Abstract
Frontotemporal lobar degeneration (FTLD) comprises two main classes of neurodegenerative diseases characterized by neuronal/glial proteinaceous inclusions (ie. proteinopathies) including tauopathies (i.e. FTLD-Tau) and TDP-43 proteinopathies (i.e. FTLD-TDP) while other very rare forms of FTLD are known such as FTLD with FUS pathology (FTLD-FUS). This review focuses mainly on FTLD-Tau and FLTD-TDP, which may present as several clinical syndromes: a behavioral/dysexecutive syndrome (behavioral-variant frontotemporal dementia); language disorders (primary progressive aphasia variants); and motor disorders (amyotrophic lateral sclerosis, corticobasal syndrome, progressive supranuclear palsy syndrome). There is considerable heterogeneity in clinical presentations of underlying neuropathology and current clinical criteria do not reliably predict underlying proteinopathies ante-mortem. In contrast, molecular etiologies of hereditary FTLD are consistently associated with specific proteinopathies. These include MAPT mutations with FTLD-Tau and GRN, C9orf72, VCP and TARDBP with FTLD-TDP. The last decade has seen a rapid expansion in our knowledge of the molecular pathologies associated with this clinically and neuropathologically heterogeneous group of FTLD diseases. Moreover, in view of current limitations to reliably diagnose specific FTLD neuropathologies prior to autopsy, we summarize the current state of the science in FTLD biomarker research including neuroimaging, biofluid and genetic analyses. We propose that combining several of these biomarker modalities will improve diagnostic specificity in FTLD through a personalized medicine approach. The goals of these efforts are to enhance power for clinical trials focused on slowing or preventing progression of spread of tau, TDP-43 and other FTLD-associated pathologies and work towards the goal of defining clinical endophenotypes of FTD.
Keywords: FTLD, TDP-43, Tau, ALS, C9orf72, GRN, MAPT
Introduction
Frontotemporal dementia (FTD) consists of a spectrum of clinical syndromes [6, 75, 135, 178, 200] associated with several underlying neurodegenerative diseases characterized by frontotemporal lobar degeneration (FTLD) [40, 140]. FTD often affects individuals younger than 65 years old and is nearly as common as Alzheimer’s disease (AD) in this age range (i.e. prevalence of ~15–22/100,000 person-years) [122, 179]. Men and women are both roughly equally affected in most population-based studies, and the disorder has a worldwide distribution [122, 174]. Many cases of FTD have a family history of a similar dementing disorder with or without amyotrophic lateral sclerosis (ALS) [127, 217]. Non-genetic environmental risk factors have been studied in only small retrospective series, but these find a possible link between a history of head trauma and increased risk of FTD [114, 184].
FTLD neuropathology may present as one of three clinical FTD syndromes: a behavioral-dysexecutive disorder - behavioral variant FTD (bvFTD) [178] - the most frequent phenotype; three clinically distinct language disorders including primary progressive aphasia (PPA) variants [75] (non-fluent/agrammatic variant, naPPA; semantic variant, svPPA and, rarely, a logopenic variant, lvPPA); in addition to motor disorders such as ALS [200], corticobasal syndrome (CBS) [6], or progressive supranuclear palsy (PSP) syndrome [135]. There is considerable heterogeneity of clinical presentations and underlying pathology, as further described below. In particular, bvFTD and CBS clinical syndromes have a range of underlying neuropathologies, while naPPA is more commonly associated with tauopathies and svPPA with TDP-43 deposition, but these associations are not absolute. Motor presentations in FTD with ALS (FTD-ALS) and PSP are reliable indications of underlying TDP-43 and tauopathy, respectively [64]. There are few autopsy studies of the recently defined lvPPA variant and in vivo imaging studies suggest that this phenotype is largely due to an atypical presentation of AD neuropathology [177]; however, forms of FTLD neuropathology have also been described with this syndrome [154]. Thus, clinical syndrome alone cannot reliably predict underlying FTLD neuropathology ante-mortem. Indeed, clinical criteria for FTD syndromes are under continuous evaluation and revision to help refine the diagnostic entities to better reflect underlying neuropathology and although broadly accepted, there is some controversy over the specific diagnostic features of FTD/PPA. Further work using well-annotated autopsy-confirmed samples and emerging biomarkers will hopefully lead to the concept of an endophenotype (i.e. clinical syndrome that predicts underlying neuropathology).
There has been a rapid increase in the past decade of knowledge about genetic etiologies of FTLD and the molecular pathologies associated with this clinically and neuropathologically heterogeneous group of diseases. FTLD neuropathology is characterized by the pathological aggregation of misfolded proteins, either in neurons or glial cells, or both. Further, increasing evidence from animal [48, 98] and cell models [84] of FTLD-Tau and to a lesser extent FTLD-TDP [173] and other neurodegenerative conditions implicate neuron-to-neuron transmission of misfolded proteins as a central process for disease progress or spread and subsequent neurodegeneration (For review please see [83]). These findings mirror hierarchical staging models of human neurodegenerative disease [29, 33, 35] and morphological studies of the spatial organization of inclusions [8]. However, AD, FTLD and other non-prion neurodegenerative diseases do not appear to be transmitted between humans and cattle like prions [100]. The central aspect of protein aggregation and spread throughout the CNS provides a promising target for therapeutic development for these currently incurable disorders and accurate as well as rapid ante mortem diagnosis is crucial for this effort.
To follow, we describe the pathological substrates of the FTLD pathologies underlying the different FTD variants and key clinical and genetic associations with a special focus on current and future efforts to improve diagnostic accuracy for development of disease-modifying therapies.
TDP-43 proteinopathies (FTLD-TDP & ALS)
Neuropathology
About 50% of all FTLD is characterized by inclusion bodies containing the transactive response (TAR) DNA-binding protein of 43 kDa (FTLD-TDP). TDP-43 was first identified in 2006 as the main constituent of ubiquitin-positive, tau-negative and α-synuclein-negative inclusions [5, 172], which was previously called FTLD with ubiquitin positive inclusions or FTLD-U [40, 140]. TDP-43 is also the characteristic inclusion found in >95% of ALS patients including nearly all sporadic cases of ALS [168, 172]. Further, there is considerable clinical overlap between ALS and FTD corresponding to the regional distribution of TDP-43 neuropathology [71] and both share common genetic etiologies [55, 168, 180]. Thus ALS and FTLD-TDP are best viewed as a clinicopathological continuum of TDP-43 proteinopathies [71, 130].
TDP-43 is a nuclear protein implicated in exon skipping and transcription regulation [18, 38, 175]. As such, TDP-43 is typically seen in most nuclei of normal cells. In disease, this protein becomes aberrantly localized to the cytoplasm where it forms cytoplasmic inclusions [172]. There are several potential mechanisms for neurodegeneration associated with TDP-43 proteinopathies (reviewed in [130]) (Figure 1) including RNA sequestration and dysfunction, loss of normal TDP-43 function through mislocalization and nuclear clearance and potential toxicity of pathological TDP-43 aggregates.
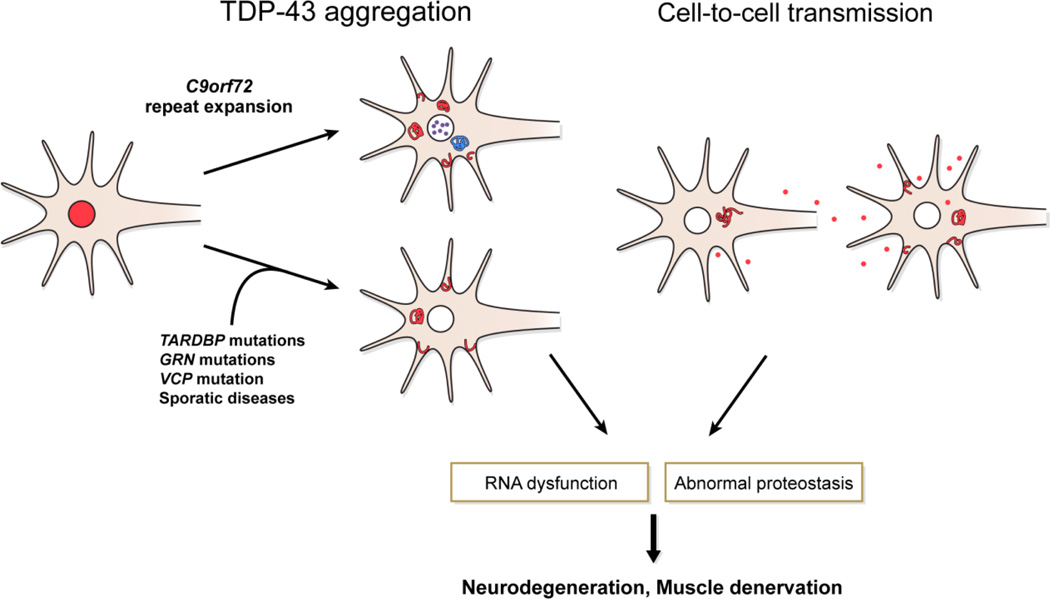
Figure 1. TDP-43 Mediated Neurodegeneration in FTLD-TDP/ALS.
Pathological TDP-43 translocation from the nucleus (red) to the cytoplasmic compartment occurs in sporadic disease and hereditary cases with C9orf72, TARDBP, GRN, and VCP mutations. VCP mutation cases also have intranuclear TDP-43 inclusions (not shown). C9orf72 mutation is associated with additional RNA foci in the nucleus (green) and cytoplasmic di-peptide repeat inclusions (blue), but the specific association with neurodegeneration is currently unclear. Neuron-to-neuron transmission is the likely mechanism for the non-random pattern of spread of neurodegeneration. These processes are linked to RNA dysfunction and abnormal proteostasis, ultimately leading to neuronal cell loss and/or muscle denervation from lower motor neuron loss. Drug-development efforts to slow or halt this process may provide novel disease modifying therapies in the future.
The neuropathology of FTLD-TDP and ALS is generally characterized by TDP-43-positive neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs), dystrophic neurites (DNs), and glial cytoplasmic inclusions (GCIs) often in association with accumulations of ubiquitin and p62 [41]. Biochemistry of postmortem brain samples of these disorders shows TDP-43 to be abnormally phosphorylated, ubiquitinated and cleaved to generate C-terminal fragments [5, 172]. Interestingly, C-terminal fragments appear to be more prominent in cortical TDP-43 deposits in comparison with lower motor neuron inclusions in the spinal cord that contain TDP-43 inclusions that are reactive to with both C-terminal and N-terminal domain specific monoclonal antibodies (MAbs) [99, 125]. The abnormal phosphorylation of the C-terminal region of the protein (pTDP-43) has led to the development of disease-specific antibodies that readily detect pathological aggregates, but leave normal TDP-43 unstained [170]. Thus, pTDP-43 immunohistochemistry (IHC) is the method of choice for detecting FTLD-TDP for routine diagnostic neuropathological evaluation [159]. Interestingly, one MAb generated against amino-acid sequence in the RNA-recognition motif (RRM) has a similar immunohistochemical staining pattern to phospho-TDP epitopes, with predominance of reactivity for pathological inclusions and minimal normal nuclear TDP-43 reactivity, suggesting the possibility of phospho-independent pathological conformers of TDP-43 [125]. Rare NCIs may be thioflavin S-positive in spinal cord indicating that they contain amyloid (i.e. beta-pleated sheets), but most TDP-43-immunoreactive inclusions are thioflavin S-negative and those in the hippocampus are never thioflavin S-positive [181]. In contrast, Bigio and co-workers found more widespread thioflavin S-positive TDP-43 inclusions in neocortical regions and dentate gyrus of the hippocampus in FTLD-TDP [21]. The reasons for these discrepancies are not clear, but they may depend on methodological differences in fixation, tissue preparation and staining techniques. Indeed, Bigio et al used a modified thioflavin-S staining protocol in their study and they also reported exuberant thioflavin-S positive astrocytosis which does not result in amyloidosis [21].
