Abstract
Biglycan accumulates in aortic valves affected by calcific aortic valve disease (CAVD), and soluble biglycan up-regulates BMP-2 expression in human aortic valve interstitial cells (AVICs) via Toll-like receptor (TLR) 2 and induces AVIC pro-osteogenic reprogramming, characterized by elevated pro-osteogenic activities. We sought to identify the factors responsible for biglycan-induced pro-osteogenic reprogramming in human AVICs. Treatment of AVICs with recombinant biglycan induced the secretion of BMP-2 and TGF-β1, but not BMP-4 or BMP-7. Biglycan up-regulated TGF-β1 expression in a TLR4-dependent fashion. Neutralization of BMP-2 or TGF-β1 attenuated the expression of ALP, osteopontin and Runx2 in cells exposed to biglycan. However, neutralization of both BMP-2 and TGF-β1 abolished the expression of these osteogenic biomarkers and calcium deposition. Phosphorylated Smad1 and Smad3 were detected in cells exposed to biglycan, and knockdown of Smad1 or Smad3 attenuated the effect of biglycan on the expression of osteogenic biomarkers. While BMP-2 and TGF-β1 each up-regulated the expression of osteogenic biomarkers, an exposure to BMP-2 plus TGF-β1 induced a greater up-regulation and results in calcium deposition. We conclude that concurrent up-regulation of BMP-2 and TGF-β1 is responsible for biglycan-induced pro-osteogenic reprogramming in human AVICs. The Smad 1/3 pathways are involved in the mechanism of AVIC pro-osteogenic reprogramming.
Keywords: BMP-2, TGF-β1, biglycan, aortic valve, osteogenic responses
Calcific aortic valve disease (CAVD) is the most common cardiovascular disorder in people of 65 years and older [1–3]. Currently, pharmacological interventions for slowing down the progression of this disease are unavailable.
Diseased aortic valve leaflets exhibit evidence of inflammation and osteogenic activity [1–4]. Valvular inflammatory and osteogenic responses appear to be the inciting events in the pathogenesis of CAVD. In addition, pro-inflammatory stimuli have been shown to promote the expression of osteogenic mediators by valvular cells in vitro [5–9]. While an interaction between the pro-inflammatory and pro-osteogenic mechanisms appear to be involve in the pathobiology of CAVD, it remains unclear what stimulus in the valvular tissue up-regulates the expression of pro-osteogenic mediators and what molecular mechanism mediates valvular osteogenic responses. Investigation of the mechanism underlying pro-osteogenic reprogramming of aortic valve cells will improve the understanding of the pathogenesis of CAVD and may lead to the development of pharmacological treatments for slowing down its progression.
Accumulating clinical and experimental studies demonstrate that the osteogenic responses of aortic valve interstitial cells (AVICs) play an important role in the development and progression of CAVD [1,10]. Expression of pro-osteogenic factors, including bone morphogenetic proteins (BMPs), by AVICs is one of the central events that are associated with the initiation and progression of the pathological changes in CAVD [11,12]. The mechanism that mediates AVIC expression of pro-osteogenic mediators remains unclear. Biglycan is a leucine-rich proteoglycan and is a component of the extracellular matrix. In the extracellular matrix, biglycan is present primarily in an insoluble form. Soluble biglycan can accumulate in tissue in response to stress and injury [13,14]. Soluble biglycan is an endogenous activator of Toll-like receptors (TLRs), particularly TLR2 and TLR4 [13,15]. Recent studies found that recombinant biglycan up-regulates the levels of phosphate transfer protein and BMP-2 in human AVICs in a TLR2-dependent fashion [14,16]. Importantly, biglycan accumulation is observed in aortic valves explanted from patients with CAVD [14,17]. Furthermore, we observed that human AVICs from diseased aortic valves express and release higher levels of biglycan and that prolonged stimulation of human AVICs with recombinant biglycan induces alkaline phosphatase (ALP) expression and calcium deposition in vitro [16]. As the expression of ALP and formation of calcium deposits are biomarkers of AVIC pro-osteogenic reprogramming [5,18], the effect of biglycan on these biomarkers indicate a potential role of soluble biglycan accumulation in CAVD progression. Currently, the factors that mediate biglycan-induced AVIC pro-osteogenic reprogramming are unknown although such reprogramming is associated with increased cellular BMP-2 levels [16].
TGF-β1 is a member of the BMP superfamily and is recognized as a potent pro-osteogenic factor [19,20]. TGF-β1 has been found to promote in vitro calcification in aortic smooth muscle cell [20] and in AVICs [21]. BMP-2 and TGF-β1 in tissue implants display synergistic effects on ectopic bone formation in vivo [22]. Several studies demonstrate that biglycan interacts with TGF-β1 and modulates its pro-osteogenic activity in osteoblasts and human umbilical vein endothelial cells [23,24]. Further, proteoglycan promotes TGF-β1 production in vascular smooth muscle cells [25]. It is possible that BMP-2 and TGF-β1 coordinately mediate AVIC pro-osteogenic reprogramming if TGF-β1 is also up-regulated by biglycan.
TGF-β1 and BMP-2 activate the Smad signaling pathways. BMP-2 primarily utilizes Smad1 while TGF-β1 utilizes Smad3 for signaling [26,27]. Phosphorylated Smad molecules translocate into the nucleus to up-regulate the expression of genes involved in osteoblast differentiation and vascular calcification [28,29]. Thus, phosphorylated Smad1 and Smad3 serve as biomarkers of BMP-2 and TGF-β1 activities [26,27]. The role of the Smad signaling pathways in biglycan-induced AVIC pro-osteogenic reprogramming needs to be elucidated.
