Abstract
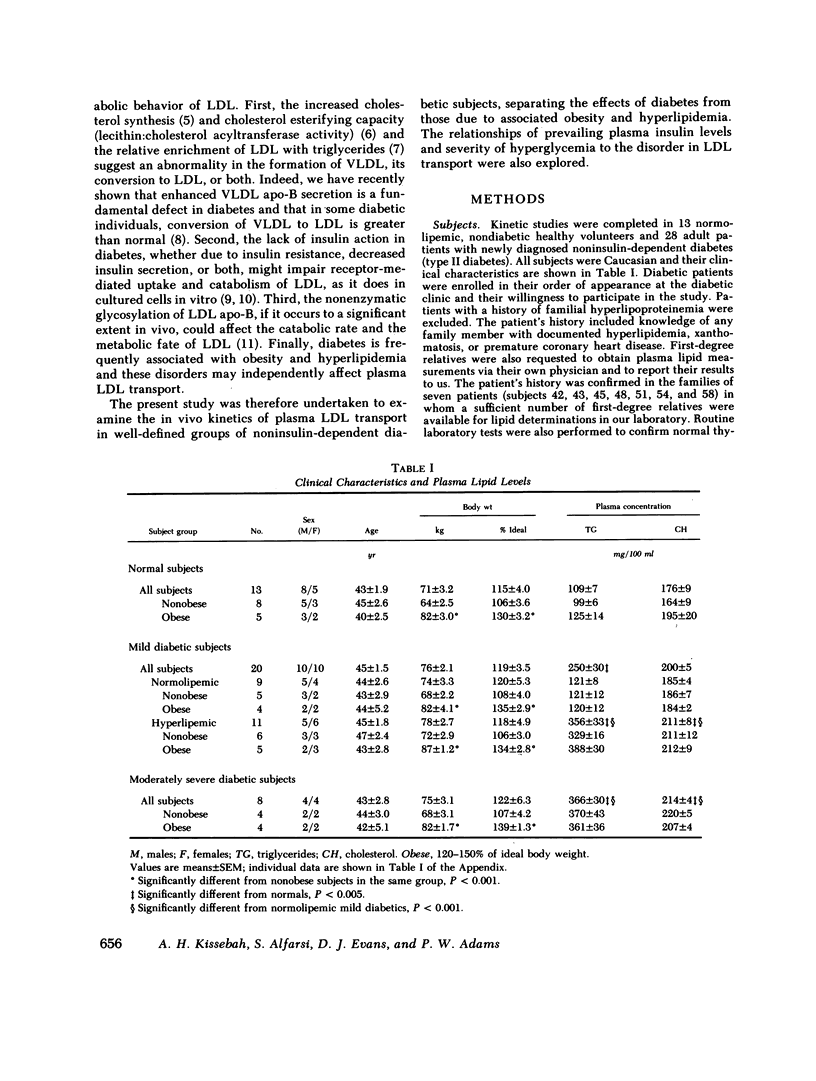
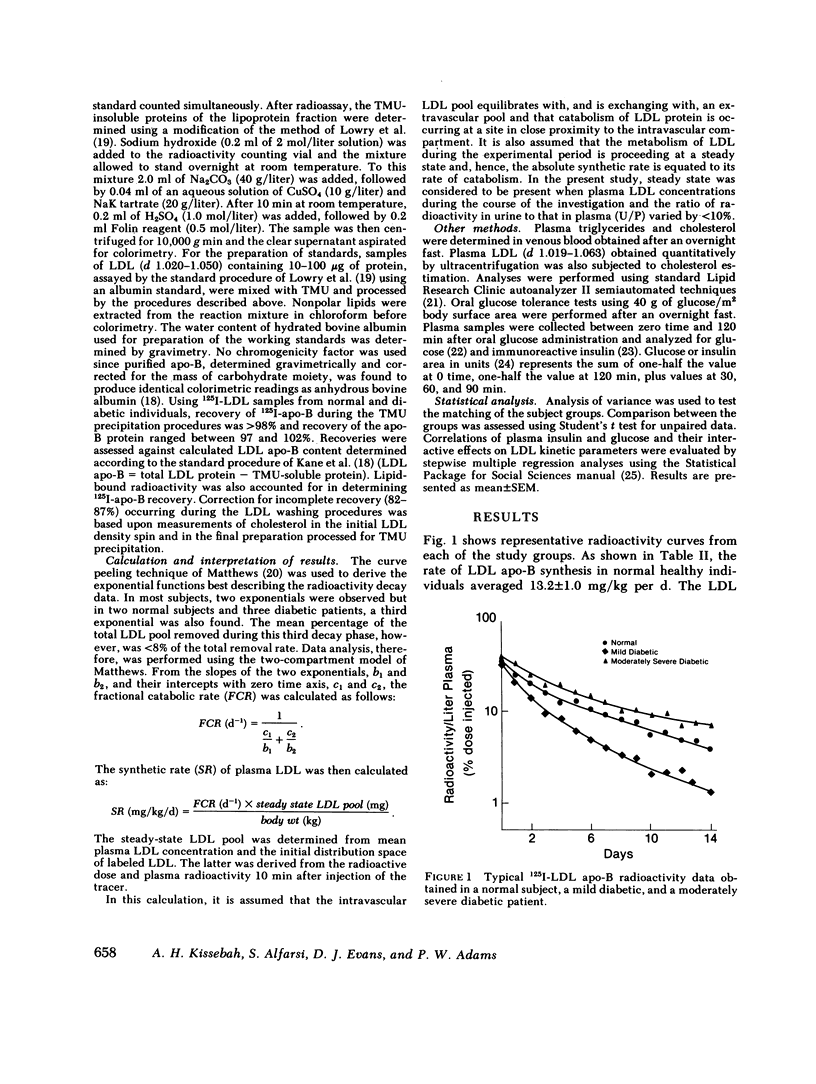
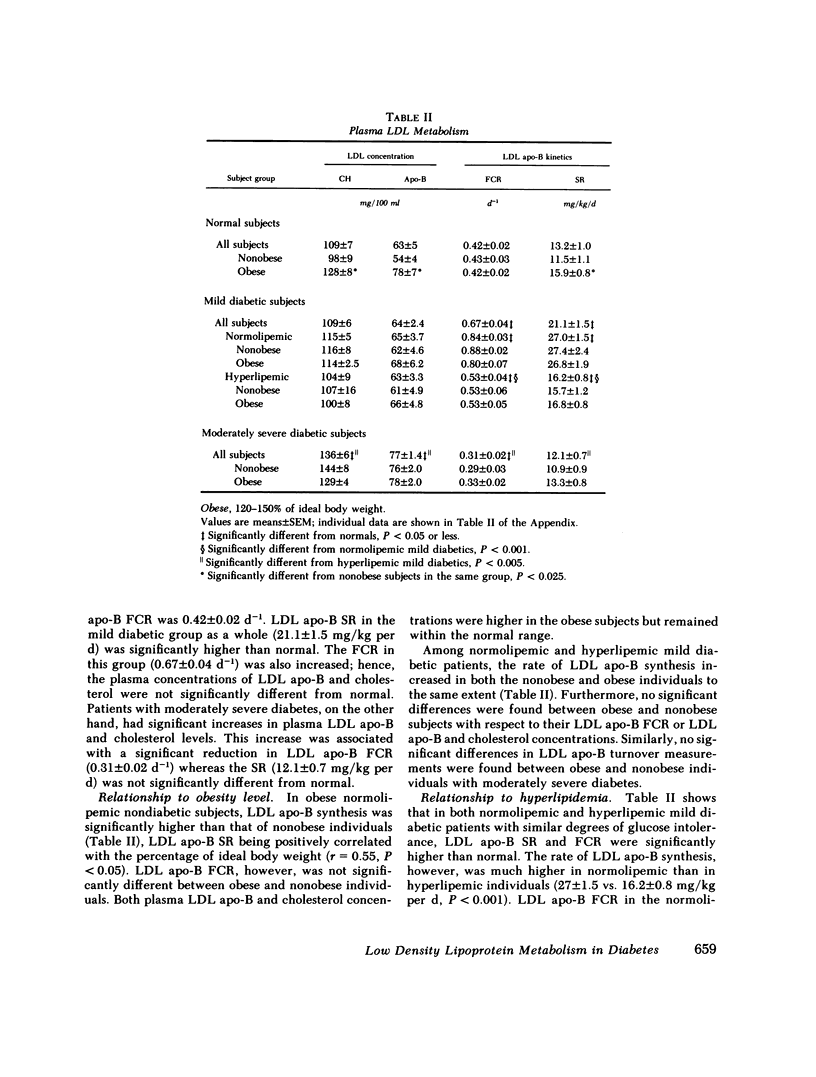
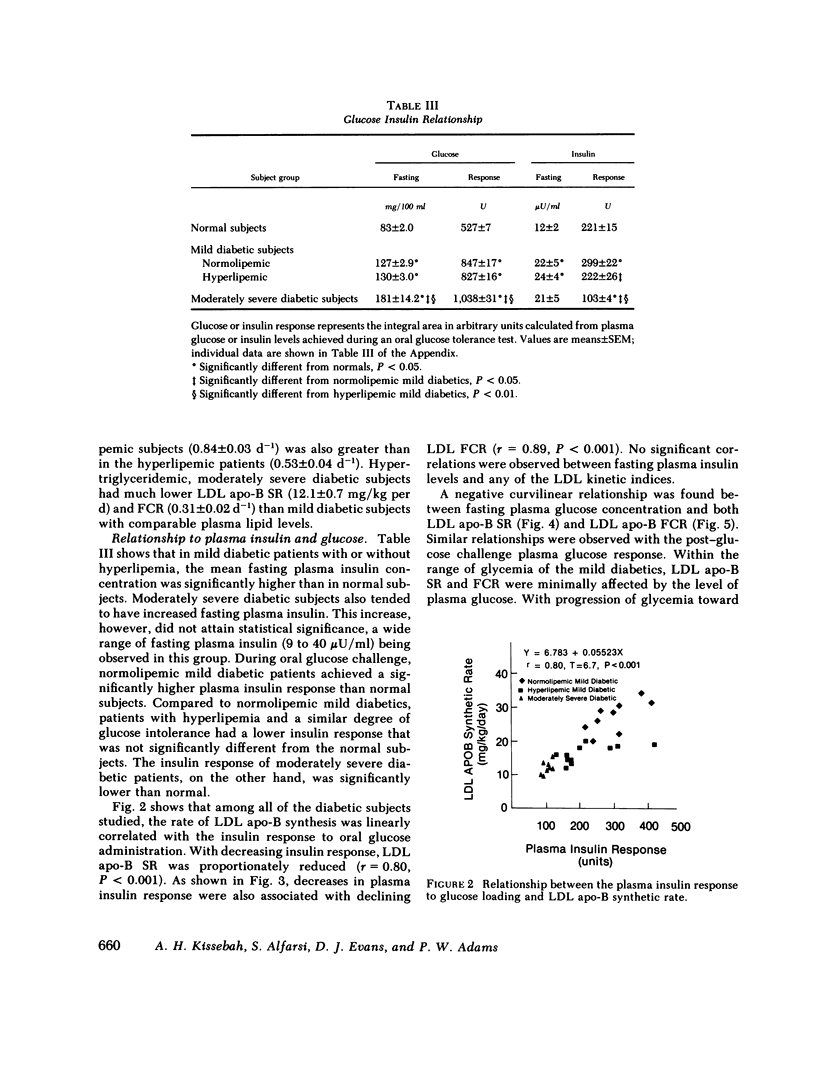
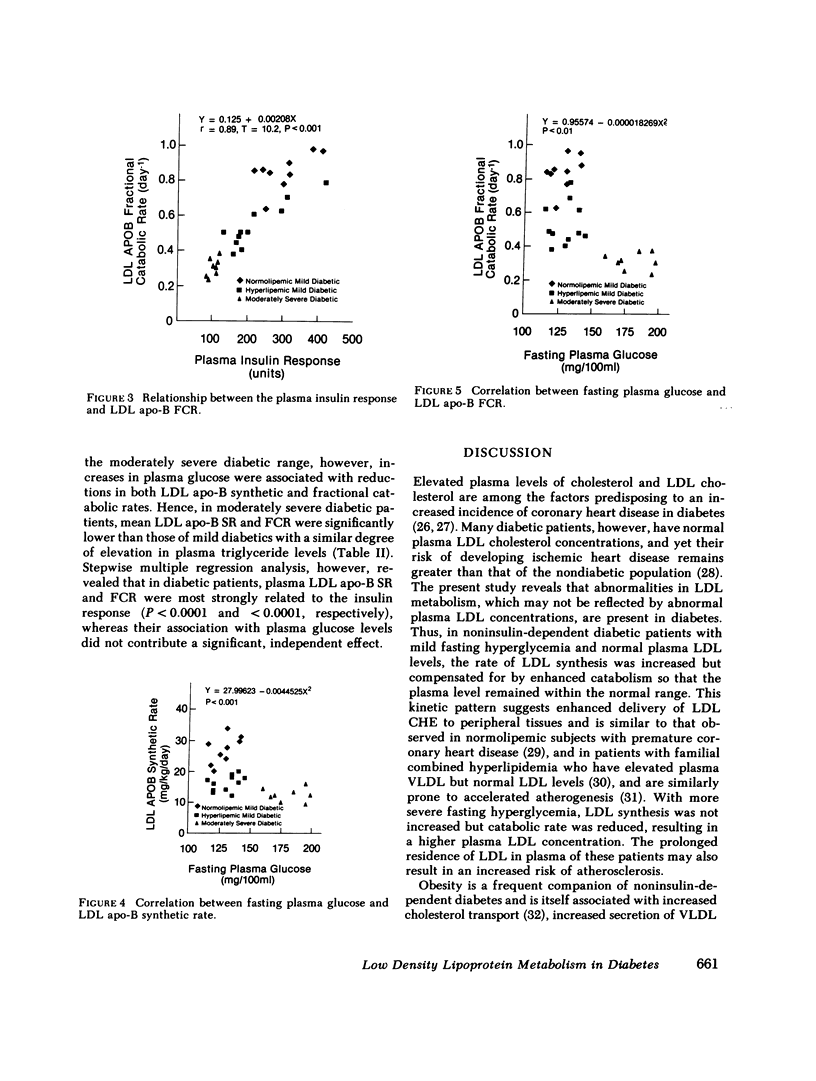
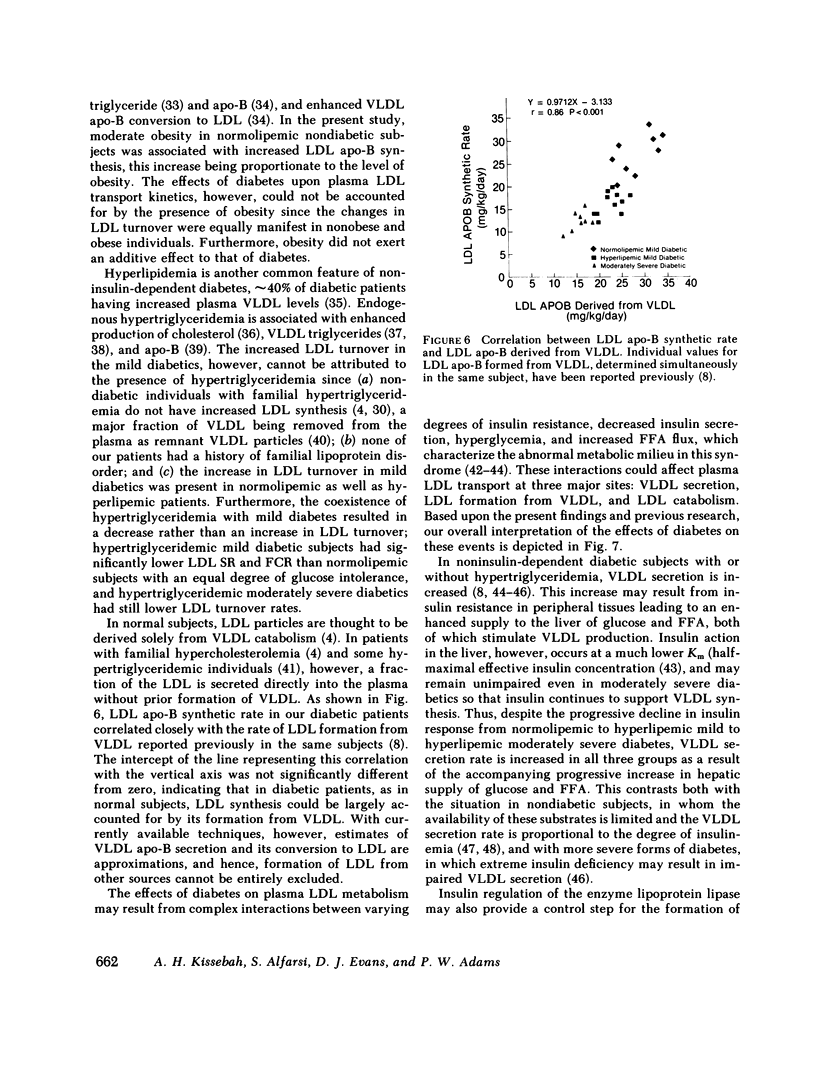
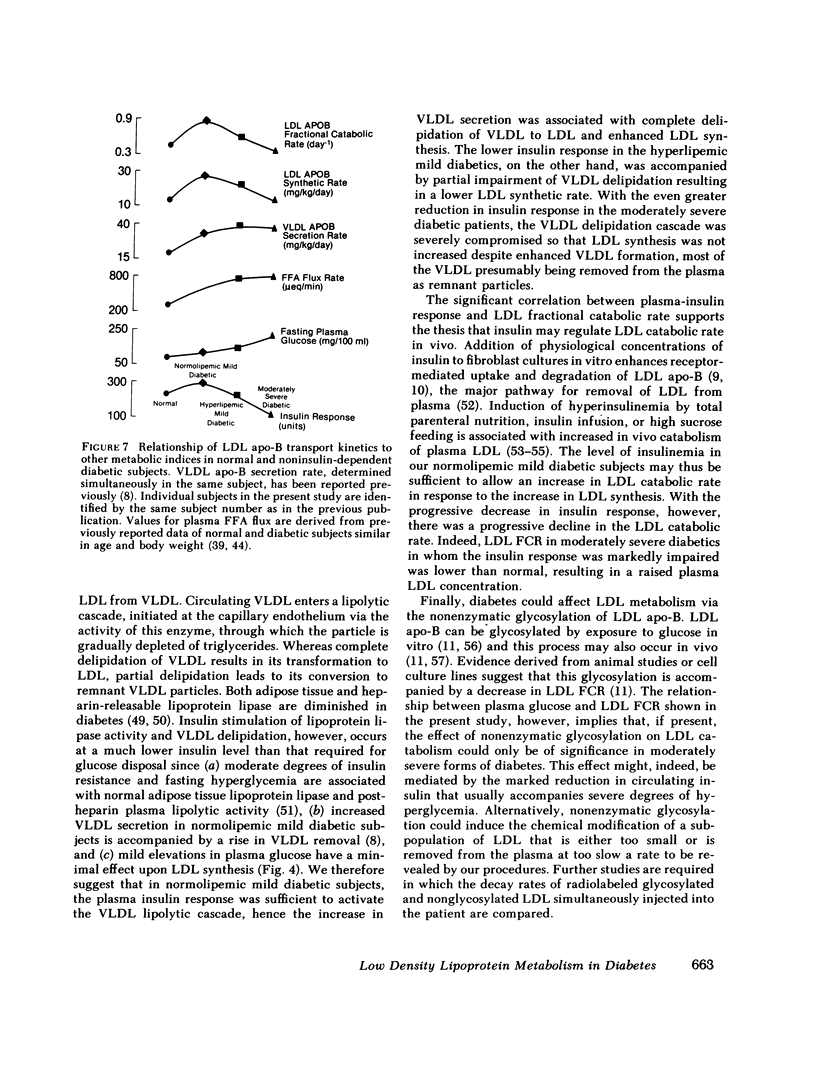
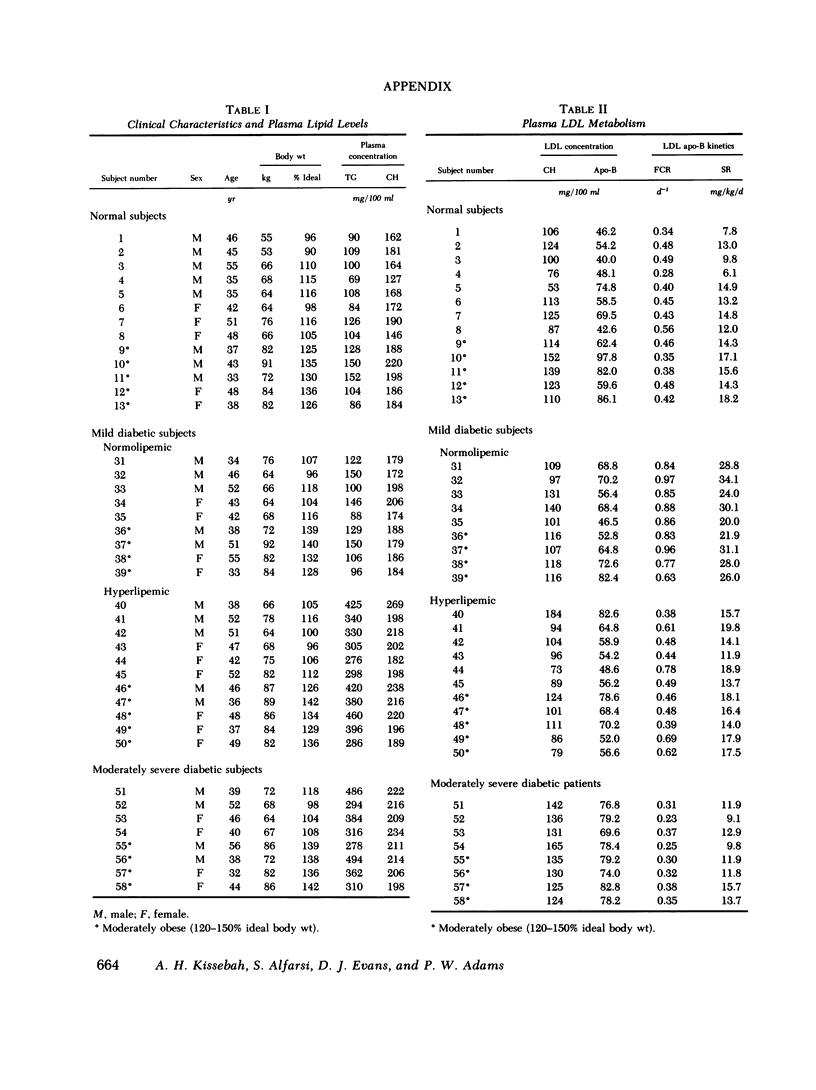
Plasma low density lipoprotein (LDL) transport kinetics were determined from the disappearance of 125I-LDL injected into age- and weight-matched groups of 13 normal subjects, 20 mild diabetics, and 8 moderately severe diabetic patients (fasting plasma glucose less than 150 and greater than 150 mg/100 ml, respectively). In mild diabetics, LDL apo-lipoprotein-B (apo-B) synthetic rate (SR) was significantly greater than normal. The fractional catabolic rate (FCR), however, was also increased so that plasma LDL concentration remained normal. In moderately severe diabetics, LDL SR was normal but FCR was reduced resulting in increased plasma LDL cholesterol and apo-B concentrations. In normal subjects, moderate obesity was associated with increased LDL secretion. In diabetic subjects, however, changes in LDL turnover were of equal magnitude in obese and nonobese patients. In normolipemic and hyperlipemic mild diabetic subjects with equal degrees of glucose intolerance, both LDL apo-B SR and FCR were greater than normal. The magnitude of these