Abstract
Background
Respiratory diseases are associated with pulmonary oxidative stress and inflammatory processes. Though studies in animal models suggest that dietary polyphenols improve lung injury, no intervention studies were carried out in humans. The aim of this study was to determine whether the intake of an anthocyanin-rich maqui extract improved H2O2 and IL-6 concentrations in exhaled breath condensates (EBCs) from asymptomatic smokers.
Findings
15 asymptomatic smokers with mild cigarette smoking (3 pack-year [2.4 - 7.7]) (mean [CI95%]) were recruited in this exploratory longitudinal study. They ingested 2 g of maqui extract (polyphenol content = 5.18 ± 2.00 g GAE/100 g; FRAP value = 27.1 ± 2.0 mmol Fe++/100 g), twice daily for two weeks. EBCs were collected before and after treatment and the changes in H2O2 and IL-6 concentrations were determined by fluorimetry and Elisa, respectively. The EBC contents of H2O2 and IL-6 H2O2 before and after treatment in smokers were also compared with those determined in single EBC samples from 8 healthy non-smokers subjects. At baseline, the H2O2 concentrations were higher and those of IL-6 lower in the smokers than in the non-smokers. Maqui extract significantly decreased H2O2 (p < 0.0002) and increased IL-6 (p < 0.004) in the EBC from smokers. The EBC concentrations of H2O2 and IL-6 after maqui administration did not differ between smokers and non-smokers.
Conclusions
Maqui extract normalizes IL-6 and H2O2 concentrations in EBC from humans with mild smoking habits. If confirmed, these results suggest that dietary polyphenols might be considered as an interesting alternative for the dietary management of respiratory disorders.
Keywords: Smokers, H2O2, IL-6, Exhaled breath condensate, Aristotelia chilensis, Anthocyanins, Respiratory diseases, Oxidative stress, Inflammation
Findings
Respiratory diseases including pneumonia, tuberculosis, asthma, cystic fibrosis, emphysema and chronic obstructive pulmonary disease are associated with oxidative and inflammatory processes generated by microorganisms, air pollutants or tobacco smoke. Epidemiological studies suggest that a high intake of fruits and vegetables exerts health-promoting effects on lung injury and function [1], probably due to the abundance of vitamins and polyphenols with antioxidant, anti-inflammatory or mitochondrial regulatory properties in these foodstuffs [2]. This is also supported by the fact that deficiencies in antioxidant vitamins are frequently described in patients suffering respiratory diseases while intervention studies indicate that vitamin supplementation might improve their health state [3,4]. On the other hand, flavonoid-rich diets are negatively associated with chronic cough and it has been shown that apple intake, as well as that of red wine, decreases the risk of asthma [5,6]. Though experimental studies indicate that polyphenol administration improves lung injury in animal models, no intervention studies about this matter were carried out in humans.
Maqui (Aristotelia chilensis) is an edible berry endemic from central and southern Chile. It exhibits one of the highest polyphenol content and antioxidant activity of all fruits including the most consumed berries, being particularly rich in the anthocyanins delphinidin and cyanidin [7-9]. Maqui has been traditionally used in the Chilean folk medicine for its antidiarrheic, anti-inflammatory and antipyretic properties; extracts of this berry are actually commercialized as nutraceuticals, mainly based on their high antioxidant activity. More recently, maqui polyphenols were shown to protect mice against ischemia–reperfusion-induced heart damage [10], to inhibit adipogenesis and inflammation in vitro [11] and to prevent LDL oxidation [12]. Maqui also improved hyperglycemia and insulin resistance in obese mice fed a high fat diet, probably through the modulation of glucose metabolism in the skeletal muscle and liver [13].
Based on these findings, we hypothesize that the intake of maqui extract improves the concentrations of hydrogen peroxide (H2O2) and IL-6 in the exhaled breath condensate (EBC) from asymptomatic smokers. We selected H2O2 and IL-6 as biological markers due to the fact that these have been widely used in many studies to evaluate the oxidative and immune changes occurring in smoker subjects and patient with lung diseases. On the other hand, the rational to carry out the study in asymptomatic smokers rather than in symptomatic heavy smokers is that it was more probable to detect a protective effect of the maqui extract in subjects with mild alteration of their pulmonary oxidative/inflammatory status.