We hypothesized that biglycan induces human AVIC pro-osteogenic reprogramming by concurrent up-regulation of BMP-2 and TGF-β1. The purpose of this study was to determine: 1) the role of BMP-2 in mediating biglycan-induced AVIC pro-osteogenic reprogramming, 2) the effect of biglycan on the release and expression of TGF-β1 by human AVICs, and 3) whether TGF-β1 plays a mechanistic role in biglycan-induced AVIC pro-osteogenic reprogramming.
Materials and Methods
Materials
Antibody against human BMP-2 was purchased from ProSci, Inc. (Poway, CA). Antibodies against human TLR2 and TLR4 were purchased from Imgenex, Inc. (San Diego, California). Antibodies against osteopontin, Runx2, phosphorylated Smad1, total Smad1, phosphorylated Smad3 and total Smad3 were purchased from Cell Signaling, Inc. (Beverly, MA). Medium 199 was purchased from Lonza (Walkersville, MD). Recombinant human biglycan (expressed by murine myeloma cell line; endotoxin-free), TGF-β1 and BMP-2, antibodies against TGF-β1 and ELISA kits for TGF-β1, BMP-2, BMP-4 and BMP-7 were purchased from R&D System (Minneapolis, MN). Specific siRNA for human Smad1 and Smad3 were purchased from Ambion, Inc. (Carlsbad, CA). HiPerFect® transfection reagent and other transfection-related reagents were purchased from Qiagen (Valencia, CA). All other chemicals and reagents were from Sigma-Aldrich Chemical Co. (St Louis, MO).
Cell isolation and treatment
Normal aortic valve leaflets were collected from the explanted hearts of 6 patients (4 males and 2 females, age 59.0±8.1 years) undergoing heart transplantation due to late stage of cardiomyopathy. These valve leaflets were thin and did not exhibit histological abnormality. All patients gave informed consent for the use of their aortic valves for this study. This study was approved by the Institutional Review Board of University of Colorado Denver.
AVICs were isolated and cultured using the previously described method [5,30]. Briefly, valve leaflets were subjected to sequential digestions with collagenase, and cells were collected by centrifugation. Cells were cultured in M199 growth medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin G, streptomycin, and amphotericin B. Cells of passage 4 to 6 were used, and cells were treated when they reached 80 to 90% confluence.
To determine the effect of soluble biglycan on TGF-β1 expression, cells were treated with recombinant human biglycan (0.05, 0.10 and 0.20 μg/ml) for 24 or 48 h. Levels of TGF-β1 in cell lysates were assessed by immunoblotting.
To determine the role of BMP-2 and TGF-β1 in biglycan-induced pro-osteogenic reprogramming, cells were treated with biglycan for 3 to 21 days in the presence of neutralizing antibodies (10 μg/ml) against human BMP-2 and/or TGF-β1. Cellular levels of ALP, osteopontin and Runx2 were analyzed at 3 days. Additional cells were treated with BMP-2 (0.10 μg/ml) [31], TGF-β1 (0.005 μg/ml) [32] or a combination of these two pro-osteogenic mediators for 3 to 21 days to determine their effect on cellular levels of ALP, osteopontin and Runx2, ALP activity and calcium deposition.
The effect of biglycan on phosphorylation of Smad1 and Smad3 was determined following stimulation of cells with biglycan for 24 to 72 h. To determine the role of the Smad1 and Smad3 signaling pathways in mediating the effect of biglycan on AVIC pro-osteogenic reprogramming, cells were pretreated with Smad1 siRNA or Smad3 siRNA and then stimulated with biglycan for 3 days.
Immunoblotting
To analyze protein levels of TGF-β1, ALP, osteopontin and Runx2, immunoblotting was performed, as described previously [33]. After visualizing protein bands with the enhanced chemiluminescence system, analysis of band density was performed using the National Institutes of Health ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
ELISA
Cell culture supernatants were collected. Levels of TGF-β1, BMP-2, BMP-4 and BMP-7 were analyzed using ELISA kits following the manufacturer’s protocol.
Gene knockdown
To knockdown Smad1 and Smad3, cells (60–70% confluent) on 24-well plates were incubated with a mixture of siRNA (50 nM) and transfection reagent (6 μl per ml of medium) for 48 h. Control cells were treated with the same concentrations of scrambled siRNA and transfection reagent.
Analysis of calcium deposits and ALP activity
Calcium deposits were examined by Alizarin Red staining. ALP activity was analyzed by chemical staining. After fixation for 15 min in 4% paraformaldehyde, cell monolayers were incubated with 0.2% Alizarin Red S solution (pH 4.2) or a mixture of 0.1 mg/ml naphthol AS-MX phosphate and 0.6 mg/ml Fast Blue BB salt (pH 8.5) at room temperature [5,16]. Alizarin red and ALP activity stains were examined and photographed with a Nikon Eclipse TS100 microscope (Tokyo, Japan). To quantitatively analyze Alizarin Red staining, stains were bleached with 10% acetic acid at 85 °C. Supernatant was analyzed spectrophotometrically at 450 nm [34].
Statistical analysis
All results are expressed as mean ± SE. Comparisons between groups were performed using a StatView software (Abacus Concepts, Calabasas, CA) with one-way analysis of variance (ANOVA) with the post hoc Bonferroni/Dunn test. An interaction was also tested if a linear trend was indicated. A difference was considered significant at P< 0.05.
