI] [PubMed] [Google Scholar]
- Reid B. L., Charlson A. J. Cytoplasmic and cell surface deoxyribonucleic acids with consideration of their origin. Int Rev Cytol. 1979;60:27–52. doi: 10.1016/s0074-7696(08)61258-9. [DOI] [PubMed] [Google Scholar]
- Rogers J. C. Characterization of DNA excreted from phytohemagglutinin-stimulated lymphocytes. J Exp Med. 1976 May 1;143(5):1249–1264. doi: 10.1084/jem.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. G., Caporale L. H., Moore M. A. Characterization of colony-stimulating activity produced by human monocytes and phytohemagglutinin-stimulated lymphocytes. Blood. 1977 Nov;50(5):811–821. [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L. The binding of human lactoferrin to mouse peritoneal cells. J Exp Med. 1976 Dec 1;144(6):1568–1580. doi: 10.1084/jem.144.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]