The variability in the morphologic types of neuronal inclusions, their distribution, density, and immunohistochemical profile has led to several proposed classifications based broadly on four pathologic subtypes which map more closely with genetic forms of FTLD-TDP but not as closely with clinical phenotypes [41, 139]. The harmonized “Type A” [139] is equivalent to type 3 of Sampathu et al, [187] and Cairns et al. [41] and is characterized by numerous short DNs and crescentic or oval NCIs, concentrated primarily in neocortical layer two (Fig. 2g). Moderate numbers of lentiform or globose NCIs are also a common but inconsistent feature of this subtype. In addition, NIIs are also present in rare sections. Harmonized “Type B” matches Sampathu et al/Cairns et al. type 2, with moderate numbers of NCIs, throughout all cortical layers, but very few DNs (Fig. 2h). Harmonized “Type C” is the same as Sampathu et al/Cairns et al. type 1, having a predominance of elongated DNs in upper cortical layers, with very few NCIs (Fig.2i). Finally, harmonized Type D, Cairns et al. type 4, refers to the pathology associated with FTLD-TDP with VCP mutation (see below) and is characterized by numerous short DNs and frequent lentiform NIIs (Fig. 2j). TDP-43 positive skein (Figure 2k) or “Lewy-like inclusions” in remaining lower motor neurons along with motor cortex TDP-43 inclusions and corticospinal tract degeneration characterize ALS pathologically. Notably, efforts to stage the spread or progression of TDP-43 pathology in FTLD-TDP and ALS-TDP have been reported using 70 µm thick tissue sections which reveals far more TDP-43 pathology than traditional thin (6–10 µm) sections, but this renders subtyping more difficult due to the greater abundance of pathology that is visualized [33, 34]. These efforts have identified a non-random hierarchical pattern of TDP-43 neuropathology in ALS and FTLD-TDP and suggest that neuron-to-neuron spread of pathological TDP-43 aggregation may be central to disease pathogenesis (reviewed by [30]). Due to current technical limitations of TDP-43 biochemistry and lack of a murine model that recapitulates all features of ALS/FTLD-TDP, cell and animal model data for transmission is currently limited but this is an area of intense research [173].
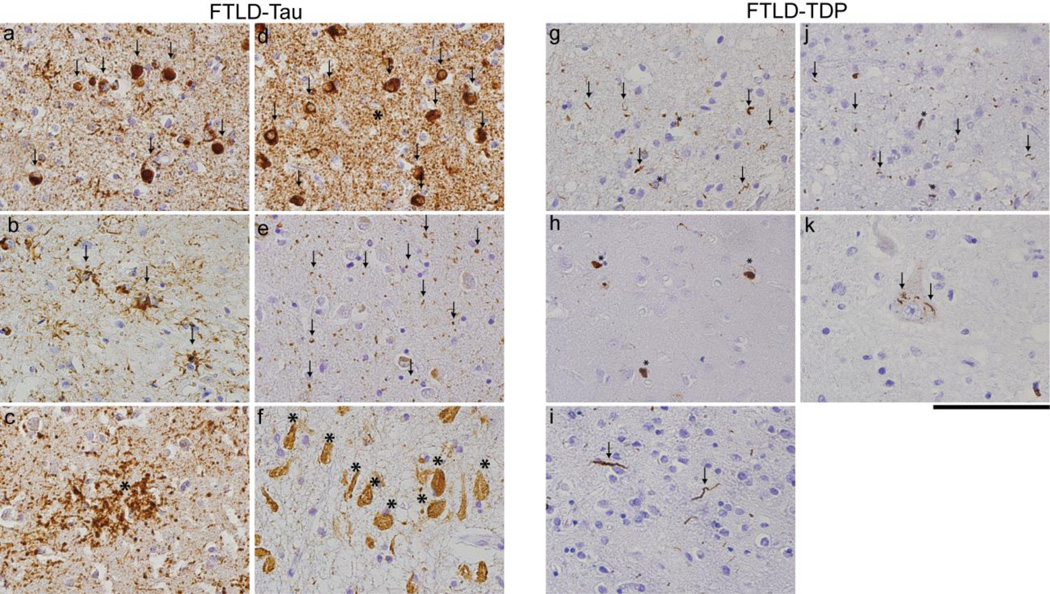
Figure 2. Neuropathological subtypes of FTLD.
Photomicrographs of FTLD-Tau (a–f) and FTLD-TDP (g–k). Images illustrate characteristic inclusion bodies including neocortical (a) round tau-positive Pick-bodies (arrows) in PiD, (b) tufted-astrocytes (arrows) in PSP, (c) astrocytic plaques (asterisk) in CBD, (d) tau-positive neuronal inclusions (arrows) and threads (asterisk) in FTLD-Tau with a MAPT mutation (p.P301L), (e) tau-positive grains (arrows) in limbic cortex in AGD, (f) extracellular ghost tangles (asterisks) in the cornu ammonis in tangle predominant dementia or primary age-related tauopathy (PART). Neocortical sections illustrate in g-j FTLD-TDP morphological subtype A (g) with superficial layer short dystrophic neurites (arrows) and neuronal cytoplasmic inclusions (asterisks) containing pathological TDP-43, (h) subtype B with mainly cytoplasmic inclusions (asterisks), (i) subtype C with long dystrophic neurites (arrows), (j) and subtype D with superficial layer lentiform intranuclear inclusions (asterisks) and short dystrophic neurites (arrows) while (k) shows skein-like inclusions (arrows) in anterior horn cell in ALS. Scale bar= 100 µm.
TDP-43 pathology is not specific to FTLD-ALS as it is also found commonly in over 50% of AD cases and related tauopathies, hippocampal sclerosis, pathological aging and other neurodegenerative diseases [4, 67, 68, 72, 166, 204, 216]. Indeed, hippocampal sclerosis of aging and TDP-43 proteinopathy appear to be closely linked [167]. Careful clinicopathological correlation studies find that co-morbid TDP-43 pathology in aging and AD may have an independent impact on cognition and neurodegeneration [111, 216]. Further, staging efforts have been made for TDP-43 in AD and they suggest a spatiotemporal progression starting in the amygdala [110] that differs from staging schemes proposed for bvFTD due to FTLD-TDP [33]. These findings suggest that TDP-43 aggregation may result from several potential mechanisms with an independent impact on cognitive function; indeed, the genetic heterogeneity of familial FTLD-TDP also implies multiple potential upstream paths (i.e. GRN, TARDBP, C9orf72, VCP, etc.) for TDP-43 mediated neurodegeneration that is central to FTLD-TDP/ALS. Future studies will help clarify the overlap of TDP-43 with other neuropathologies that characterize different neurodegenerative disorders, and perhaps future TDP-43 directed therapies may be of utility in AD cases with dual-pathology. Thus, TDP-43 specific biomarkers are of critical importance.
Genetics
FTLD-TDP is extraordinarily diverse from a genetic standpoint (Figure 3). Four main molecular etiologies of autosomal dominantly-inherited pathogenic mutations have been identified for TDP-43 proteinopathies: variably but abnormally long expansions of a hexanucleotide (GGGGCC) repeat in the chromosome 9 open reading frame 72 gene (C9orf72) [55, 143, 180] is the most frequent genetic cause of familial FTD, FTD-ALS and ALS; mutations in the progranulin gene (GRN) [13, 53, 162] is the second most frequent genetic cause of familial FTLD-TDP while mutations in valosin-containing protein gene (VCP) [213, 214] and TAR DNA-binding protein gene (TARDBP) [73, 113, 206] are less common causes of familial FTLD-TDP and/or ALS. Although each genetic cause is characterized neuropathologically by the presence of TDP-43-immunoreactive inclusions, the morphology, IHC, distribution of the inclusion bodies, and clinical phenotype varies between the different genotypes.
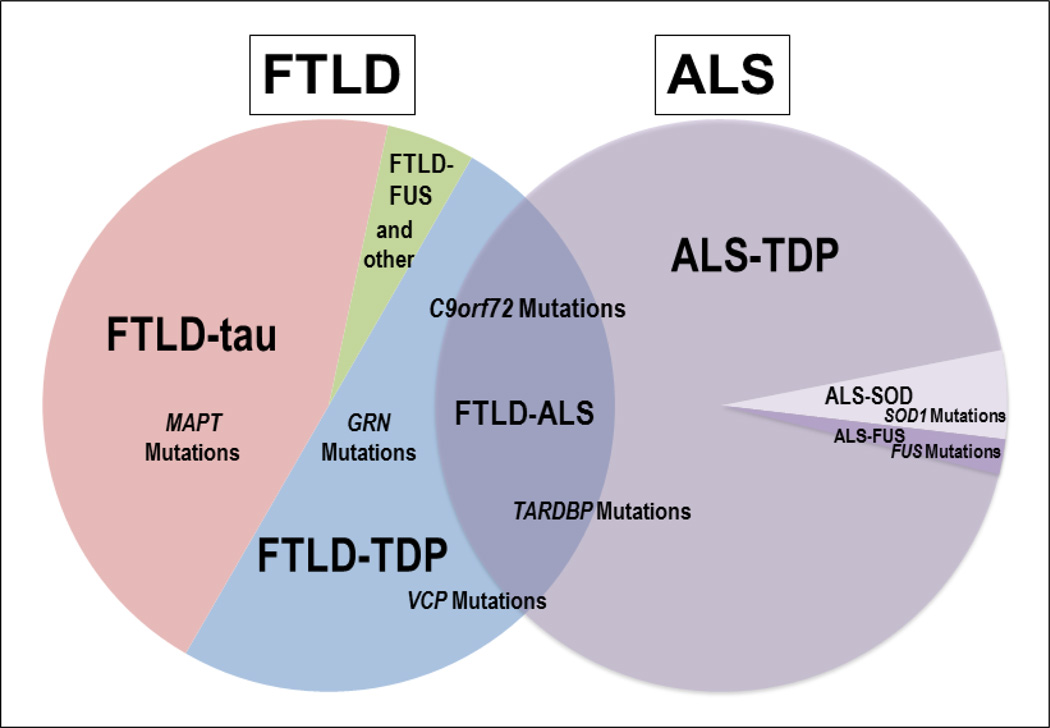
Figure 3. Genetic Associations in FTLD/ALS.
Relative frequencies of neuropathological subtypes and associated molecular etiologies of FTLD and ALS are depicted. FTLD-Tau represents roughly 45% of all FTLD and mutations in MAPT are the sole known cause of hereditary forms of this disorder. FTLD-TDP accounts for roughly 50% of all FTLD and hereditary forms are associated with pathogenic mutations in GRN, C9orf72, TARDBP and VCP and rare other genes. ALS is associated with TDP-43 neuropathology in >95% of cases and there is considerable clinicopathological and genetic overlap of FTLD-TDP and ALS as demonstrated by the overlapping Venn diagrams. Placement of gene names reflect these associations, with FTLD-ALS/ALS cases more associated with C9orf72 and TARDBP while less commonly linked to VCP and rarely GRN. TARDBP is rarely associated with FTLD without co-morbid ALS. A minority of ALS is associated with pathogenic mutations in SOD1 and FUS, while FTLD-FUS also may occur a sporadic condition. Extremely rare cases of FTLD (other) are associated with pathogenic mutations in CHMP2B and FTLD-U neuropathology.