Study design
The study was approved by the Ethics Committee of the Faculty of Medicine, University of Chile; each volunteer signed an informed consent before its inclusion in the protocol. Exclusion criteria included pregnancy, acute or chronic digestive or respiratory pathologies, type-2 diabetes, autoimmune or allergic diseases, acute or chronic intake of drugs or vitamin supplements. This was an exploratory, open and uncontrolled study carried out in 15 asymptomatic smokers who exhibited a low intake of fruits and vegetables (<200 g/d, i.e. lower than half of the WHO recommendations) and a moderate cigarette consumption (mean [CI95%]) (smoking habits: 8 cigarettes/day [7 - 15]; smoking history: 6 y [5 - 13 y]; number of pack-year: 3 [2.4 - 7.7]). The volunteers had to ingest 2 g of a maqui extract (a gift of Nativ for Life, Santiago, Chile) twice daily for two weeks. The extract displayed a total polyphenol content of 5.18 ± 2.00 g of Gallic Acid Equivalents (GAE)/100 g and a FRAP (Ferric Reducing Antioxidant Power) value of 27.1 ± 2.0 mmol Fe++/100 g, as determined in our laboratory [14,15]. The nutritional composition of the extract per 2 g-serving was: energy: 5 kcal; protein: 0.1 g; fat: 0.2 g; carbohydrate: 0.6 g; total fiber: 1 g and sodium: 0.4 mg. During this two-week period, digestive symptoms and stool frequency and consistency were daily registered by the volunteers using an ad hoc form and the Bristol Scale for stool evaluation. The subjects continued smoking cigarettes during the treatment period. The samples of EBC were collected before and after the administration of the maqui extract in each of the participants. For comparison, a single sample of EBC was also obtained from each one of 8 non-smokers healthy subjects; these subjects did not receive any maqui extract.
Determination of H2O2 and IL-6 in exhaled breath condensate
The collection of EBC samples was carried out as described elsewhere [16]. Subjects had to breathe for 10 min at a normal frequency and tidal volume in an EcoScreen apparatus (Jaeger, Wurzburg, Germany) equipped with a mouthpiece and a two-way non re-breathing valve also serving as a saliva trap. The condensates (~ 1 mL) were maintained frozen until use. The concentrations of H2O2 in EBC was measured in a 96-wells microplate by using Amplex® Red reagent (Life Technologies, Carlsbad, CA, USA). Amplex Red, in the presence of peroxidase, reacts with H2O2 in a 1:1 stoichiometry to produce resorufin, a red fluorescent compound with an absorption/emission maxima of 570/585 nm. Fluorescence was determined in a Synergy HT™ multi-detection Microplate reader (BioTek Instruments, USA); the limit of detection of the method was 4 nM (i.e. 200 fmoles/well). IL-6 concentrations were determined using a commercial human IL-6 Elisa kit (Pierce, USA, respectively); the sensibility of the assay was <1 pg/ml. The coefficients of variation intra- and inter-assay for these determinations was <15%.
Statistical methods
Results were presented as means [CI95%]. Normality of the variables was determined by the Shapiro-Wilk test. Changes in H2O2 and IL-6 values after treatment in the smokers were analyzed by paired Student's t-test. As the variances of both groups were not homogenous, H2O2 and IL-6 values between groups were compared by using the non-parametric Mann-Whitney U test.