Results
Neutralization of BMP-2 reduces, but does not abrogate biglycan-induced expression of ALP, osteopontin and Runx2
BMP-2 is an important mediator of AVIC calcification [18]. To determine the role of BMP-2 in biglycan-induced expression of ALP, osteopontin and Runx2, we stimulated cells with 0.10 μg/ml of recombinant human biglycan in the presence of a specific neutralizing antibody against BMP-2. Neutralization of BMP-2 attenuated the increase in protein levels of ALP, osteopontin and Runx2 after 3 days of biglycan stimulation while non-immune IgG had no effect (Figure 1). However, the levels of these osteogenic biomarkers in cells treated with BMP-2-neutralizing antibody remained significantly higher than those in untreated controls (baseline). It appears that BMP-2 has a partial role in mediating the effect of biglycan on AVIC expression of ALP, osteopontin and Runx2.
Figure 1. Neutralization of BMP-2 diminishes biglycan-induced expression of ALP, osteopontin and Runx2.
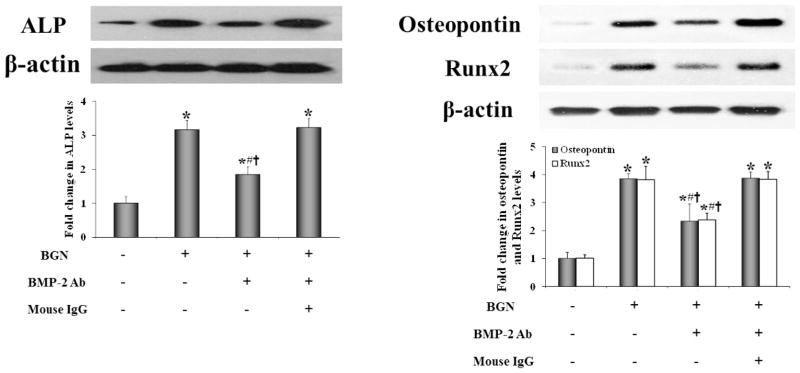
Human AVICs were treated with biglycan (BGN, 0.10 μg/ml) for 3 days in the presence of a neutralizing antibody (Ab) against BMP-2 or non-immune IgG. Neutralization of BMP-2 reduces protein levels of ALP, osteopontin and Runx2 in cells treated with biglycan. n=4 isolates from different donors; *P<0.05 vs. untreated control; #P<0.05 vs. BGN alone; †P<0.05 vs. BGN+non-immune IgG.
Biglycan up-regulates TGF-β1 expression
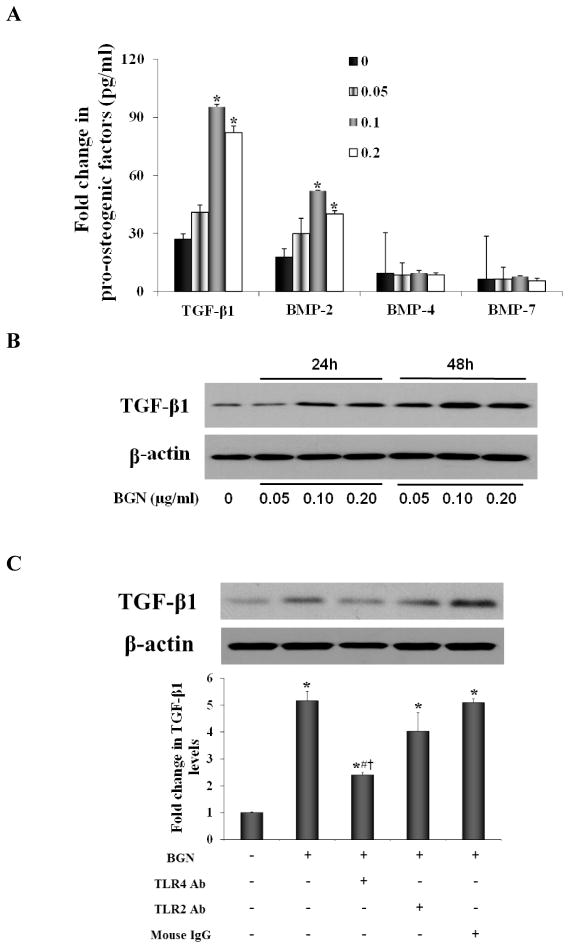
To identify additional pro-osteogenic factors, we determined the effect of recombinant biglycan on the secretion of TGF-β1, BMP-2, BMP-4 and BMP-7 by human AVICs. As shown in Figure 2A, biglycan induced the secretion of TGF-β1 and BMP-2, but had no effect on the secretion of BMP-4 and BMP-7. To examine whether the increased secretion of TGF-β1 is associated with up-regulation of its protein levels in the cells, we analyzed TGF-β1 protein in cell lysates after biglycan stimulation for 24 and 48 h. Treatment with biglycan increased the protein levels of TGF-β1 at 24 h, and higher levels of this pro-osteogenic factor were observed in cell lysate at 48 h. (Figure 2B). Thus, biglycan up-regulates expression of TGF-β1 in human AVICs and promotes the release of this pro-osteogenic factor.
Figure 2. Biglycan up-regulates TGF-β1 expression and secretion.
Human AVICs were treated with biglycan (BGN; 0.05, 0.10 and 0.20 μg/ml) for 24 h to 48h. A. At 48 h, levels of TGF-β1 and BMP-2, but not BMP-4 and BMP-7 are markedly increased in culture medium. B. A representative immunoblot shows that cellular TGF-β1 protein levels are elevated at 24 h and 48 h. C. A representative immunoblot and densitometric data show that neutralization of TLR4 markedly reduces TGF-β1 protein levels following biglycan treatment (BGN, 0.10 μg/ml) for 48 h, while neutralization of TLR2 had a minor effect. n=4 isolates from different donors; *P<0.05, vs. untreated control; #P<0.05 vs. BGN alone; † P<0.05 vs. BGN + non-immune IgG; Ab =neutralizing antibody.