Sporadic Disease and Genetic Risk Factors
One genome wide association study (GWAS) was performed using only FTLD-TDP patients with either a pathologically confirmed TDP-43 pathology or a GRN mutation and genome wide significance was detected for a single gene, transmembrane protein 106B (TMEM106B) on chromosome 7 [207]. Although this risk factor has not been replicated in all followed up studies using clinically-derived cohorts, perhaps due to the underlying pathologic heterogeneity amongst clinically defined cohorts, the most significant TMEM106b association was in FTLD-TDP patients carrying GRN mutations [52, 62, 183, 207, 208]. An international GWAS including all subtypes of clinical FTLD was recently completed and found two novel single nucleotide polymorphisms (SNPs) associated with disease possibly related to immune function and lysosomal pathways and autophagy [59]. Finally, C9orf72 expansion is seen in a small subset of sporadic ALS (~5–10%) and less commonly FTD (~5%) [143]. Thus, the contribution of genetic modifiers to phenotypic variation in genetic and sporadic FTLD-TDP is evolving and these discrepancies highlight the importance of autopsy-confirmation for genetic and biomarker discovery studies in FTLD.
Progranulin (GRN)
Mutations in GRN located on chromosome 17q21 are the molecular genetic basis of about one quarter of all familial cases of FTLD-TDP [13, 15, 53, 162]. Pathogenic mutations in GRN are mainly nonsense and splice site mutations resulting in the loss of one GRN allele (i.e. null mutations); some mutations, however, are missense mutations causing mis-trafficking within the cell and a functional haploinsufficiency; both mechanisms result in progranulin protein haploinsufficieny. More than 70 different pathogenic mutations in GRN have been reported. Further, recent studies show that microRNA-132 and microRNA-212 repress TMEM106B expression through shared microRNA-132/212 binding sites in the TMEM106B 3'UTR and that endogenous neuronal TMEM106B proteins colocalize with progranulin proteins in late endo-lysosomes, while TMEM106B overexpression increases intracellular levels of progranulin. Thus, TMEM106B is an FTLD-TDP risk gene that alters progranulin pathways [44]. GRN mutation cases are exclusively associated with TDP-43 “subtype A” [139]. Interestingly, progranulin protein is not found in TDP-43 inclusions [140], but GRN mRNA expression from the normal allele is increased in cortical areas of neurodegeneration in GRN mutation carriers and this may be mediated by reactive proliferation of microglia in affected brain regions [45] . Low serum progranulin levels are found in the serum/plasma of GRN mutation carriers [61, 195] and thus provide a promising biomarker for potential emerging progranulin-restorative therapies [26].
C9orf72 hexanucleotide expansion
The expansion of a hexanucleotide (GGGGCC) repeat in a non-coding region of the C9orf72 gene was recently discovered [55, 180] and is the most common molecular etiology of hereditary and sporadic ALS and/or FTLD-TDP. C9orf72 encodes a protein of unknown function. Pathologic expansion repeats extend from approximately 30 to more than 1,000, and there appears to be no direct association between the severity of disease and expansion size above the normal range. These analyses may be confounded by differences in C9orf72 expansion in peripheral blood and various regions of CNS, as some correlations of repeat length with demographic features in FTD have been described for some specific brain regions [205]. The C9orf72 expansion is more common in patients with familial ALS and FTD-ALS than familial FTLD. Notably, however, TMEM106B, the risk gene for FTLD-TDP, has also been identified as a genetic modifier of FTD with C9orf72 expansions with the minor allele protective of developing FTD, but not MND [205]. Interestingly, the genotype that confers increased risk for developing FTLD-TDP has been associated with later age at onset and death in C9orf72 expansion carriers with FTD [69].
Neuropathologically, the majority of C9orf72 mutation cases have TDP subtype B [22, 141, 198] but unlike other TDP-43 proteinopathies, cases with the hexanucleotide expansion also have additional proteinacious inclusions of unclear clinical significance that are not reactive for TDP-43 (Figure 1). C9orf72 cases have small p62-positive NCIs and rare NIIs in cerebellar granular neurons and p62-immunoreactive star-shaped NCIs and occasional punctate NIIs in the hippocampus [2]. C9orf72 cases also have additional ubiquilin-positive pathology in cerebellum and hippocampus, and the presence of these at autopsy predicts the occurrence of pathological hexanuculceotide C9orf72 expansions [36, 103]. There are also foci of RNA aggregations in neuronal nuclei in these regions [55, 158]. Finally, the hexanucleotide repeat region is bi-directionally translated by an unconventional repeat-associated non-ATG translation of the expanded C9orf72 transcript to form aggregating dipeptide-repeat (DPR) proteins (poly-(Gly-Ala), poly-(Gly-Pro) and poly-(Gly-Arg), poly-(Pro-Ala) and poly-(Pro-Arg)) which also are predictive of C9orf72 expansion [10, 70, 161]. Indeed, DPR proteins are highly co-localized in p62-positive, TDP-43 negative, inclusions in FTLD-ALS spectrum cases with C9orf72 repeat expansion [144, 161] and share a similar morphology and regional distribution; although DPR are more widespread [10]. Double-labelling immunofluorescence studies of ubiquilin and DPR are lacking but the regional distribution and minimal co-localization with TDP-43 suggest a similar relationship to DPR as p62. Interestingly, there does not appear to be a correlation between DPR pathology and neurodegeneration [138]; however, recent cell and Drosophila model experiments suggest a potential toxicity of DPR protein accumulation distinct from RNA foci-associated gene dysregulation [124, 157, 219]. Indeed, substantial DPR pathology has been reported in early/pre-symptomatic C9orf72 autopsy cases with an absence or minimal TDP-43 neuropathology [11, 176]. Further, DPR proteins are detectable in the cerebrospinal fluid of C9orf72 mutation carriers and could serve as a useful biomarker for C9orf72 associated TDP-43 proteinopathies [201]. Finally, transcriptional silencing of mutant C9orf72 due to promoter hypermethylation is associated with lower RNA foci and DPR aggregate burden in human brains, and later age of death in FTD suggesting that expression of the mutant gene is indeed deleterious [136, 185]. Further work is needed to clarify the link between C9Orf72 expansion, p62, ubuiquillin, DPR aggregation, RNA foci and TDP-43 aggregation with neurodegeneration; however, presently it is TDP-43 accumulation that is most closely linked with neurodegeneration in ALS/FTLD-TDP [36, 138].
TARDBP
The discovery of mutations in TARDBP on chromosome 1 indicated that abnormal TDP-43 is sufficient to cause neurodegeneration [206], thereby confirming the initial discovery of the linkage of TDP-43 pathology to FTLD and ALS [172]. However, mutations in TARDBP account for only a small number, less than 4%, of FALS cases, and are rare causes of FTD. In limited autopsy studies, TDP-43 proteinopathy seen in TARDBP mutation cases is similar to that seen in sporadic ALS/FTLD-TDP; however, there may be more extensive proteinopathy outside motor areas than in sporadic cases [42].
VCP
VCP is located on chromosome 9p13.3-p12 and several pathogeneic missense mutations have been linked to a rare phenotype of hereditary inclusion body myopathy (IBM) associated with Paget disease of bone (PDB) and early-onset frontotemporal dementia (IBMPFD) [213, 214]. More recently, mutations in VCP have also been reported in patients with an ALS without dementia phenotype [108]. Human VCP (also called p97, ter94, or CDC48) is a 644 amino acid protein encoded by a gene with 17 exons. It is a member of the AAA-ATPase superfamily involved in multiple functions including: vesicle transport and fusion, 26S proteasome function, and assembly of peroxisomes [54, 155]. The neuropathology in FTLD-TDP with a VCP mutation is a unique subtype of FTLD-TDP, subtype D [139], characterized by numerous NIIs (Figure 2j). Identification of pTDP-43, but not VCP, within ubiquitin-positive inclusions supports the hypothesis that VCP mutations lead to a dominant-negative loss or alteration of VCP function culminating in impaired degradation of TDP-43.
Finally, it is noteworthy that in addition to the mutations noted above that cause ALS/FTLD-TDP, multiple pathogenic mutations in four other genes (including those encoding ataxin-2, optineurin, NIPA1 and angiogenin) for ALS and/or FTLD-TDP have been discovered that also are linked to TDP-43 pathology thereby suggesting that ALS and FTLD share similar disease mechanisms all of which involve TDP-43 pathology [86, 115, 119, 145].
Clinicopathological Correlations
FTLD-TDP can present clinically as bvFTD, FTD-ALS, CBS or PPA [64] (Figure 4). Of note, the majority of svPPA cases are associated with FTLD-TDP [78], in particular “subtype C” [139, 182]; while roughly half of bvFTD [64, 88, 182] and ~15% of CBS have TDP-43 neuropathology [133]. FTLD-TDP can also less commonly present with slow hesitant speech, consistent with lvPPA [154] and motor speech difficulties consistent with naPPA [78, 120, 196]. A small percentage of patients with FTLD-TDP neuropathology may present clinically with an amnestic disorder similar to AD, especially those with an older onset and co-morbid hippocampal sclerosis at autopsy [167]. The development of clinical motor neuron disease in FTD patients is highly associated with underlying TDP-43 neuropathology and is a poor prognostic marker [96].
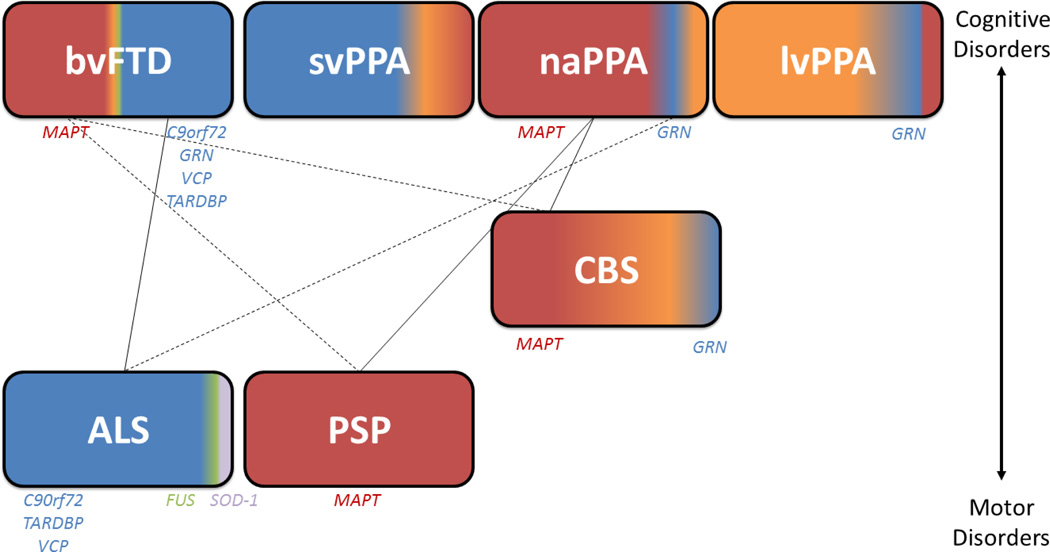
Figure 4. Clinicopathological and Genetic Associations in FTLD/ALS.