Results
The characteristics of both groups of subjects are described in Table 1. Before initiating maqui administration, the EBC concentration of IL-6 in smokers tended to be lower than those detected in the non-smokers (3.64 pg/ml [2.59 – 4.69] vs. 5.71 pg/ml [3.59 – 7.82], respectively; p = 0.057) and that of H2O2 higher (88.6 nM [67.6 – 109.5] vs. 52.5 nM [24.3 – 80.7], respectively; p = 0.059) (Figure 1). Although various studies reported higher pulmonary concentrations of IL-6 in smokers, they are generally carried out in older subjects, with a more elevated consumption of cigarettes and a longer smoking history, than the subjects who participated in our study [14]. Our results confirm those from McCrea et al. [17] who described that the IL-6 concentrations in bronchoalveolar lavage fluid (BALF) are lower in healthy smokers than in nonsmokers, and that BALF macrophages isolated from these smokers released less IL-6 when stimulated with LPS. Similar results have been reported in a number of studies that described a lower release of bioactive IL-6 and other pro-inflammatory cytokines from alveolar macrophages in smokers than in nonsmokers [18-22]. This immune alteration might explain the delayed rate of bacterial clearance in mice exposed to cigarette smoke and posteriorly infected with P. aeruginosa [23] and the higher susceptibility to pulmonary infections in smokers. Various components of tobacco including acrolein, nicotine, tar, hydroquinone and catechol have been shown to inhibit the immune response [24]. In addition, it has also been suggested that exposure of alveolar macrophages to tobacco smoke results in a hyporesponsive state similar to endotoxin tolerance, due to the inhibition of the TLR2/4-induced expression of pro-inflammatory cytokines and the impaired activation of IRAK-1, p38, and NF-ķB [25]. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone, a component of tobacco smoke has also been shown to decrease IL-6 from bronchial and alveolar epithelial cells [26].
Table 1.
Characteristics of the control (non-smoker) and smoker groups
| Non-smokers (n = 8) | Smokers (n = 15) | |
|---|---|---|
| Female (%) | 62.5 | 40 |
| Age (y) | 22.0 ± 1.1 | 26.4 ± 8.6 |
| BMI (kg/m2) | 23.1 ± 3.4 | 25.0 ± 1.8 |
Figure 1.
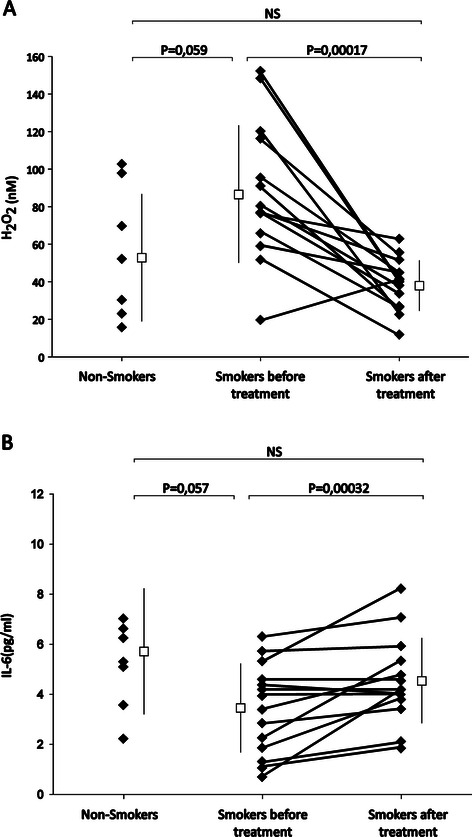
Concentrations of H2O2(A) and IL-6(B) in exhaled breath condensates from non-smoker controls subjects and smokers before and after the two-week administration of maqui extract. The squares and vertical bars indicate the means and CI95%.