We have found that stimulation of TLR2 or TLR4 induces osteogenic responses in human AVICs [5]. In a recent study [16], we observed that biglycan interacts with both TLR2 and TLR4 in human AVICs. To determine the role of these two innate immune receptors in mediating biglycan-induced TGF-β1 expression, we applied neutralizing antibodies to block TLR2 and TLR4. Interestingly, neutralization of TLR4 reduced TGF-β1 protein levels in cells exposed to biglycan (Figure 2C). In contrast, neutralization of TLR2 had a minor effect on TGF-β1 levels (Figure 2C). Our previous study shows that biglycan up-regulates BMP-2 levels in human AVICs primarily through TLR2. The results of the present study indicate that TLR4 has a major role in mediating TGF-β1 expression induced by biglycan in human AVICs.
Neutralization of both TGF-β1 and BMP-2 abolishes the effect of biglycan on pro-osteogenic reprogramming in AVICs
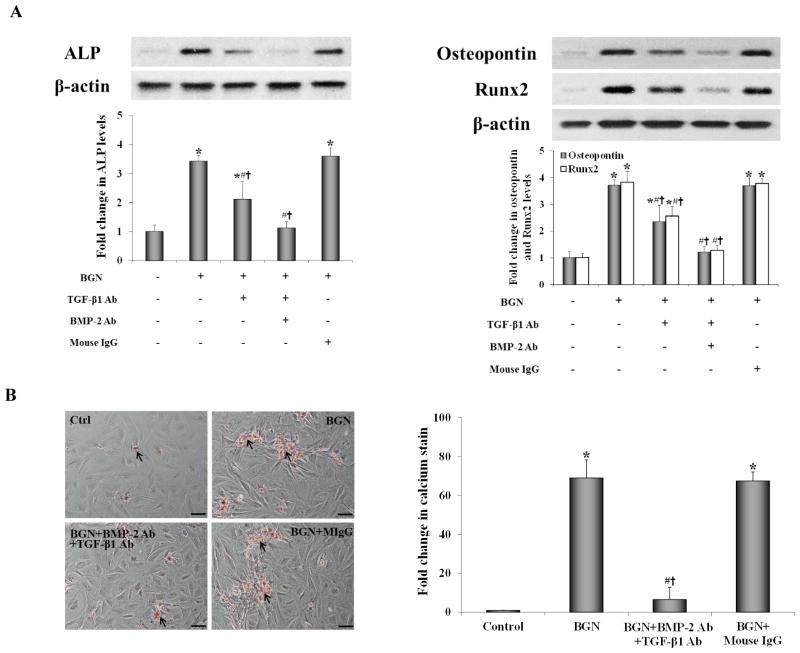
To determine whether both TGF-β1 and BMP-2 are involved in mediating biglycan-induced AVIC pro-osteogenic reprogramming in human AVICs, we applied antibodies to neutralize TGF-β1 and BMP-2. Neutralization of TGF-β1 attenuated the increase in protein levels of ALP, osteopontin and Runx2 in AVICs exposed to biglycan. Neutralization of both TGF-β1 and BMP-2 abrogated the increase in protein levels of these three osteogenic biomarkers in AVICs exposed to biglycan, resulting in comparable levels of these biomarkers to those of untreated controls (Figure 3A). Importantly, biglycan-induced calcium deposition was also abrogated by concurrent neutralization of TGF-β1 and BMP-2 (Figure 3B). Collectively, these data show that neutralizing both TGF-β1 and BMP-2 abolishes the effect of biglycan on AVIC pro-osteogenic reprogramming expressed as up-regulated expression of ALP, osteopontin and Runx2 and formation of calcium deposits.
Figure 3. Neutralization of both BMP-2 and TGF-β1 abolishes the effect of biglycan on AVIC pro-osteogenic reprogramming.
Cells were treated with biglycan (BGN, 0.10 μg/ml) for 3 or 21 days in the presence of neutralizing antibodies (Ab) against BMP-2 and TGF-β1. A. Representative immunoblots and densitometric data show that neutralization of both BMP-2 and TGF-β1 normalizes ALP, osteopontin and Runx2 protein levels examined at 3 days. B. Representative images of Alizarin Red staining (scale bar = 200 μm) and spectrophotometric data show that neutralization of both BMP-2 and TGF-β1 abolishes the formation of calcium deposits at 21 days. n=4 isolates in each group; *P<0.05 vs. untreated control; #P<0.05 vs. BGN alone; †P<0.05 vs. BGN+non-immune IgG; MIgG=mouse IgG.
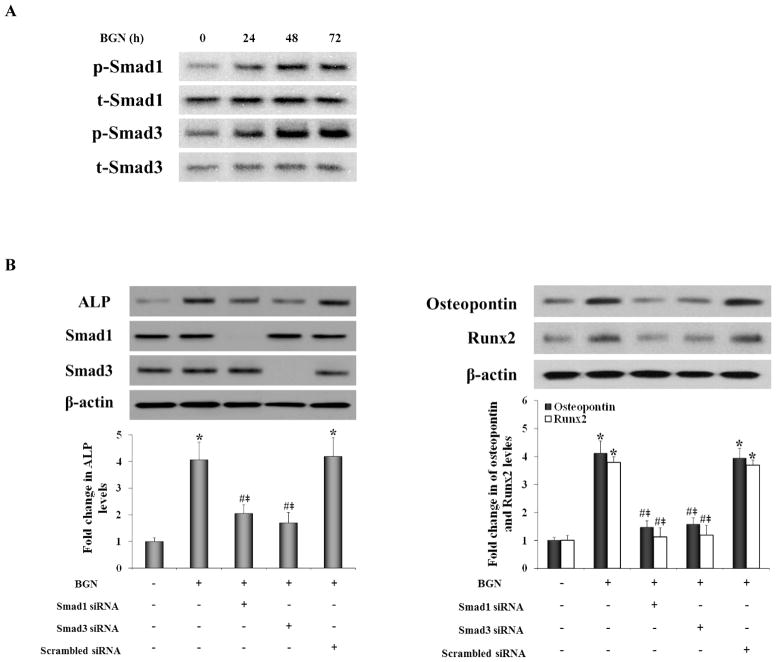
Knockdown of Smad1 and Smad3 reduces the expression of ALP, osteopontin and Runx2 in AVICs exposed to biglycan
TGF-β1 and BMP-2 signaling activities are evaluated by phosphorylation of Smad3/1 in osteoblasts and mesenchymal precursor cells [27]. We examined the effect of biglycan on the phosphorylation of Smad3 and Smad1. We found that biglycan induced the phosphorylation of both Smad3 and Smad1 (Figure 4A). To understand whether the Smad signaling pathways utilized by TGF-β1 and BMP-2 mediate AVIC expression of ALP, osteopontin and Runx2, we determined the effect of knockdown of Smad3 and Smad1 on the levels of ALP, osteopontin and Runx2 following stimulation with biglycan. The results in Figure 4B show that knockdown of either Smad3 or Smad1 reduced the levels of ALP, osteopontin and Runx2 following biglycan treatment. Thus, Smad signaling mediated by TGF-β1 and BMP-2 is required for up-regulation of expression of ALP, osteopontin and Runx2 by biglycan in human AVICs.