The schematic portrays relative frequencies of neuropathological subtypes of FTLD and pathogenic mutations associated with FTD clinical phenotypes arranged with predominant cognitive syndromes above and predominant motor disorders below (CBS is intermediate with largely mixed cognitive/motor features). Common associations between syndromes (i.e. ALS-bvFTD, PSP-naPPA) are identified with solid lines and dashed line represent less common co-morbid syndromes (i.e. ALS-naPPA, PSP-bvFTD, CBS-bvFTD). FTLD-Tau pathology (red) is found in virtually all PSP cases and the majority of naPPA. FTLD-Tau is also found in a significant proportion of CBS and bvFTD and rare in svPPA. TDP-43 pathology (blue) is found in almost all ALS and the majority of svPPA, while roughly half of bvFTD cased harbor FTLD-TDP at autopsy while FTLD-TDP pathology is less commonly found in naPPA and CBS. Atypical presentations of AD are seen in a significant proportion of CBS and less commonly in svPPA and naPPA, but very rarely in bvFTD. Finally, a small percentage of ALS has FUS or SOD-1 (green) pathology at autopsy and FUS is a rare substrate for bvFTD. Genetic etiologies linked to clinical phenotypes are written below in order of frequency; svPPA is largely a sporadic condition.
Hereditary forms of FTLD-TDP have specific associations with clinical phenotypes of FTD. The most frequent clinical presentation of FTLD-TDP with GRN mutation is bvFTD [128]; although there is considerable heterogeneity between patients within and between families, including language dysfunction, consistent with PPA variants while extrapyramidal symptoms (parkinsonism and CBS) are less frequent and ALS is extremely rare [43]. Mutant GRN has been associated with lvPPA as well [154]. C9orf72 expansion may present with ALS and/or several clinical FTD syndromes, most commonly bvFTD but also PPA [22, 103, 141]; interestingly, neuropsychiatric features uncommon to bvFTD have been described [198]. In addition, clinical cases of AD with FTLD-TDP with varying degrees of AD neuropatholoigic change or unknown neuropathology have been associated with C9orf72 expansions [14, 85, 142]. Other reported c9orf72 clinical phenotypes include neuropsychiatric disease [20], Huntington’s disease-like presentation [87] and multiple system atrophy [74]. C9orf72 expansion carriers with clinical ALS have a shorter disease duration than sporadic cases [39, 103] and C9orf72 expansion carriers with FTD may have a more rapid cognitive decline associated with more severe cortical atrophy compared with other forms of FTLD-TDP [103]; however, cases of slowly progressive C9orf72 mutation positive FTD with minimal cortical atrophy have also been reported [117]. Indeed, several studies find additional areas of cortical atrophy in C9orf72 FTD in the thalamus, parietal lobes and cerebellum on neuroimaging [103, 141, 190], while some cases may have minimal atrophy and non-progressive clinical symptoms [22, 117]. Further, C9orf72 ALS-FTD may have a longer disease duration than ALS-FTD without a mutation [190]; although a wide range of age at onset, death and disease duration has been reported [22, 93, 141, 193].Thus, significant heterogeneity exists for C9orf72-associated cases with potential multiple genetic or other modifying factors. Although the TARDBP mutations are most frequently associated with ALS and ALS-FTD clinical phenotypes, additional features of chorea and PSP-like presentations may be seen in patients with TARDBP mutations. Indeed, patients with “ALS-plus” symptoms (i.e. extrapyramidal, autonomic, oculomotor or cerebellar dysfunction) are more likely to harbor a pathogenic mutation in TARDBP, C9orf72 or VCP compared with sporadic cases [148].
Tauopathies (FTLD-Tau)
Neuropathology
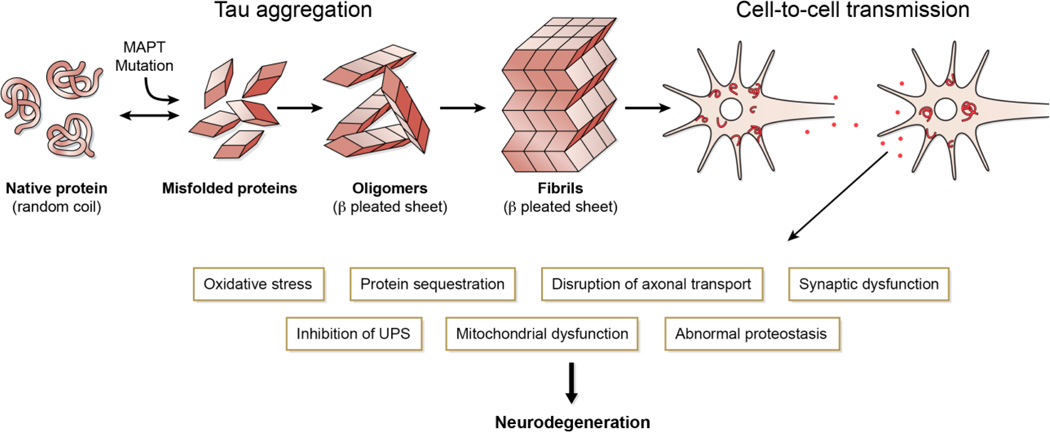
Roughly 45% of FTLD is caused by a diverse class of neurodegenerative diseases characterized by neuronal and glial inclusions composed of the microtubule-binding protein, tau (FTLD-Tau) (Figure 3). The discovery of multiple pathogenic mutations in MAPT associated with diverse FTD syndromes, formerly known as FTDP-17 and now called FTLD-Tau with MAPT mutation (see below), has led to the unequivocal evidence that tau abnormalities alone are sufficient to cause neurodegenerative disease (similar to previously described TARDBP mutations in ALS/FTLD-TDP) (Figure 5).
Figure 5. Tau-Mediated Neurodegeneration in FTLD-Tau.
Tau misfolding and aggregation into to beta-pleated sheet containing oligomers and fibrils occurs in familial FTLD-Tau due to MAPT mutations and in FTLD-Tau. This process results in loss of microtubule binding function and formation of cytosolic tau inclusions (red). Animal- and cell-model data suggest neuron-to-neuron transmission is central to disease pathogenesis and propagation. This process leads to multiple areas of cell-dysfunction (boxes).
As reviewed recently [134, 218], tau proteins are low molecular- weight MAPs that are abundant in the central nervous system (CNS), where they are expressed predominantly in axons, and at very low or negligible levels in astrocytes and oligodendrocytes. Human tau proteins are encoded on a single gene located on chromosome 17q21 with 16 exons leading to the generation of 6 different CNS tau isoforms generated by alternative splicing of 11 of these exons in the messenger RNA (mRNA) transcript. In the adult human brain, alternative splicing of exons 2, 3, and 10 generates 6 tau isoforms ranging from 352 to 441 amino acids in length, which differ by the presence of either 3 or 4 microtubule (MT) binding repeats (3R tau or 4R tau, respectively) consisting of repeat sequences of 31 or 32 amino acids each that are encoded by exons 9 to 12. Additionally, alternative splicing of exons 2 and 3 leads to the absence (0N) or presence of inserted sequences of 29 (1N) or 58 (2N) amino acids in the amino-terminal third of the molecule thereby resulting in 4R0N, 4R1N, 4R2N, 3R0N, 3R1N and 3R2N tau proteins at a 1:1 ratio of 3R to 4R tau in the adult CNS.
Tau functions by binding to and stabilizing MT and this process is regulated by phosphorylation. Several protein kinases and protein phosphatases have been implicated in regulating the phosphorylation state and thus the function of tau. The phosphorylation sites are clustered in regions flanking the MT-binding repeats, and increasing tau phosphorylation at multiple sites regulates MT binding [32]. More recently, tau has also been shown to be modified by acetylation [49, 156]. However, in both sporadic and familial tauopathies, tau is hyperphosphorylated and acetylated and it is this “abnormal” tau that is the principle component of the filamentous aggregates in neurons and glia that are the pathological hallmarks of these disorders. Similar to phosphorylation, acetylation of tau at the lysine 280 residue (i.e. acK280) in the second MT-binding motif of 4R tau also disrupts the MT-binding function of tau, but in addition also promotes tau aggregation in vitro [49]. Other lysine residues in the MT-binding motif may inhibit tau-polymerization and phosphorylation at these residues [50] and may inhibit degradation of abnormal tau [156]. The acK280 modification is disease-specific for pathological tau in tauopathies and is not present in normal control CNS tissue [49, 101, 102]. Comparison with multiple tau epitopes across various stages of AD neuropathology suggests a close association of acK280 modification with the amyloid properties (i.e. Thioflavin-S reactive) of AD tangles but also in Thioflavin-S negative inclusions in FTLD-Tau [101, 102]. Thus, a potential interplay between phosphorylation and acetylation modifications in tau may perturb normal tau function and promote pathological aggregation in various tauopathies. Finally, in the disease state, tau also may be nitrated [91] and glycated [129] which may contribute to disease pathogenesis.
FTLD-Tau can be subdivided into several neuropathological diagnoses and classified based on the predominant tau isoforms that are present in the inclusion bodies (i.e. 3R, 4R or equal 3R:4R ratio).
3R Tauopathy: Pick’s Disease
The sole 3R predominant tauopathy is Pick’s disease (PiD), which historically referred to clinical FTD in general but now this term is reserved for the neuropathological diagnosis described here due to the neuropathological heterogeneity of FTD (Figure 4). On gross-examination, there is often severe “knife-edge” atrophy of the frontotemporal neocortex. The diagnostic histological feature of PiD is the Pick body [163] (Figure 2a). Pick bodies are well-circumscribed, spherical, argyrophilic, and tau-immunoreactive neuronal intracytoplasmic inclusions. In addition, there are swollen achromatic so-called “ballooned” neurons or Pick cells, neuronal loss, and astrocytosis. Pick bodies are found most abundantly in the granule cells of the dentate gyrus. Pick bodies are found at lower densities in the pyramidal neurons of the frontal and temporal neocortex. The distribution of Pick bodies may be uni- or bilaminar, and this difference may reflect the stage of progression of the disease [9]. A prominent band may be seen in layer II and upper layer III, and a band in layer IV. These neurons can be contrasted with those in AD, in which NFT are found predominantly in the large pyramidal neurons of layers III and V, the major cortico-cortical projecting neurons. Spatial pattern analysis has shown that Pick bodies appear in regular clusters throughout affected cortical areas [7]. Pick bodies are best identified using tau-directed immunohistochemistry. They have a similar staining pattern to NFTs, but the immunohistochemical and biochemical profile of tau in Pick disease is different from that in AD: In Pick disease, IHC shows that 3R tau isoforms predominate in Pick bodies [16, 56] and biochemical western blot studies support these IHC findings [221]. A subset of inclusions are thioflavin S-positive and among these, are the ones that that contain 4R-tau immunoreactivity by IHC with acetylation-specific antibodies directed at K280 [101]. Ultrastructurally, Pick bodies contain 15 nm diameter filaments and do not appear to have a limiting membrane [163]. Ballooned-neurons can be labeled with antibodies specific for the heat-shock protein, β-crystallin. The significance of ballooned neurons in the pathogenesis of PiD is unclear and they are not present in all cases. Finally, PiD also contains numerous tau-positive glial inclusions in grey and white matter.