Our results also confirm previous studies indicating higher H2O2 levels in EBC from smokers [27]. The smoker subjects consumed the maqui extract daily for two weeks. The treatment was well tolerated by the subjects and no side-effect, including digestive symptoms, were reported during the study. At the end of the treatment period, a significant decrease (by 57.3%) of H2O2 concentrations (37.8 nM [30.0 – 45.8]; p = 0.0002) and a significant increase of IL-6 concentrations (by 30.8%) (4.76 pg/ml [3.72 - 5.79]; p = 0.0032) were observed. Such changes in H2O2 and IL-6 were detected in 92.8% and 80% of the smokers, respectively; they were inversely correlated (R = 0.59; p = 0.027). At the end of the period of maqui administration, the EBC concentrations of these molecules in the smoker subjects did not differ from these detected in the non-smokers. Some studies have described the ability of polyphenols to act as immunostimulant and more specifically to increase IL-6 expression. Sage phenolic compounds, for example, increased IL-6 and TNF release by the macrophage-like cells RAW264.7 infected by Leishmania [28] while grape seed extract increased IL-6 expression and secretion in astrocytes, improving their resistance against H2O2-induced oxidative damage [29]. To our knowledge, our results show for the first time that the intake of a polyphenol-rich extract exerts beneficial effects in the respiratory tract of humans, normalizing the concentrations of IL-6 and H2O2 EBC in asymptomatic smokers with moderate cigarette smoking. Such protective effects have been described in animal models of respiratory diseases with different dietary polyphenols. For example, resveratrol was shown to improve lung injury induced by staphylococcal enterotoxin-B in mice [30] while the administration of a walnut extract restored the levels of glutathione reductase and catalase and reduced the xanthine oxidase activity in lung tissues of rats treated with cigarette smoke extract [31]. Another widely used dietary polyphenol, curcumin, was shown to attenuate the pulmonary inflammation and emphysema induced by intratracheal porcine pancreatic elastase or cigarette smoke in mice [32]. Polyphenols from red wine and red fruits were also shown to cause bronchodilation and to suppress airway inflammation in an animal model of asthma induced by ovalbumin sensitization [33]. Berry anthocyanins have been shown to be absorbed through the intestinal mucosa in in vitro system (Caco-2 cells) and in human volunteers [34,35], therefore making possible that these molecules may reach the respiratory tract and exert their antioxidant and anti-inflammatory activities. This was recently supported by the observations from Aquil et al. who detected the presence of different anthocyanins in the lung tissue from blueberry-fed mice [36].
Conclusions
These preliminary results suggest that the intake of a dietary polyphenol-rich maqui extract increases the EBC concentrations of IL-6 and decrease those of H2O2 in humans with mild smoking habits. Such changes could be beneficial for the subject as it could improve their resistance to respiratory infections while lowering oxidative stress in their lungs. These results support the realization of randomized, double-blind, placebo-controlled clinical trials in higher numbers of subjects, smokers or patients with respiratory diseases, to confirm such effect. If so, dietary polyphenols might be considered as an interesting alternative for the dietary management of these pathologies.
Acknowledgments
We would like to thank all the volunteers who participated in the study and Prof. Oscar Brunser for critically revising the manuscript. Finally we thank Nativ for Life for providing the maqui extract.
Abbreviations
- CI95%
Confidence Interval 95%
- EBC
Exhaled breath condensate
- H2O2
Hydrogen peroxide
- IL-6
Interleukin-6
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MG was the Principal Investigator and contributed to the study design, data analysis and the writing of the manuscript. DV, DA and AS contributed to the recruitment of the volunteers, collection of the EBC and data analysis. DV, DA and EE carried out the determination of H2O2 and IL-6 en the EBC samples. CC analyzed the polyphenol content and antioxidant activity of the maqui extract and participated to the data analysis and manuscript writing. AS contributed to study design, the recruitment and follow up of the subjects and manuscript preparation. All authors read and approved the manuscript.
Contributor Information
Daniela Vergara, Email: dani.vergara.h@gmail.com.
Daniela Ávila, Email: davila1990@hotmail.com.
Elizabeth Escobar, Email: mari.elizabeth.escobar.sagredo@gmail.com.
Catalina Carrasco-Pozo, Email: catalinacarrasco@med.uchile.cl.
Andrés Sánchez, Email: andres.sanchez.cordoba@gmail.com.
Martin Gotteland, Email: mgottela@med.uchile.cl.