Figure 4. Smad1 and Smad3 are required for biglycan-induced the expression of ALP, osteopontin and Runx2.
A. Cells were stimulated with biglycan (BGN, 0.10 μg/mL) for 24 to 72 h. Representative immunoblots shows biglycan induces the phosphorylation of Smad1 and Smad3. B. Representative immunoblots and densitometric data show that knockdown of either Smad1 or Smad3 reduced the expression of ALP, osteopontin and Runx2 following treatment with biglycan. n=4 isolates in each group; *P<0.05 vs. untreated control; #P<0.05 vs. BGN alone; ‡P<0.05 vs. BGN+scrambled siRNA.
An exposure to TGF-β1 and BMP-2 results in pro-osteogenic reprogramming in AVICs
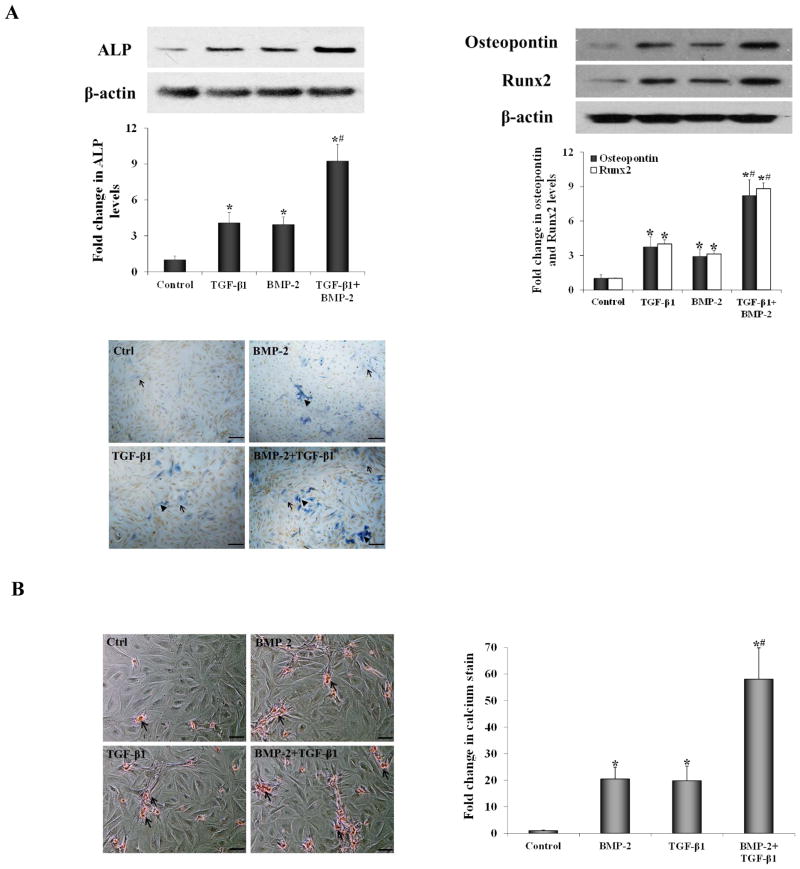
To determine whether TGF-β1 and BMP-2 induce AVIC pro-osteogenic reprogramming comparable to that induced by biglycan, we incubated human AVICs with BMP-2 and TGF-β1 and analyzed protein levels of ALP, osteopontin and Runx2 at day 3, ALP enzyme activity at day 7 and calcium deposits at day 21. As shown in Figure 5A, the levels of ALP, osteopontin and Runx2, as well as ALP enzyme activity, increased following stimulation with either BMP-2 or TGF-β1. Prolonged stimulation with BMP-2 or TGF-β1 induced calcium deposition (Figure 5B). However, greater expression of ALP, osteopontin and Runx2, as well as greater ALP activity and calcium deposition, were observed in cells exposed to a combination of BMP-2 and TGF-β1. And the fold change in levels of ALP, osteopontin and Runx2 was greater than the sum of BMP-2 treatment alone and TGF-β1 treatment alone. It appears that BMP-2 and TGF-β1 display a synergy in the induction of AVIC pro-osteogenic reprogramming.
Figure 5. Treatment with BMP-2 plus TGF-β1 induces pro-osteogenic reprogramming.