4R Tauopathies: Corticobasal Degeneration
Similar to PiD, current nomenclature of FTLD-Tau reserves the term corticobasal degeneration (CBD) for the neuropathological diagnosis of the 4R tauopathy [57] described below. Corticobasal syndrome (CBS) refers to the clinical diagnosis of patients who present with an asymmetric Parkinsonian disorder [6], which was originally linked to CBD. This clinical syndrome is now known to encompass several potential underlying neuropathologies outside of CBD [133], which has necessitated the change in nomenclature (see below). On gross examination, the brain is atrophied asymmetrically in the posterior frontal and parietal lobes; both the pre- and postcentral gyri may be affected. There is also pallor of the substantia nigra in the majority of cases. Microscopically, neuron loss may be more severe in the outer cortical laminae and generate status spongiosus. The white matter underlying the affected areas of cortex may be rarefied and display a reactive astrocytosis. Ballooned neurons are often readily seen throughout the neocortex. There is usually severe neuronal loss and accompanying astrocytosis in the substantia nigra. A characteristic feature is the intraneuronal basophilic inclusion. These “corticobasal inclusions” are argyrophilic and fibrillar, and are labeled by anti-ubiquitin and anti-tau antibodies. Histologically, they resemble the NFTs of PSP. Ultrastructurally, the filaments in these CBD inclusions are mainly straight, with a diameter of 15 nm [212]. In addition to these corticobasal inclusions, small neuronal tau-positive inclusions and neuropil threads can be found in the superficial layers of the cortex.
The most prominent microscopic features of this disorder are clusters of astrocytic tau-positive processes that coalesce to form astrocytic plaques (Figure 2c). There are also tau-positive inclusions in oligodendrocytes, referred to as “coiled bodies.” Both the astrocytic and oligodendroglial inclusions in CBD are labeled by anti-ubiquitin and anti-tau antibodies. The tau protein in CBD is predominantly 4R tau, and these findings are supported by biochemical western blot studies [65, 223]. Moreover, CBD inclusions are robustly reactive for the acK280 acetylation modification of tau [102]. Interestingly, CBD inclusions do not react with amyloid-binding dyes and lack several tau epitopes linked to more “mature” and extracellular ghost tangles in AD such as C-terminal truncation epitopes [17, 82]. These morphological and biochemical differences in tangle formation between CBD and PSP (see below) as well as with AD are currently unclear, but provide a possible avenue for future efforts in biomarker discovery to differentiate these tauopathies ante-mortem.
4R Tauopathies: Progressive Supranuclear Palsy
PSP pathologically is also a 4R predominant tauopathy with significant white matter pathology predominantly in the brainstem and subcortical structures [220, 223]. Macroscopically, the substantia nigra and locus coeruleus often appear pale and cortical atrophy is variable. Histology reveals tangles, neuropil threads, glial inclusions, neuronal loss, and astrocytosis [57]. The predominant hallmarks of PSP are the 4R tau positive tangles and tufted astrocytes (Figure 2b). The NFTs are found in the substantia nigra, globus pallidus, subthalamic nucleus, nucleus basalis of Meynert, pretectal area, tegmentum of the midbrain and pons, locus coeruleus, raphé nuclei, and the nuclei of various cranial nerves as well as the cerebellar dentate nucleus. The tangles are readily seen by silver impregnation methods but are best visualized by tau IHC. Electron microscopy demonstrates that the tangles contain straight filaments of 12–15 nm, which in turn are composed of six or more protofilaments of 2–5 nm [160]. Paired helical filaments (PHFs) similar to those seen in AD, and intermediate forms have been described. Many astrocytes have inclusions called tufted astrocytes, and the cell bodies containing these inclusions may be tuft-shaped or less frequently thorn-shaped. Oligodendrocytes may also contain tau-positive “coiled-body” inclusions. PSP inclusions are largely negative for thioflavin-S and robustly positive for the acK280 acetylation modification in tau [102]. Similar to CBD, PSP cases lack several “late” C-terminal truncation tau epitopes seen in AD neuropathology [17, 81].
Recently, globular glial tauopathies (GGT) have been described in 22 cases as a new neuropathological entity [1]. GGT is a 4R tauopathy with astrocytic and oligodendridic tau inclusions similar to PSP but they differ by the lack Gallyas-reactivity. Further there is often minimal subcortical neuronal loss in the dentate and subthalamic nuclei and very extensive white matter pathology. Three GGT types have been delineated corresponding to involvement in fronotemporal (Type I), motor/corticospinal tract (Type II) or both (Type III) corresponding to a range of clinical syndromes including ALS, FTD and PSP [1].
4R Tauopathies: Argyrophilic Grain Disease (AGD)
The term ‘argyrophilic grain’ derives from its appearance using some (e.g. Gallyas), but not all, silver impregnation methods; however, they are best visualized using 4R isoform specific anti-tau antibodies (Figure 2e). Grains are small (4–8 µm diameter), round or spindle-shaped structures found mainly in the cortical neuropil and to a lesser degree in the underlying white matter. Grains are found mainly in dendrites and dendritic branches and some axons. Other non-specific lesions include: pre-tangle neurons, coiled bodies, tau-immunoreactive astrocytes, swollen achromatic, or ‘ballooned,’ neurons, NFTs, and neuropil threads. The tau-immunoreactivity in astrocytes is more diffuse than the compact fibrillar, tau-immunoreactive inclusions of tufted astrocytes of PSP. Ultrastructurally, grains contain straight filaments of 10–20 nm diameter and tubular structures of 25 nm diameter. Although AGD may be found in the absence of other diseases, and it is mainly a 4R tauopathy [222, 223] and it is most frequently occurs with more common neurodegenerative disorders including AD, the 4-repeat (4R) tauopathies, PSP and CBD, and other molecular pathologies including dementia with Lewy bodies, Parkinson disease dementia, and Parkinson disease. AGD may also be found as a comorbidity in older patients with hippocampal sclerosis. Various staging schemes have been proposed based on the density and distribution of lesions in the medial temporal lobe, adjacent structures, neocortex and subcortical nuclei [60, 186]. Grains in AGD show reactivity with acK280 acetylation modification of tau [101], but may lack other potential acetylation epitopes [76].
3R/4R Tauopathies: Primary age-related tauopathy (PART)
PART is a 3R/4R tauopathy that may readily be distinguished from AD by the presence of NFT in medial temporal lobe structures and the complete or nearly complete absence of Aβ plaques. This distinction is now recognized in the recent National Institute on Aging-Alzheimer’s Association (NIA-AA) diagnostic criteria for AD [159] and thus, since the neuropathology can occur with minimal cognitive symptoms the term “tangle predominant senile dementia” has been replaced with PART [51].The most characteristic finding is the presence of neuronal loss, gliosis, and frequent NFT, including extracellular NFT, called ghost tangles, in the hippocampus, parahippocampal gyrus, and entorhinal cortex (Figure 2f). This is a common finding in patients of advanced age [51]. In more advanced disease, NFT may be seen in the nucleus basalis of Meynert, the amygdala, periaqueductal gray matter, locus coeruleus, and other regions; but NFT are rare in the isoocortex of PART. Biochemical studies indicate that fractions enriched for insoluble tau reveal no difference in the tau isoform ratio (3R:4R) in PART as is the case in AD [188] and tangles are similarly detected using phosphorylation-dependent tau specific MAbs. Fine structural analysis of the NFT reveals mainly PHFs similar to those seen in AD. In addition, acK280 reactivity in PART is also similar to acetylation of tau in AD [101]. Tangle predominant senile dementia was previously categorized as FTLD-Tau [140] and there is considerable pathological overlap between PART, including lack of an association with APOE 4 genotype, suggesting a pathway of disease independent from AD [51]; however, these biochemical and topographic distribution similarities to AD, together with varying rates of progression of Aβ plaque and tau neurofibrillary pathology have suggested by some that PART is not a separate process from AD [31]. These discrepancies are a matter of ongoing study and debate.
Genetics
Risk Factors in Sporadic FTLD-Tau Disease
Two extended haplotypes (H1, H2) cover the human MAPT gene, and there is complete disequilibrium between polymorphisms that span the gene (which covers approximately 100 kb of DNA). This suggests that the establishment of the two haplotypes was an ancient event, and that either recombination is suppressed in this region, or recombinant genes are selected against. The more common haplotype (H1) is significantly overrepresented in patients with PSP [12] and CBD [92], but there is no difference between the MAPT H2 haplotype or H2/H2 genotype frequency in PiD cases when compared with control subjects (Morris et al., 2002a). To date, no specific genetic locus has been associated with AGD. However, a 40 kb deletion at 17p13.2 encompassing the cystinosin, lysosomal cystine transporter (CTNS) gene has recently been described suggesting that this may be a candidate gene for AGD [211]. No mutation has been reported in MAPT in PART, but haplotype analysis demonstrates a strong association with the MAPT H1 haplotype. Next-generation re-sequencing of MAPT followed by association analysis showed an association between PART and two polymorphisms in the MAPT 3' untranslated region (UTR). These results suggest that haplotype-specific variation in the MAPT 3' UTR underlies an Aβ-independent mechanism for neurodegeneration in PART [188]. There are discrepant findings of an association of PART and APOE genotype and the potential overlap of PART with AD remains uncertain [31].
To identify common genetic variation contributing to PSP, a GWAS found significant signals associated with PSP risk in syntaxin 6 (STX6), eukaryotic translation initiation factor 2-α kinase 3 (EIF2AK3), and myelin-associated oligodendrocyte basic protein (MOBP) genes [89]. Two independent variants in MAPT affect risk for PSP, one of which influences MAPT brain expression. The genes implicate proteins for vesicle-membrane fusion at the Golgi-endosomal interface, the endoplasmic reticulum unfolded protein response and, and a myelin structural component.
Hereditary FTLD-Tau: FTLD-Tau with a MAPT mutation
FTLD-Tau with MAPT mutations (Figure 3), previously called FTDP-17, is now distinguished from chromosome 17-linked families who have a mutation in the GRN (Figure 3). MAPT mutations, of which more than 40 have been identified as pathogenic, cause tau dysfunction by several mechanisms [63, 90]. First, intronic and some exonic mutations affect the alternative splicing of exon 10 and consequently alter the relative proportions of 3R and 4R tau which may disturb normal tau function and lead to increased cytoplasmic tau and inclusion formation. Missense mutations impair the ability of tau to bind MTs and to promote MT assembly. Finally, some mutations also promote the assembly of tau into pathological amyloid filaments.
Familial cases with MAPT mutations typically have atrophy of the frontal and temporal lobes and microscopically show neuronal loss, astrocytosis, microvacuolation, and swollen neurons. There is a spectrum of tau pathology associated with MAPT mutations, including intraneuronal neurofibrillary -tangle-like inclusions (Figure 2d), neuronal globose tangle-like inclusions, intraneuronal Pick body–like inclusions, astrocytic tangle-like inclusions, and oligodendroglial inclusions resembling coiled bodies and dystrophic neurites. Mutations in MAPT generate a heterogeneous biochemical phenotype as well: mutations may generate predominantly either 3R or 4R tau, or a combination of the two. Thus, an extraordinarily wide-range of tau pathology has been observed in these familial cases and aside from tau inclusion pathology, there is no unifying or distinct neuropathological finding to diagnose these familial conditions at autopsy [63]. Indeed, on occasion cases may appear pathologically consistent with sporadic tauopathies (i.e. PiD, CBD. PSP) and require genetic testing for diagnosis as disorders caused by MAPT mutations. Tau inclusions are similarly hyperphosphorylated in hereditary tauopathies as in sporadic disease (i.e. PiD, CBD, PSP). In addition, p.P301L and IVS10+16 mutation cases, which contain predominantly 4R tau isoforms, have robust reactivity for ack280, despite the absence of thioflavin-S reactive amyloid tau inclusions [101].