increases, however, was lower in the hyperlipemic individuals. Stepwise regression analysis revealed that both LDL SR and FCR correlated positively and linearly with insulin response to glucose loading, but negatively and curvilinearly with fasting plasma glucose and glucose response. We propose that in noninsulin-dependent diabetes, mild hyperglycemia is accompanied by increased LDL turnover, despite normal plasma LDL levels, whereas moderately severe hyperglycemia is associated with decreased LDL catabolism, resulting in increased plasma LDL levels. These changes cannot be attributed to the presence of obesity or hypertriglyceridemia, and may relate to varying degrees of insulin resistance and decreased insulin secretion affecting plasma very low density lipoprotein (VLDL) secretion, VLDL conversion to LDL, and LDL catabolism. Both increased LDL turnover in mild diabetes and delayed removal of LDL in moderately severe diabetes could increase cholesterol ester availability to peripheral tissues, and may result in an increased risk of atherosclerosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. W., Kissebah A. H., Harrigan P., Stokes T., Wynn V. The kinetics of plasma free fatty acid and triglyceride transport in patients with idiopathic hypertriglyceridaemia and their relation to carbohydrate metabolism. Eur J Clin Invest. 1974 Jun;4(3):149–161. doi: 10.1111/j.1365-2362.1974.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Angelin B., Einarsson K., Hellström K., Leijd B. Bile acid kinetics in relation to endogenous tryglyceride metabolism in various types of hyperlipoproteinemia. J Lipid Res. 1978 Nov;19(8):1004–1016. [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med. 1977 Jun 16;296(24):1365–1371. doi: 10.1056/NEJM197706162962401. [DOI] [PubMed] [Google Scholar]
- Brunzell J. D., Schrott H. G., Motulsky A. G., Bierman E. L. Myocardial infarction in the familial forms of hypertriglyceridemia. Metabolism. 1976 Mar;25(3):313–320. doi: 10.1016/0026-0495(76)90089-5. [DOI] [PubMed] [Google Scholar]
- Chait A., Bierman E. L., Albers J. J. Low-density lipoprotein receptor activity in cultured human skin fibroblasts. Mechanism of insulin-induced stimulation. J Clin Invest. 1979 Nov;64(5):1309–1319. doi: 10.1172/JCI109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A., Bierman E. L., Albers J. J. Regulatory role of insulin in the degradation of low density lipoprotein by cultured human skin fibroblasts. Biochim Biophys Acta. 1978 May 25;529(2):292–299. doi: 10.1016/0005-2760(78)90072-3. [DOI] [PubMed] [Google Scholar]
- Chait A., Foster D., Miller D. G., Bierman E. L. Acceleration of low-density lipoprotein catabolism in man by total parenteral nutrition. Proc Soc Exp Biol Med. 1981 Oct;168(1):97–104. doi: 10.3181/00379727-168-41240. [DOI] [PubMed] [Google Scholar]