References
- 1.Romieu I. Nutrition and lung health. Int J Tuberc Lung Dis. 2005;9:362–74. [PubMed] [Google Scholar]
- 2.Carrasco Pozo C1, Mizgier ML, Speisky H, Gotteland M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem Biol Interact. 2012;195:199–205. doi: 10.1016/j.cbi.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Thornton KA, Mora-Plazas M, Marín C, Villamor E. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. J Nutr. 2014;144:496–503. doi: 10.3945/jn.113.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen K, Chen XR, Zhang L, Luo HY, Gao N, Wang J, et al. Effect of simultaneous supplementation of vitamin A and iron on diarrheal and respiratory tract infection in preschool children in Chengdu City, China. Nutrition. 2013;29:1197–203. doi: 10.1016/j.nut.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen SO, Sterne JA, Thompson RL, Songhurst CE, Margetts BM, Burney PG. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med. 2001;164:1823–8. doi: 10.1164/ajrccm.164.10.2104061. [DOI] [PubMed] [Google Scholar]
- 6.Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN Study. Am J Respir Crit Care Med. 2001;164:61–4. doi: 10.1164/ajrccm.164.1.2010025. [DOI] [PubMed] [Google Scholar]
- 7.Fredes C, Yousef GG, Robert P, Grace MH, Lila MA, Gómez M, et al. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J Sci Food Agric. 2014;94:2639–48. doi: 10.1002/jsfa.6602. [DOI] [PubMed] [Google Scholar]
- 8.Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010; 2010:bap024. doi:10.1093/database/bap024. [DOI] [PMC free article] [PubMed]
- 9.Phenol Explorer – Database on polyphenol content in foods. http://phenol-explorer.eu/.
- 10.Cespedes C, El-Hafidi M, Pavon N, Alarcon J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008;107:820–9. doi: 10.1016/j.foodchem.2007.08.092. [DOI] [Google Scholar]
- 11.Schreckinger ME, Wang J, Yousef G, Lila MA, Gonzalez De Mejia E. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J Agric Food Chem. 2010;58:8966–76. doi: 10.1021/jf100975m. [DOI] [PubMed] [Google Scholar]
- 12.Miranda-Rottmann S, Aspillaga AA, Pérez DD, Vasquez L, Martinez AL, Leighton F. Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J Agric Food Chem. 2002;50:7542–7. doi: 10.1021/jf025797n. [DOI] [PubMed] [Google Scholar]
- 13.Rojo LE, Ribnicky L, Poulev A, Rojas-Silva P, Kuhn P, Dorn R, et al. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis) Food Chem. 2012;131:387–96. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 15.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenol and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–78. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 16.Carpagnano GE, Kharitonov SA, Foschino-Barbaro MP, Resta O, Gramiccioni E, Barnes PJ. Increased inflammatory markers in the exhaled breath condensate of cigarette smokers. Eur Respir J. 2003;21:589–93. doi: 10.1183/09031936.03.00022203. [DOI] [PubMed] [Google Scholar]
- 17.McCrea KA, Ensor JE, Nall K, Bleecker ER, Hasday JD. Altered cytokine regulation in the lungs of cigarette smokers. Am J Respir Crit Care Med. 1994;150:696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 18.Sauty A, Mauel J, Philippeaux MM, Leuenberger P. Cytostatic activity of alveolar macrophages from smokers and nonsmokers: role of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha. Am J Respir Cell Mol Biol. 1994;11:631–7. doi: 10.1165/ajrcmb.11.5.7946392. [DOI] [PubMed] [Google Scholar]
- 19.Dandrea T, Tu B, Blomberg A, Sandström T, Sköld M, Eklund A, et al. Differential inhibition of inflammatory cytokine release from cultured alveolar macrophages from smokers and non-smokers by NO2. Hum Exp Toxicol. 1997;16:577–88. doi: 10.1177/096032719701601005. [DOI] [PubMed] [Google Scholar]
- 20.Mikuniya T, Nagai S, Tsutsumi T, Morita K, Mio T, Satake N, et al. Proinflammatory or regulatory cytokines released from BALF macrophages of healthy smokers. Respiration. 1999;66:419–26. doi: 10.1159/000029425. [DOI] [PubMed] [Google Scholar]