Clinicopathological correlations
FTLD-Tau comprises approximately half of all cases with a bvFTD clinical syndrome [64, 88, 182] (Figure 4) and this includes PiD and FTLD-Tau with a MAPT mutation, and less commonly CBD and PSP. Further, PiD most often presents clinically with bvFTD but also has been reported in association with PPA and CBS phenotypes [182]. In a large autopsy series of patients with clinical CBS, CBD comprised only 35% of cases, with 13% having PSP neuropathology and 23% with AD (in addition to the afore-mentioned ~15% with FTLD-TDP) [133]. Conversely, CBD neuropathology can often present with cognitive syndromes in the absence of motor features of CBS [165]. Thus, CBS is a very heterogeneous clinical syndrome and recent clinical criteria have been proposed to help identify clinical features that may identify underlying CBD neuropathology [6]. In contrast, the clinical syndrome of PSP, and in particular the supranuclear vertical gaze palsy and presence of early postural instability, is highly associated with PSP neuropathology [135, 182]. As such, PSP patients are an attractive patient population for emerging tau-directed therapies; however, despite the specificity of these clinical features, the criteria are not very sensitive and patients with PSP neuropathology may have other clinical manifestations. Indeed, CBS and PSP patients may present with behavioral changes consistent with bvFTD or non-fluent motor speech difficulties consistent with naPPA, prior to, or after the development of the movement disorder. Therefore, the presence of extrapyramidal symptoms suggestive of CBS/PSP in bvFTD or naPPA likely reflect an underlying tauopathy in most cases [64]. Finally, the majority of naPPA patients are found to have underlying FTLD-Tau in most autopsy series [78, 88, 109, 120, 154, 182] but a significant proportion may have underlying FTLD-TDP [78, 120, 154, 196] or AD at autopsy [3, 78, 120, 154]. FTLD-Tau with MAPT mutations are extremely heterogeneous and have been associated largely with bvFTD and PPA, but PSP and CBS clinical phenotypes have also been described [63].
The associations of PART and AGD with specific clinical symptoms of dementia are less defined. AGD has a varied clinical presentation with episodic memory loss observed in most subjects, but behavioral abnormalities, personality changes and emotional and mood imbalance similar to bvFTD have also been described [60] . Finally, PART is usually a late-onset (>80 years) amnestic disorder [107] although some cases may have bvFTD clinical features as well or be clinically silent [51].
Other forms of FTLD including FTLD-FUS, FTLD-U and dementia lacking distinctive histopathology (DLDH)
Following the discovery of mutations in TARDBP in FALS, the search for other RNA/DNA binding proteins led to the discovery of mutations in FUS in other FALS kindreds and that the ubiquitinated inclusion bodies in these cases contained FUS protein [123, 209]. Interestingly, the inclusion bodies of another group of FTLD-U entities was also found to be characterized by inclusion bodies containing FUS protein, but in the absence of FUS mutations and these include: basophilic inclusion body disease (BIBD) [164], neuronal intermediate filament (IF) inclusion disease (NIFID) [171], and atypical FTLD-U [203] (Figure 2l). Collectively FTLD-FUS accounts for <5% of all FTLD (Fig. 2). Both FUS and TDP-43 are RNA-binding proteins and have similar structures and both are involved in transcriptional regulation. Neuropathologically, there are similarities; TDP-43 and FUS migrate from their normal nuclear location to the cytoplasm where they form relatively insoluble aggregates. In vitro, several of the mutations appear to disrupt the import of TDP-43 or FUS into the nucleus which may result in it’s a nuclear loss of function as well as a potential gain of toxic function as FUS aggregates in the cytoplasm. The family of three FET (FUS, EWS1, and TAF15) RNA-binding proteins which are expressed in all tissues and almost all cell types are all components of the inclusions in these sporadic FUS diseases [169]. Clinically FTLD-FUS with atypical FTLD-U often presents with bvFTD at a younger with atypical neuropsychological features [197, 203] while rare reports of FTLD-FUS with NIFID and BIBD include a more varied age at onset and clinical phenotype [126, 131, 197].
Advances in the genetics and molecular pathology of FTLD have consigned most cases previously described as FTLD-U (which was formerly known as DLDH) or FTLD-U plus ALS to FTLD-FUS or FTLD-TDP proteinopathy [140]. Today, very few cases in autopsy series have FTLD with inclusions containing proteins of the ubiquitin-proteasome system (FTLD-UPS) that are tau TDP-43 or FUS negative inclusions. At present, only one rare disease entity is assigned to this entity and that is FTLD-UPS with charged multivesicular body protein 2B (CHMP2B) gene mutation (Figure 2m). Human CHMP2B is a component of the endosomal secretory complex, which becomes dysregulated by the gene defects. There have been very few neuropathologic studies of this rare autosomal dominantly inherited disease.
FTLD not otherwise specified (FTLD-NOS) is an entity reserved for cases where the molecular pathology is not known, or that the case has not been investigated using anti-ubiquitin, tau, FUS, or TDP-43 antibodies [140]. Historically, this entity included dementia lacking distinctive histology DLDH cases [121]. Most of these cases have now been screened with molecular pathology-specific antibodies and most cases now have been re-assigned to one of the FTLD entities described above. There remain, however, rare cases with the stereotypical features of FTLD, but without any inclusions having been detected. The nosology of these cases remains uncertain. A recent entity referred to as FTD “phenocopy” has emerged to describe minimally progressive FTD cases that may represent decompensated psychiatric disorders or other non-neurodegenerative disease etiologies but autopsy studies are lacking [118].
FTLD BIOMARKER STUDIES
Due to the complex clinciopathologic relationships in FTLD (Figure 4) there is an urgent need for disease-specific biomarkers to improve ante-mortem diagnostics. Several modalities have been employed for FTLD biomarker development including neuroimaging, biofluid, genetic and clinical measures. A desirable biomarker will have sufficient sensitivity and specificity for FTLD-specific neuropathology and optimally have low-cost and minimal invasiveness.
As mentioned, differentiation of FTLD neuropathology from atypical AD is a critical first step, as this would change current clinical management since approved AD therapies may worsen FTD [26]. Clinical features of early episodic memory loss and visusopatial impairment are suggestive of underlying AD in patients with an FTD behavioral disorder [178] or PPA [75]; however clinical measures require extensive training and have ceiling effects which may limit use in clinical trials. Biofluid and neuroimaging biomarkers would be advantageous to follow as surrogate end-points of potential disease-modifying therapies. Well-studied cerebrospinal fluid (CSF) biomarkers such as total-tau (t-tau) and amyloid-beta (Aβ1–42) can accurately distinguish autopsy-confirmed AD from controls [191] and FTLD [19, 105, 202], with AD cases having higher t-tau: Aβ1–42 ratio. Indeed, CSF t-tau: Aβ1–42 ratio may provide a substantial improvement over clinical diagnosis in differentiating atypical AD from FTD [105, 202]. Further, AD is predominantly a grey matter (GM) disease, compared with the significant white matter (WM) involvement in FTLD, and as such, diffusion tensor imaging (DTI) approaches appear to approach meaningful levels of diagnostic accuracy in differentiating AD from FTLD in autopsied cases [150–152]. There are still limitations in the wide-spread use of CSF biomarkers for AD in clinical practice based on intra- and inter-lab sources of variation at pre-analytical, analytical and post-analytical stages [116, 192, 210], but there are international cooperative efforts to standardize these assays [147]. In vivo imaging of amyloid-beta [47] may also be a useful tool to identify atypical cases of AD with an FTD clinical phenotype; however this is not specific and a significant proportion of FTLD cases may have low levels of co-morbid AD neuropathology [202]. Thus, FTLD-specific biomarkers are crucial.
After exclusion of atypical AD cases there is still considerable heterogeneity of FTLD neuropathology and a reasonable next step in diagnostic algorithm would be differentiation of the two main classes of FTLD neuropathology: FTLD-Tau from FTLD-TDP, as disease protein-targeted therapies are already in development such as those targeting tau [37, 218]. Since FTLD-TDP does not have significant phospho-tau pathology there may be less phosphorylated tau (p-tau) released into the CSF compared to FTLD-Tau. Indeed, although both FTLD-Tau and FTLD-TDP have lower levels of p-tau and t-tau compared to controls, FTLD-TDP and ALS have lower levels of p-tau and p-tau:t-tau ratio compared with FTLD-Tau [79, 97]. CSF measurements of neurofilament light chains, a marker of axonal injury and neuronal loss, has found elevated levels in clinical FTD cohorts compared with controls and other neurodegenerative diseases [189, 194], with potential prognostic utility suggested by association with FTD disease severity in one study [189]. Further, exploratory proteomics-based approach has identified several other potential CSF biomarker candidates for FTLD-TDP [94] and others have developed assays to detect specific forms of tau [23, 24, 137] which may be helpful in differentiating FTLD-Tau and subtypes within this group. Plasma [66] and CSF [199] measurements of TDP-43 pathology have yet to find specificity to differentiate TDP-43 proteinopathies from controls; however novel MAbs directed at various epitopes on TDP-43 [125] may prove useful for future biomarker studies. Novel biofluid analytes will require further validation in future studies with large autopsy-confirmed samples and require efforts for reducing inter-lab sources of variability before wide-spread clinical use.
Both FTLD-Tau and FTLD-TDP are associated with widespread ventromedial and dorsolateral frontal and anterior temporal GM loss compared with healthy control patients using magnetic resonance imaging (MRI). Direct comparison of neuropathological groups finds subtle differences in MRI cortical atrophy patters that may be helpful in diagnosis (reviewed by [215]). Based on neuropathological observations of higher relative WM burden in FTLD-Tau compared to FTLD-TDP, comparisons of DTI imaging in autopsy-confirmed cases finds diagnostic accuracy for FTLD-Tau and the WM degeneration was confirmed on neuropathological examination of subjects who were imaged ante mortem [149]. Finally, the recent development of tau-specific radioligands [46, 146] holds great promise for a non-invasive method to identify FTLD-Tau cases and studies to demonstrate this are currently ongoing. Since the current clinical definitions of bvFTD, CBS and PPA variants do not correspond to a particular neuropathology (Fig. 4) it is not possible to compare clinical diagnostic accuracy with FTLD-Tau or FTLD-TDP specific biomarkers. Instead, prospective studies using these emerging biomarkers will be critical in refining clinical criteria to develop endophenotypes through identification of key clinical features that predict FTLD-Tau or FTLD-TDP neuropathology (e.g. bvFTD-Tau vs bvFTD-TDP).