- Cramp D. G. New automated method for measuring glucose by glucose oxidase. J Clin Pathol. 1967 Nov;20(6):910–912. doi: 10.1136/jcp.20.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidge N. H., Poulis P. Metabolic heterogeneity in the formation of low density lipoprotein from very low density lipoprotein in the rat: evidence for the independent production of a low density lipoprotein subfraction. J Lipid Res. 1978 Mar;19(3):342–349. [PubMed] [Google Scholar]
- Garcia M. J., McNamara P. M., Gordon T., Kannel W. B. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974 Feb;23(2):105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- Greenfield M., Kolterman O., Olefsky J., Reaven G. M. Mechanism of hypertriglyceridaemia in diabetic patients with fasting hyperglycaemia. Diabetologia. 1980 Jun;18(6):441–446. doi: 10.1007/BF00261698. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Mok H. Y., Zech L., Steinberg D., Berman M. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest. 1979 Jun;63(6):1274–1283. doi: 10.1172/JCI109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Janus E. D., Nicoll A., Wootton R., Turner P. R., Magill P. J., Lewis B. Quantitative studies of very low density lipoprotein: conversion to low density lipoprotein in normal controls and primary hyperlipidaemic states and the role of direct secretion of low density lipoprotein in heterozygous familial hypercholesterolaemia. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):149–159. doi: 10.1111/j.1365-2362.1980.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Sata T., Hamilton R. L., Havel R. J. Apoprotein composition of very low density lipoproteins of human serum. J Clin Invest. 1975 Dec;56(6):1622–1634. doi: 10.1172/JCI108245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Kurup I. V. Nonenzymatic glycosylation of human plasma low density lipoprotein. Evidence for in vitro and in vivo glucosylation. Metabolism. 1982 Apr;31(4):348–353. doi: 10.1016/0026-0495(82)90109-3. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Adams P. W., Wynn V. Inter-relationship between insulin secretion and plasma free fatty acid and triglyceride transport kinetics in maturity onset diabetes and the effect of phenethylbiguanide (phenformin). Diabetologia. 1974 Apr;10(2):119–130. doi: 10.1007/BF01219667. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Adams P. W. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in man: normolipemic subjects, familial hypertriglyceridemia and familial combined hyperlipidemia. Metabolism. 1981 Sep;30(9):856–868. doi: 10.1016/0026-0495(81)90064-0. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Adams P. W., Wynn V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia. 1976 Dec;12(6):563–571. doi: 10.1007/BF01220632. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Evans D. J., Adams P. W. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in non-insulin-dependent diabetes mellitus. Diabetes. 1982 Mar;31(3):217–225. doi: 10.2337/diab.31.3.217. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. I., Lees R. S., Fredrickson D. S. The nature of pre beta (very low density) lipoproteins. J Clin Invest. 1966 Jan;45(1):63–77. doi: 10.1172/JCI105324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B., Chait A., Wootton I. D., Oakley C. M., Krikler D. M., Sigurdsson G., February A., Maurer B., Birkhead J. Frequency of risk factors for ischaemic heart-disease in a healthy British population. With particular reference to serum-lipoprotein levels. Lancet. 1974 Feb 2;1(7849):141–146. doi: 10.1016/s0140-6736(74)92438-6. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mancini M., Rivellese A., Rubba P., Riccardi G. Plasma lipoproteins in maturity onset diabetes. Nutr Metab. 1980;24 (Suppl 1):65–73. doi: 10.1159/000176372. [DOI] [PubMed] [Google Scholar]
- Mattock M. B., Fuller J. H., Maude P. S., Keen H. Lipoproteins and plasma cholesterol esterification in normal and diabetic subjects. Atherosclerosis. 1979 Dec;34(4):437–449. doi: 10.1016/0021-9150(79)90068-6. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Reardon M., Fidge N. H. Sucrose-induced changes in VLDL- and LDL-B apoprotein removal rates. Metabolism. 1979 May;28(5):531–535. doi: 10.1016/0026-0495(79)90193-8. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M. Plasma triglyceride transport kinetics in diabetes mellitus. Metabolism. 1973 Jan;22(1):1–22. doi: 10.1016/0026-0495(73)90024-3. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Farquhar J. W., Reaven G. M. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974 Oct;57(4):551–560. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Pykälistö O. J., Smith P. H., Brunzell J. D. Determinants of human adipose tissue lipoprotein lipase. Effect of diabetes and obesity on basal- and diet-induced activity. J Clin Invest. 1975 Nov;56(5):1108–1117. doi: 10.1172/JCI108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Hill D. B., Gross R. C., Farquhar J. W. Kinetics of triglyceride turnover of very low density lipoproteins of human plasma. J Clin Invest. 1965 Nov;44(11):1826–1833. doi: 10.1172/JCI105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckless J. P., Betteridge D. J., Wu P., Payne B., Galton D. J. High-density and low-density lipoproteins and prevalence of vascular disease in diabetes mellitus. Br Med J. 1978 Apr 8;1(6117):883–886. doi: 10.1136/bmj.1.6117.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen R. J., Willis P. W., 3rd, Fajans S. S. Atherosclerosis in diabetes mellitus. Correlations with serum lipid levels, adiposity, and serum insulin level. Arch Intern Med. 1972 Dec;130(6):833–843. doi: 10.1001/archinte.130.6.833. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Birge C., Miller J. P., Kessler G., Santiago J. Apolipoprotein B levels and altered lipoprotein composition in diabetes. Diabetes. 1974 Oct;23(10):827–834. doi: 10.2337/diab.23.10.827. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Bicker S., Lorimer A. R., Packard C. J. Receptor-mediated low density lipoprotein catabolism in man. J Lipid Res. 1979 Nov;20(8):999–1006. [PubMed] [Google Scholar]
- Tobey T. A., Greenfield M., Kraemer F., Reaven G. M. Relationship between insulin resistance, insulin secretion, very low density lipoprotein kinetics, and plasma triglyceride levels in normotriglyceridemic man. Metabolism. 1981 Feb;30(2):165–171. doi: 10.1016/0026-0495(81)90167-0. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Mahoney E. M., Branks M. J., Fisher M., Elam R., Steinberg D. Nonenzymatic glucosylation of low-density lipoprotein alters its biologic activity. Diabetes. 1982 Apr;31(4 Pt 1):283–291. doi: 10.2337/diab.31.4.283. [DOI] [PubMed] [Google Scholar]
- Wynn V., Doar J. W. Some effects of oral contraceptives on carbohydrate metabolism. Lancet. 1966 Oct 1;2(7466):715–719. doi: 10.1016/s0140-6736(66)92978-3. [DOI] [PubMed] [Google Scholar]