- 21.Dubar V, Gosset P, Aerts C, Voisin C, Wallaert B, Tonnel AB. In vitro acute effects of tobacco smoke on tumor necrosis factor alpha and interleukin-6 production by alveolar macrophages. Exp Lung Res. 1993;19:345–59. doi: 10.3109/01902149309064351. [DOI] [PubMed] [Google Scholar]
- 22.Soliman DM, Twigg HL., 3rd Cigarette smoking decreases bioactive interleukin-6 secretion by alveolar macrophages. Am J Physiol. 1992;263:L471–8. doi: 10.1152/ajplung.1992.263.4.L471. [DOI] [PubMed] [Google Scholar]
- 23.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stämpfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2004;170:1164–71. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]
- 24.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–84. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Cowan MJ, Hasday JD, Vogel SN, Medvedev AE. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol. 2007;179:6097–106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- 26.Proulx LI, Gaudreault M, Turmel V, Augusto LA, Castonguay A, Bissonnette EY. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone, a component of tobacco smoke, modulates mediator release from human bronchial and alveolar epithelial cells. Clin Exp Immunol. 2005;140:46–53. doi: 10.1111/j.1365-2249.2005.02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak D, Antczak A, Krol M, Pietras T, Shariati B, Bialasiewicz P, et al. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. Eur Respir J. 1996;9:652–7. doi: 10.1183/09031936.96.09040652. [DOI] [PubMed] [Google Scholar]
- 28.Radtke OA, Foo LY, Lu Y, Kiderlen AF, Kolodziej H. Evaluation of sage phenolics for their antileishmanial activity and modulatory effects on interleukin-6, interferon and tumour necrosis factor-alpha-release in RAW 264.7 cells. Z Naturforsch C. 2003;58:395–400. doi: 10.1515/znc-2003-5-618. [DOI] [PubMed] [Google Scholar]
- 29.Fujishita K, Ozawa T, Shibata K, Tanabe S, Sato Y, Hisamoto M, et al. Grape seed extract acting on astrocytes reveals neuronal protection against oxidative stress via interleukin-6-mediated mechanisms. Cell Mol Neurobiol. 2009;29:1121–9. doi: 10.1007/s10571-009-9403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amcaoglu Rieder S, Nagarkatti P, Nagarkatti M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. Brit J Pharmacol. 2012;167:1244–58. doi: 10.1111/j.1476-5381.2012.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qamar W, Sultana S. Polyphenols from Juglans regia L. (walnut) kernel modulate cigarette smoke extract induced acute inflammation, oxidative stress and lung injury in Wistar rats. Hum Exp Toxicol. 2011;30:499–506. doi: 10.1177/0960327110374204. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Odajima N, Moriyama C, et al. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L614–23. doi: 10.1152/ajplung.90443.2008. [DOI] [PubMed] [Google Scholar]
- 33.Joskova M, Sadlonova V, Nosalova G, Novakova E, Franova S. Polyphenols and their components in experimental allergic asthma. Adv Exp Med Biol. 2013;756:91–8. doi: 10.1007/978-94-007-4549-0_12. [DOI] [PubMed] [Google Scholar]
- 34.Yi W, Akoh CC, Fischer J, Krewer G. Absorption of anthocyanins from blueberry extracts by Caco-2 human intestinal cell monolayers. J Agric Food Chem. 2006;54:5651–8. doi: 10.1021/jf0531959. [DOI] [PubMed] [Google Scholar]
- 35.Milbury PE, Vita JA, Blumberg JB. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr. 2010;140:1099–104. doi: 10.3945/jn.109.117168. [DOI] [PubMed] [Google Scholar]
- 36.Aqil F, Vadhanam MV, Jeyabalan J, Cai J, Singh IP, Gupta RC. Detection of anthocyanins/anthocyanidins in animal tissues. J Agric Food Chem. 2014;62:3912–5. doi: 10.1021/jf500467b. [DOI] [PMC free article] [PubMed] [Google Scholar]