Hereditary forms of FTLD provide a unique opportunity for biomarker discovery as pathogenic mutations do reliably predict underlying neuropathology, in contrast to clinical syndrome. Detection of hereditary cases is aided by use of a formal pedigree analysis to identify symptomatic individuals with a high likelihood of having an underlying FTLD-pathogenic mutation [217]. Further, study of pre-symptomatic individuals within families that harbor pathogenic mutations may be useful to understand the longitudinal progression of biomarkers in early stages of disease [26]. Indeed, there are signs of network dysfunction in pre-symptomatic GRN [58] and C9orf72 mutation carriers [132]. As afore mentioned, serum progranulin levels [195] and CSF DPR levels [201] may prove to be useful biomarkers for GRN and C9orf72 mutation cases, respectively. While these hereditary forms of FTLD may be attractive for clinical trial development for therapeutics specific for the mutation (e.g. progranulin restorative therapy), it is unclear if inclusion of hereditary cases with sporadic disease would influence disease outcome measurements for more broad tau or TDP-directed therapies. For example, C9orf72 disease contains additional protein inclusions [2, 10, 36, 161], additional clinical symptoms [148, 198] and possibly a worse prognosis compared with sporadic forms of the disease [39, 103]. Further, FTLD-Tau with MAPT mutations usually have a much earlier age at onset. Thus, disease modifying therapeutic trials targeting tau or TDP would most likely benefit from a stratified analytic approach, similar to APOE genotype in AD clinical trials. Finally, SNPs may also provide a potential non-invasive method to help improve diagnostics in sporadic disease. Simultaneous evaluation of multiple SNPs from autopsy-confirmed FTLD GWAS [89, 207] finds several SNPs over-expressed in FTLD-Tau or FTLD-TDP in a clinically mixed group of sporadic autopsy-conformed cases [153]. In a study of sporadic bvFTD, the risk allele in FTLD-Tau associated SNP in MOBP was associated with a shorter disease duration and WM loss on DTI in the midbrain and long association fibers [104]. These studies highlight the potential usefulness of SNP genotyping as diagnostic and prognostic markers, although future studies in large populations of FTD patients with known pathology from diverse ethnic backgrounds are needed for confirmation of these associations. In addition, next-generation sequencing advancements will most likely reveal multiple new variants associated with forms of FTLD for future studies.
Most likely a combination of markers, rather than a single marker alone will have sufficient sensitivity and specificity to accurately diagnose the underlying molecular etiology of FTLD. Indeed, a combination of neuropsychological measures with neuroimaging data improves diagnostic accuracy in PPA [95]. Further, AD-associated biofluid analytes are highly correlated with regional GM density on MRI in FTLD/ALS; Low p-tau levels correlate with degeneration in motor area GM and WM in ALS [79] and low t-tau levels are associated with frontal and temporal regional atrophy in FTLD patients [80]. Indeed, in a mixed AD and FTLD cohort, GM density was predictive of CSF t-tau:Aβ levels, and predicted CSF-values based on GM density in ventromedial prefrontal (low t-tau:Aβ) and posterior neocortical regions (high t-tau:Aβ) was accurate in identifying underlying neuropathology, suggesting quantitative MRI could potentially serve as a surrogate for CSF biomarker measures [150].
Finally, FTLD-associated GWAS-derived SNPs predictive of FTLD-Tau or FTLD-TDP were found to correlate with measures of GM and WM degeneration, suggesting that genetic variants may influence anatomic degeneration [153]. Thus, multimodal assessments provide converging evidence for biomarker validation. Future work integrating multiple modalities in large data-sets of well-annotated autopsy-confirmed cases will be critical for defining clinically useful diagnostic algorithms for FTLD. Due to the relative rarity of these disorders compared with AD, large multi-center efforts will be necessary. Recent international multi-center clinical trials of bvFTD [27] and PSP [28] have proven the feasibility of such efforts. Indeed, longitudinal observational studies are currently underway in Europe (i.e. Genetic Frontotemporal Dementia Initiative- GENFI) and the US (i.e. Advancing Research and Treatment for Frontotemporal Lobar Degeneration Consortium- ARTFL) and Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects- LEFFTDS).
CONCLUSIONS
The accumulation of different pathologically misfolded proteins in diverse inclusion bodies is a common feature of both FTLD-Tau and FTLD-TDP that comprise the sporadic and familial neurodegenerative disorders presenting with the clinical spectrum of FTLD/ALS. The discovery of mutations in MAPT, leading to abnormal filamentous inclusions, demonstrates that tau dysfunction is sufficient to produce neurodegenerative disease. Similarly, the discovery of mutations in TARDBP in familial ALS indicate that TDP-43 dysfunction is sufficient to cause disease. The causal links between GRN, VCP, and C9orf72 mutations and TDP-43 proteinopathy are indirect and require further research to be elucidated. The identification of additional gene mutations in FTLD or polymorphisms, such as TMEM106B, at distinct genetic loci that either cause or are risk factors for disease will provide additional insights into disease pathogenesis, as well as the development of novel strategies for treatment and prevention. Notably, the evidence that tau pathology can be transmitted in animal models opens up new avenues to pursue mechanistic studies of disease progression as well as novel strategies to block the spread of tau pathology and it will be interesting to determine if TDP-43, FUS and other FET pathologies can be transmitted in laboratory animals to create compelling model systems to study the pathogenesis of these FTLD pathologies [83, 112].
Finally, since a current limitation in clinical practice is the inability to reliably diagnose specific FTLD neuropathologies prior to autopsy, we expect that a multi-modal approach utilizing, clinical, genetic, neuroimaging and biofluid FTLD-specific biomarkers will be central to accurately diagnose FTLD-spectrum pathology ante mortem [25, 26, 77, 106]. This approach will require discovery of new more informative biomarkers for FTLD, but this will certainly enhance power for clinical trials focused on slowing or preventing transmission of tau, TDP-43 and other FTLD-associated pathologies and work towards the goal of defining clinical endophenotypes of FTD.
Acknowledgments
Support for this work was provided by grants from the National Institute on Aging of the National Institutes of Health (PO1-AG03991 and P50-AG05681) and from the Alzheimer’s Drug Discovery Foundation to NJC and P30-AG10124 (JQT and VMV), PO1-AG17586 (JQT, VMV, and VM-YL), PO1-AG032953 (JQT, VMV, and VM-YL) and NS088341 (DJI). We would also like to thank the members of the Knight Alzheimer’s Disease Research Center, Washington University, St. Louis, MO, and the Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia, PA, who contributed to the work, and the many patients studied and their families, for making the research reviewed here possible.
References
- 1.Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B, Giaccone G, Hatanpaa KJ, Holton JL, Josephs KA, et al. Globular glial tauopathies (GGT): consensus recommendations. Acta neuropathologica. 2013;126:537–544. doi: 10.1007/s00401-013-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta neuropathologica. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 3.Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer's disease. Brain : a journal of neurology. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 4.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Annals of neurology. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochemical and biophysical research communications. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong R, Cairns N, Lantos P. The spatial patterns of Pick bodies, Pick cells and Alzheimer's disease pathology in Pick's disease. Neuropathology : official journal of the Japanese Society of Neuropathology. 1999;19:64–70. doi: 10.1046/j.1440-1789.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong RA, Cairns NJ. Different molecular pathologies result in similar spatial patterns of cellular inclusions in neurodegenerative disease: a comparative study of eight disorders. J Neural Transm. 2012;119:1551–1560. doi: 10.1007/s00702-012-0838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong RA, Cairns NJ, Lantos PL. Laminar distribution of pick bodies, pick cells and Alzheimer disease pathology in the frontal and temporal cortex in Pick's disease. Neuropathology and applied neurobiology. 1999;25:266–271. doi: 10.1046/j.1365-2990.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 10.Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baborie A, Griffiths TD, Jaros E, Perry R, McKeith IG, Burn DJ, Masuda-Suzukake M, Hasegawa M, Rollinson S, Pickering-Brown S, et al. Accumulation of dipeptide repeat proteins predates that of TDP-43 in Frontotemporal Lobar Degeneration associated with hexanucleotide repeat expansions in C9ORF72 gene. Neuropathology and applied neurobiology. 2014 doi: 10.1111/nan.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Human molecular genetics. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 13.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 14.Beck J, Poulter M, Hensman D, Rohrer JD, Mahoney CJ, Adamson G, Campbell T, Uphill J, Borg A, Fratta P, et al. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. American journal of human genetics. 2013;92:345–353. doi: 10.1016/j.ajhg.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, et al. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alzheimer disease and associated disorders. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- 16.Bell K, Cairns NJ, Lantos PL, Rossor MN. Immunohistochemistry distinguishes: between Pick's disease and corticobasal degeneration. Journal of neurology, neurosurgery, and psychiatry. 2000;69:835–836. doi: 10.1136/jnnp.69.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry RW, Sweet AP, Clark FA, Lagalwar S, Lapin BR, Wang T, Topgi S, Guillozet-Bongaarts AL, Cochran EJ, Bigio EH, et al. Tau epitope display in progressive supranuclear palsy and corticobasal degeneration. J Neurocytol. 2004;33:287–295. doi: 10.1023/B:NEUR.0000044190.96426.b9. [DOI] [PubMed] [Google Scholar]
- 18.Bhardwaj A, Myers MP, Buratti E, Baralle FE. Characterizing TDP-43 interaction with its RNA targets. Nucleic acids research. 2013;41:5062–5074. doi: 10.1093/nar/gkt189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian H, Van Swieten JC, Leight S, Massimo L, Wood E, Forman M, Moore P, de Koning I, Clark CM, Rosso S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieniek KF, van Blitterswijk M, Baker MC, Petrucelli L, Rademakers R, Dickson DW. Expanded C9ORF72 hexanucleotide repeat in depressive pseudodementia. JAMA neurology. 2014;71:775–781. doi: 10.1001/jamaneurol.2013.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bigio EH, Wu JY, Deng HX, Bit-Ivan EN, Mao Q, Ganti R, Peterson M, Siddique N, Geula C, Siddique T, et al. Inclusions in frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) and amyotrophic lateral sclerosis (ALS), but not FTLD with FUS proteinopathy (FTLD-FUS), have properties of amyloid. Acta neuropathologica. 2013;125:463–465. doi: 10.1007/s00401-013-1089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain : a journal of neurology. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borroni B, Gardoni F, Parnetti L, Magno L, Malinverno M, Saggese E, Calabresi P, Spillantini MG, Padovani A, Di Luca M. Pattern of Tau forms in CSF is altered in progressive supranuclear palsy. Neurobiology of aging. 2009;30:34–40. doi: 10.1016/j.neurobiolaging.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Borroni B, Malinverno M, Gardoni F, Alberici A, Parnetti L, Premi E, Bonuccelli U, Grassi M, Perani D, Calabresi P, et al. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology. 2008;71:1796–1803. doi: 10.1212/01.wnl.0000335941.68602.39. [DOI] [PubMed] [Google Scholar]
- 25.Boxer AL, Gold M, Huey E, Gao FB, Burton EA, Chow T, Kao A, Leavitt BR, Lamb B, Grether M, et al. Frontotemporal degeneration, the next therapeutic frontier: Molecules and animal models for frontotemporal degeneration drug development. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012 doi: 10.1016/j.jalz.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boxer AL, Gold M, Huey E, Hu WT, Rosen H, Kramer J, Gao FB, Burton EA, Chow T, Kao A, et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: The next therapeutic frontier) Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012 doi: 10.1016/j.jalz.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boxer AL, Knopman DS, Kaufer DI, Grossman M, Onyike C, Graf-Radford N, Mendez M, Kerwin D, Lerner A, Wu CK, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet neurology. 2013;12:149–156. doi: 10.1016/S1474-4422(12)70320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, Doody RS, Lees A, Golbe LI, Williams DR, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet neurology. 2014;13:676–685. doi: 10.1016/S1474-4422(14)70088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Del Tredici K. Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nature reviews Neurology. 2013;9:708–714. doi: 10.1038/nrneurol.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta neuropathologica. 2014 doi: 10.1007/s00401-014-1356-1. [DOI] [PubMed] [Google Scholar]