Cells ware treated with BMP-2 (0.100 μg/ml), TGF-β1 (0.005 μg/ml) or both of these two factors for 3 to 21 days. A. Cellular levels of ALP, osteopontin and Runx2 examined at 3 days and ALP activity stain (scale bar = 50 μm) examined at 7 days were up-regulated, particularly in cells exposed to BMP-2+TGF-β1 (Arrow = moderate activity and arrow head = high activity). B. Representative images of Alizarin Red staining (scale bar = 200 μm) and spectrophotometric data show that calcium deposits were present in cells treated with BMP-2 or TGF-β1, especially in those treated with BMP-2+TGF-β1 for 21 days. n=4 isolates; *P<0.05 vs. untreated control; #P<0.05 vs. BMP-2 alone or TGF-β1 alone.
Discussion
We recently reported that extracellular soluble biglycan interacts with TLR2 and TLR4 in human AVICs, and prolonged stimulation of human AVICs with biglycan induces pro-osteogenic reprogramming, characterized as the expression of osteogenic biomarkers (ALP, osteopontin and Runx2) and formation of calcium deposits, which is associated with TLR2-mediated up-regulation of BMP-2 [16]. In the present study, we found that BMP-2 has a partial role in mediating biglycan-induced AVIC reprogramming. In addition, we found that biglycan up-regulates TGF-β1 expression in human AVICs through TLR4. Both BMP-2 and TGF-β1 are required for AVIC reprogramming induced by biglycan. Further, BMP-2 and TGF-β1 synergistically induce the expression of osteogenic biomarkers and calcium deposition in human AVICs.
Biglycan has been found to accumulate in calcific aortic valves and may play a role in AVIC calcification [14,35]. BMP-2 is involved in bone formation, as well as in vascular and heart valve calcification [36,37]. Our previous studies found recombinant human biglycan induces BMP-2 expression in human AVICs through TLR2 [16]. However, the role of BMP-2 in biglycan-induced pro-osteogenic reprogramming in human AVICs remains unclear. To verify pro-osteogenic reprogramming, we examined the expression of ALP (a biomarker of early osteoblastic differentiation), osteopontin (an osteogenic biomarker) and Runx2 (an osteogenic transcription factor). The results show that neutralization of BMP-2 reduces, but not abolishes, the up-regulated expression of ALP, osteopontin and Runx2 in AVICs exposed to biglycan. In addition, AVICs exposed to recombinant BMP-2 display moderately up-regulated expression of ALP, osteopontin and Runx2, and moderate calcium deposition. Thus, BMP-2 plays a partial role in biglycan-induced AVIC pro-osteogenic reprogramming, and BMP-2 alone is insufficient to induce the pro-osteogenic changes seen in cells exposed to biglycan. It appears that other pro-osteogenic factors are also involved in mediating AVIC reprogramming induced by biglycan.
Among the BMPs, BMP-2, -4, and -7 have greater pro-osteogenic activity [29] and are identified in aortic valves explanted from patients with CAVD [37]. TGF-β1 is also found in aortic valves explanted from patients with CAVD and has a potent pro-osteogenic effect on AVICs [37,38]. To identify additional pro-osteogenic factors induced by biglycan, we analyzed the levels of BMP-2, BMP-4, BMP-7 and TGF-β1 in the culture medium of cells exposed to biglycan. Interestingly, we observed that biglycan increases the levels of BMP-2 and TGF-β1, but has no effect on the levels of BMP-4 and BMP-7. The increase in extracellular levels of TGF-β1 appears to be a result of up-regulated expression of this pro-osteogenic factor since biglycan increased cellular levels of TGF-β1 protein. Such changes in extracellular and cell-associated levels of TGF-β1 are similar to those for BMP-2 as increased cellular levels of BMP-2 are observed in AVICs exposed to biglycan [16].
Chronic inflammation and calcification are important pathological changes associated with the progression of CAVD [1,2,4]. A number of studies, including ours, demonstrate that pro-inflammatory stimuli induce the expression of pro-osteogenic mediators in human AVICs [5–9]. The current consensus is that an inflammatory milieu in aortic valves affected by the disease promotes a shift of valvular cells towards a pro-osteogenic phenotype, exacerbating mineral deposition. However, it is unclear what pro-inflammatory factors are responsible and how a pro-inflammatory signaling modulates AVIC osteogenic response. TLRs recognize danger-associated molecular patterns (DAMPs) and invoke a pro-inflammatory response in cells [39]. Interestingly, previous studies found that stimulation of TLR2 or TLR4 in human AVICs induces up-regulation of pro-osteogenic mediators [5,6,30]. Several endogenous agents, including heat shock proteins and soluble biglycan, could activate TLR signaling, and are termed DAMPs [39,40]. Among the DAMPs, biglycan is particularly interesting since it is over-expressed in sclerotic and calcified aortic valve leaflets [14,17]. It is likely that soluble biglycan accumulates in diseased aortic valve tissue and promotes CAVD progression. In this regard, recent studies found that biglycan induces the expression of phospholipid transfer protein and BMP-2 in human AVICs through TLR2 [14,16]. Furthermore, biglycan is capable of inducing human AVIC calcification in vitro [16]. Our recent study found that biglycan is co-immmunoprecipitated with TLR2 and TLR4 in human AVICs incubated with biglycan [16]. It is possible that TLR2 and/or TLR4 play a role in mediating biglycan-induced TGF-β1 expression in human AVICs. Our results show that neutralization of TLR4 results in a greater reduction in TGF-β1 levels in cells exposed to biglycan while neutralization of TLR2 has a minimal effect. Taken together, these results and our previous findings demonstrate that in human AVICs, biglycan induces the expression of BMP-2 and TGF-β1 through TLR2 and TLR4, respectively. These findings support our notion that these two innate immunoreceptors regulate osteogenic responses in human AVICs [5,16,30,41].
A partial role of BMP-2 in biglycan-induced AVIC pro-osteogenic reprogramming and the up-regulation of TGF-β1 expression by biglycan indicate that TGF-β1 may play a role in the mechanism underlying AVIC reprogramming induced by biglycan. Indeed, neutralization of TGF-β1 alone reduces the expression of ALP, osteopontin and Runx2, and the formation of calcium deposits. In contrast, neutralization of both TGF-β1 and BMP-2 abolishes the effect of biglycan on the expression of osteogenic biomarkers and the formation of calcium deposits. Thus, TGF-β1 also plays a role in mediating biglycan-induced pro-osteogenic reprogramming in human AVICs. It appears that TGF-β1 and BMP-2 are in concert to mediate the effect of biglycan. Further evidence is obtained from the experiments using recombinant TGF-β1 and BMP-2. While TGF-β1 and BMP-2 each up-regulates protein levels of ALP, osteopontin and Runx2, as well as ALP enzyme activity and calcium deposition, greater changes in these osteogenic biomarkers are induced by a combination of TGF-β1 and BMP-2. Although a low level of TGF-β1 is applied in the present study, the calcification change caused by BMP-2 plus TGF-β1 is much greater than the sum of BMP-2 alone and TGF-β1 alone. Therefore, the results show that a combination of BMP-2 and TGF-β1 is sufficient to induce the osteogenic changes seen in cells exposed to biglycan. The effect of TGF-β1 and BMP-2 on AVIC pro-osteogenic reprogramming is not simply additive. It seems that these two pro-osteogenic factors act synergistically. Concurrent up-regulation of TGF-β1 and BMP-2 is critical for the induction of AVIC pro-osteogenic reprogramming by biglycan. Other studies show that BMPR-IB formation promoted by TGF-β1 enhances BMP-2-induced osteogenic functions in bone marrow stromal cells in vitro [42]. It remains unclear from the present study how TGF-β1 and BMP-2 interact in the induction of AVIC reprogramming. Further studies are needed to address the underlying mechanism.
Signaling transduction for TGF-β1 and BMP-2 in osteoblasts is primarily mediated by the Smad-dependent pathways, including Smad2/3 for TGF-β1 and Smad1/5/8 for BMP-2 [27]. Phosphorylation of Smad3/1 serves as markers of TGF-β1 and BMP-2 signaling activities [27]. Our results show that biglycan-induced AVIC reprogramming is accompanied by the phosphorylation of both Smad3 and Smad1. Knockdown of either Smad3 or Smad1 reduces protein levels of ALP, osteopontin and Runx2 in AVICs exposed to biglycan. Our results demonstrate that TGF-β1 and BMP-2 activities are required for biglycan-induced pro-osteogenic reprogramming in human AVICs and indicate that the classical signaling pathways utilized by these two pro-osteogenic factors mediate the effect of biglycan.
Previous studies have found an interaction of biglycan and TLRs with pro-osteogenic mediators. In this regard, the work by Ye and colleagues demonstrate that glycosaminoglycan chains of biglycan promote osteoblast differentiation induced by BMP-4 [43]. In addition, Seki and colleagues reported that activation of TLR4 enhances TGF-β signaling in hepatic stellate cells and exaggerates hepatic fibrosis [44]. These studies indicate that endogenous agents and innate immunereceptors modulate cellular osteogenic responses. As biglycan accumulation is associated with CAVD, and soluble biglycan can function as DAMP, it is likely that biglycan plays a role in TLR-mediated pro-osteogenic reprogramming of AVICs. Our findings in human AVICs demonstrate that biglycan up-regulates the expression of BMP-2 and TGF-β1 via TLR2/4. Both BMP-2 and TGF-β1 are required for the induction of pro-osteogenic reprogramming by biglycan in human AVICs, and the classical Smad signaling pathways play an important role in mediating the pro-osteogenic effects of biglycan. These novel findings support the notion that endogenous molecules, by functioning as DAMPs, may promote aortic valve calcification and CAVD progression via induction of AVIC pro-osteogenic reprogramming.
Conclusions
Biglycan is capable of up-regulating the expression of BMP-2 and TGF-β1 in human AVICs through innate immunoreceptors. Concurrent up-regulation of BMP-2 and TGF-β1 mediates biglycan-induced AVIC pro-osteogenic reprogramming, and these two pro-osteogenic factors utilize the classical Smad signaling pathways to exert their effects on human AVICs.
Limitations
One of the limitations of this study is its relatively small sample size (6 AVIC isolates from normal aortic valves). To check the reproducibility of the observations, all experiments were repeated using cells from 4 different donors. In addition, the concentrations of recombinant BMP-2 and TGF-β1 applied may be quite different from their concentrations in the micro-environments surrounding AVICs in valvular tissue. The results obtained merely show that these two factors are capable of inducing the pro-osteogenic reprogramming in human AVICs.
Biglycan up-regulates BMP-2 and TGF-β1 in human aortic valve cells through TLRs.
Both BMP-2 and TGF-β1 are required for aortic valve cell pro-osteogenic reprogramming.
Smad signaling pathways are involved in mediating the pro-osteogenic effects of biglycan.
Acknowledgments
b) Sources of Funding: This study was supported in part by National Institutes of Heart, Lung and Blood Grant HL106582.
Footnotes
c) Disclosure: None
References
- 1.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cote N, Mahmut A, Bosse Y, Couture C, Page S, Trahan S, Boulanger MC, Fournier D, Pibarot P, Mathieu P. Inflammation Is Associated with the Remodeling of Calcific Aortic Valve Disease. Inflammation. 2013;36:573–581. doi: 10.1007/s10753-012-9579-6. [DOI] [PubMed] [Google Scholar]
- 3.Helske S, Oksjoki R, Lindstedt KA, Lommi J, Turto H, Werkkala K, Kupari M, Kovanen PT. Complement system is activated in stenotic aortic valves. Atherosclerosis. 2008;196:190–200. doi: 10.1016/j.atherosclerosis.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Fullerton DA, Su X, Ao L, Cleveland JC, Meng X. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of Toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J Am Coll Cardiol. 2009;53:491–500. doi: 10.1016/j.jacc.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 6.Lopez J, Fernandez-Pisonero I, Duenas AI, Maeso P, Roman JA, Crespo MS, Garcia-Rodriguez C. Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol. 2012;158:18–25. doi: 10.1016/j.ijcard.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 7.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, Brueckmann M, Vahl C, Hagl S, Haase KK, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–211. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 8.Kaden JJ, Kilic R, Sarikoc A, Hagl S, Lang S, Hoffmann U, Brueckmann M, Borggrefe M. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med. 2005;16:869–872. [PubMed] [Google Scholar]
- 9.Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K. Tumor necrosis factor-alpha accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. 2011;337:16–23. doi: 10.1124/jpet.110.177915. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulos A, Kaoukis A, Papadaki H, Pyrgakis V. Pathophysiologic mechanisms of calcific aortic stenosis. Ther Adv Cardiovasc Dis. 2012;6:71–80. doi: 10.1177/1753944712439337. [DOI] [PubMed] [Google Scholar]
- 11.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derbali H, Bosse Y, Cote N, Pibarot P, Audet A, Pepin A, Arsenault B, Couture C, Despres JP, Mathieu P. Increased biglycan in aortic valve stenosis leads to the overexpression of phospholipid transfer protein via Toll-like receptor 2. Am J Pathol. 2010;176:2638–2645. doi: 10.2353/ajpath.2010.090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song R, Zeng Q, Ao L, Yu JA, Cleveland JC, Zhao KS, Fullerton DA, Meng X. Biglycan Induces the Expression of Osteogenic Factors in Human Aortic Valve Interstitial Cells via Toll-Like Receptor-2. Arterioscler Thromb Vasc Biol. 2012;32:2711–2720. doi: 10.1161/ATVBAHA.112.300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens EH, Saltarrelli JG, Baggett LS, Nandi I, Kuo JJ, Davis AR, Olmsted-Davis EA, Reardon MJ, Morrisett JD, Grande-Allen KJ. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc Pathol. 2010;20:334–342. doi: 10.1016/j.carpath.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–357. [PubMed] [Google Scholar]
- 19.Yetkin E, Waltenberger J. Molecular and cellular mechanisms of aortic stenosis. Int J Cardiol. 2009;135:4–13. doi: 10.1016/j.ijcard.2009.03.108. [DOI] [PubMed] [Google Scholar]
- 20.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, Gorman JH, 3rd, Gorman RC, Levy RJ. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83:946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Si X, Jin Y, Yang L. Induction of new bone by ceramic bovine bone with recombinant human bone morphogenetic protein 2 and transforming growth factor beta. Int J Oral Maxillofac Surg. 1998;27:310–314. doi: 10.1016/s0901-5027(05)80622-8. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302 (Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. Faseb J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 25.Yan J, Stringer SE, Hamilton A, Charlton-Menys V, Gotting C, Muller B, Aeschlimann D, Alexander MY. Decorin GAG synthesis and TGF-beta signaling mediate Ox-LDL-induced mineralization of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:608–615. doi: 10.1161/ATVBAHA.110.220749. [DOI] [PubMed] [Google Scholar]
- 26.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsunobu T, Torigoe K, Ishikawa M, de Vega S, Kulkarni AB, Iwamoto Y, Yamada Y. Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev Biol. 2009;332:325–338. doi: 10.1016/j.ydbio.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 30.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC, Fullerton DA. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294:C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC, Fullerton DA. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138:1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 33.Ao L, Zou N, Cleveland JC, Fullerton DA, Meng X. Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: Mediating KC and MCP-1 expression induced by extracellular HSC70. Am J Physiol Heart Circ Physiol. 2009;297:H21–H28. doi: 10.1152/ajpheart.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan CM, Zhang X, James AW, Mari Kim T, Sun N, Wu B, Ting K, Soo C. NELL-1 increases pre-osteoblast mineralization using both phosphate transporter Pit1 and Pit2. Biochem Biophys Res Commun. 2012;422:351–357. doi: 10.1016/j.bbrc.2012.04.077. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann S, Walther T, Kempfert J, Rastan A, Garbade J, Dhein S, Mohr FW. Mechanical strain and the aortic valve: influence on fibroblasts, extracellular matrix, and potential stenosis. Ann Thorac Surg. 2009;88:1476–1483. doi: 10.1016/j.athoracsur.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 38.Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis. 2008;17:689–699. [PMC free article] [PubMed] [Google Scholar]
- 39.Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14:2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 41.Zeng Q, Jin C, Ao L, Cleveland JC, Jr, Song R, Xu D, Fullerton DA, Meng X. Cross-talk between the toll-like receptor 4 and notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation. 2012;126:S222–S230. doi: 10.1161/CIRCULATIONAHA.111.083675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Mariani FV, Harland RM, Luo K. Ski represses bone morphogenic protein signaling in Xenopus and mammalian cells. Proc Natl Acad Sci U S A. 2000;97:14394–14399. doi: 10.1073/pnas.97.26.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye Y, Hu W, Guo F, Zhang W, Wang J, Chen A. Glycosaminoglycan chains of biglycan promote bone morphogenetic protein-4-induced osteoblast differentiation. Int J Mol Med. 2012;30:1075–1080. doi: 10.3892/ijmm.2012.1091. [DOI] [PubMed] [Google Scholar]
- 44.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]