- 32.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- 33.Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Fang L, Van Deerlin VM, Ludolph AC, Lee VM, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta neuropathologica. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of neurology. 2013 doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brettschneider J, Tredici KD, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of neurology. 2013 doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, et al. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta neuropathologica. 2012;123:825–839. doi: 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunden KR, Trojanowski JQ, Smith AB, 3rd, Lee VM, Ballatore C. Microtubule-stabilizing agents as potential therapeutics for neurodegenerative disease. Bioorganic & medicinal chemistry. 2014;22:5040–5049. doi: 10.1016/j.bmc.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. The Journal of biological chemistry. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 39.Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet neurology. 2012;11:232–240. doi: 10.1016/S1474-4422(12)70014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta neuropathologica. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. The American journal of pathology. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cairns NJ, Perrin RJ, Schmidt RE, Gru A, Green KG, Carter D, Taylor-Reinwald L, Morris JC, Gitcho MA, Baloh RH. TDP-43 proteinopathy in familial motor neurone disease with TARDBP A315T mutation: a case report. Neuropathology and applied neurobiology. 2010;36:673–679. doi: 10.1111/j.1365-2990.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen-Plotkin AS, Martinez-Lage M, Sleiman PM, Hu W, Greene R, Wood EM, Bing S, Grossman M, Schellenberg GD, Hatanpaa KJ, et al. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Archives of neurology. 2011;68:488–497. doi: 10.1001/archneurol.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L, Busch JI, Akle S, Grossman M, Van Deerlin V, et al. TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:11213–11227. doi: 10.1523/JNEUROSCI.0521-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen-Plotkin AS, Xiao J, Geser F, Martinez-Lage M, Grossman M, Unger T, Wood EM, Van Deerlin VM, Trojanowski JQ, Lee VM. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta neuropathologica. 2010;119:111–122. doi: 10.1007/s00401-009-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C, Shankle WR, Lerner AJ, Su MY, Elizarov A, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. Journal of Alzheimer's disease : JAD. 2014;38:171–184. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- 47.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet neurology. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 48.Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cook C, Carlomagno Y, Gendron TF, Dunmore J, Scheffel K, Stetler C, Davis M, Dickson D, Jarpe M, DeTure M, et al. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Human molecular genetics. 2014;23:104–116. doi: 10.1093/hmg/ddt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta neuropathologica. 2014 doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, Bertelsen S, Mayo K, Norton JB, Morris JC, et al. Association of TMEM106B gene polymorphism with age at onset in granulin mutation carriers and plasma granulin protein levels. Archives of neurology. 2011;68:581–586. doi: 10.1001/archneurol.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 54.Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nature cell biology. 2001;3:740–744. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- 55.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delacourte A, Robitaille Y, Sergeant N, Buee L, Hof PR, Wattez A, Laroche-Cholette A, Mathieu J, Chagnon P, Gauvreau D. Specific pathological Tau protein variants characterize Pick's disease. Journal of neuropathology and experimental neurology. 1996;55:159–168. doi: 10.1097/00005072-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) Journal of molecular neuroscience : MN. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dopper EG, Rombouts SA, Jiskoot LC, den Heijer T, de Graaf JR, de Koning I, Hammerschlag AR, Seelaar H, Seeley WW, Veer IM, et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2014;83:e19–e26. doi: 10.1212/WNL.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 59.Ferrari R, Hernandez DG, Nalls MA, Rohrer JD, Ramasamy A, Kwok JB, Dobson-Stone C, Brooks WS, Schofield PR, Halliday GM, et al. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet neurology. 2014;13:686–699. doi: 10.1016/S1474-4422(14)70065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain : a journal of neurology. 2008;131:1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 61.Finch N, Baker M, Crook R, Swanson K, Kuntz K, Surtees R, Bisceglio G, Rovelet-Lecrux A, Boeve B, Petersen RC, et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain : a journal of neurology. 2009;132:583–591. doi: 10.1093/brain/awn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M, Crook R, Hunter T, Ghidoni R, Benussi L, et al. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011;76:467–474. doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forman M, Trojanoswki JQ, Lee VM-Y. Hereditary Tauopathies and Idiopathic Frontotemporal Dementias. Cambridge University Press, City; 2004. [Google Scholar]
- 64.Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, et al. Frontotemporal dementia: clinicopathological correlations. Annals of neurology. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forman MS, Zhukareva V, Bergeron C, Chin SS, Grossman M, Clark C, Lee VM, Trojanowski JQ. Signature tau neuropathology in gray and white matter of corticobasal degeneration. The American journal of pathology. 2002;160:2045–2053. doi: 10.1016/S0002-9440(10)61154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, Snowden JS, Allsop D, Mann DM. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer's disease and frontotemporal lobar degeneration. Acta neuropathologica. 2008;116:141–146. doi: 10.1007/s00401-008-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP. TAR-DNA binding protein 43 in Pick disease. Journal of neuropathology and experimental neurology. 2008;67:62–67. doi: 10.1097/nen.0b013e3181609361. [DOI] [PubMed] [Google Scholar]
- 68.Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta neuropathologica. 2009;117:151–158. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 69.Gallagher MD, Suh E, Grossman M, Elman L, McCluskey L, Van Swieten JC, Al-Sarraj S, Neumann M, Gelpi E, Ghetti B, et al. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta neuropathologica. 2014;127:407–418. doi: 10.1007/s00401-013-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gendron TF, Bieniek KF, Zhang YJ, Jansen-West K, Ash PE, Caulfield T, Daughrity L, Dunmore JH, Castanedes-Casey M, Chew J, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta neuropathologica. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Archives of neurology. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, Garruto RM, Perl DP, Galasko D, Lee VM, et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta neuropathologica. 2008;115:133–145. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 73.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, 3rd, Bigio EH, Caselli R, et al. TDP-43 A315T mutation in familial motor neuron disease. Annals of neurology. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldman JS, Quinzii C, Dunning-Broadbent J, Waters C, Mitsumoto H, Brannagan TH, 3rd, Cosentino S, Huey ED, Nagy P, Kuo SH. Multiple system atrophy and amyotrophic lateral sclerosis in a family with hexanucleotide repeat expansions in C9orf72. JAMA neurology. 2014;71:771–774. doi: 10.1001/jamaneurol.2013.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grinberg LT, Wang X, Wang C, Sohn PD, Theofilas P, Sidhu M, Arevalo JB, Heinsen H, Huang EJ, Rosen H, et al. Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta neuropathologica. 2013;125:581–593. doi: 10.1007/s00401-013-1080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grossman M. Biomarkers to identify the pathological basis for frontotemporal lobar degeneration. Journal of molecular neuroscience : MN. 2011;45:366–371. doi: 10.1007/s12031-011-9597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nature reviews Neurology. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grossman M, Elman L, McCluskey L, McMillan CT, Boller A, Powers J, Rascovsky K, Hu W, Shaw L, Irwin DJ, et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA neurology. 2014;71:442–448. doi: 10.1001/jamaneurol.2013.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grossman M, Farmer J, Leight S, Work M, Moore P, Van Deerlin V, Pratico D, Clark CM, Coslett HB, Chatterjee A, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Annals of neurology. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 81.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiology of aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 82.Guillozet-Bongaarts AL, Glajch KE, Libson EG, Cahill ME, Bigio E, Berry RW, Binder LI. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta neuropathologica. 2007;113:513–520. doi: 10.1007/s00401-007-0209-6. [DOI] [PubMed] [Google Scholar]
- 83.Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nature medicine. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo JL, Lee VM. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS letters. 2013;587:717–723. doi: 10.1016/j.febslet.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harms M, Benitez BA, Cairns N, Cooper B, Cooper P, Mayo K, Carrell D, Faber K, Williamson J, Bird T, et al. C9orf72 hexanucleotide repeat expansions in clinical Alzheimer disease. JAMA neurology. 2013;70:736–741. doi: 10.1001/2013.jamaneurol.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hart MP, Brettschneider J, Lee VM, Trojanowski JQ, Gitler AD. Distinct TDP-43 pathology in ALS patients with ataxin 2 intermediate-length polyQ expansions. Acta neuropathologica. 2012;124:221–230. doi: 10.1007/s00401-012-0985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hensman Moss DJ, Poulter M, Beck J, Hehir J, Polke JM, Campbell T, Adamson G, Mudanohwo E, McColgan P, Haworth A, et al. C9orf72 expansions are the most common genetic cause of Huntington disease phenocopies. Neurology. 2014;82:292–299. doi: 10.1212/WNL.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM. Clinicopathological correlates in frontotemporal dementia. Annals of neurology. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 89.Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, Rademakers R, de Silva R, Litvan I, Riley DE, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature genetics. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 91.Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM, Trojanowski JQ. Nitration of tau protein is linked to neurodegeneration in tauopathies. The American journal of pathology. 2003;163:1021–1031. doi: 10.1016/S0002-9440(10)63462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- 93.Hsiung GY, DeJesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E, Butler R, Leung B, Fok A, Rutherford NJ, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain : a journal of neurology. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu WT, Chen-Plotkin A, Grossman M, Arnold SE, Clark CM, Shaw LM, McCluskey L, Elman L, Hurtig HI, Siderowf A, et al. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology. 2010;75:2079–2086. doi: 10.1212/WNL.0b013e318200d78d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu WT, McMillan C, Libon D, Leight S, Forman M, Lee VM, Trojanowski JQ, Grossman M. Multimodal predictors for Alzheimer disease in nonfluent primary progressive aphasia. Neurology. 2010;75:595–602. doi: 10.1212/WNL.0b013e3181ed9c52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu WT, Seelaar H, Josephs KA, Knopman DS, Boeve BF, Sorenson EJ, McCluskey L, Elman L, Schelhaas HJ, Parisi JE, et al. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Archives of neurology. 2009;66:1359–1364. doi: 10.1001/archneurol.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu WT, Watts K, Grossman M, Glass J, Lah JJ, Hales C, Shelnutt M, Van Deerlin V, Trojanoswki JQ, Levey AI. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology. 2013 doi: 10.1212/01.wnl.0000436625.63650.27. in press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K, Neumann M, Clark CM, Elman LB, Miller BL, Grossman M, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. The American journal of pathology. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, Lee VM, Trojanowski JQ. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA neurology. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Irwin DJ, Cohen TJ, Grossman M, Arnold SE, McCarty-Wood E, Van Deerlin VM, Lee VM, Trojanowski JQ. Acetylated tau neuropathology in sporadic and hereditary tauopathies. The American journal of pathology. 2013;183:344–351. doi: 10.1016/j.ajpath.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX, Lee VM, Trojanowski JQ. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain : a journal of neurology. 2012;135:807–818. doi: 10.1093/brain/aws013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Irwin DJ, McMillan CT, Brettschneider J, Libon DJ, Powers J, Rascovsky K, Toledo JB, Boller A, Bekisz J, Chandrasekaran K, et al. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2013;84:163–169. doi: 10.1136/jnnp-2012-303507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Irwin DJ, McMillan CT, Suh E, Powers J, Rascovsky K, Wood EM, Toledo JB, Arnold SE, Lee VM, Van Deerlin VM, et al. Myelin oligodendrocyte basic protein and prognosis in behavioral-variant frontotemporal dementia. Neurology. 2014;83:502–509. doi: 10.1212/WNL.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Irwin DJ, McMillan CT, Toledo JB, Arnold SE, Shaw LM, Wang LS, Van Deerlin V, Lee VM, Trojanowski JQ, Grossman M. Comparison of cerebrospinal fluid levels of tau and Abeta 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Archives of neurology. 2012;69:1018–1025. doi: 10.1001/archneurol.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Irwin DJ, Trojanowski JQ, Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Frontiers in aging neuroscience. 2013;5:6. doi: 10.3389/fnagi.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta neuropathologica. 2007;113:107–117. doi: 10.1007/s00401-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 108.Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain : a journal of neurology. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW. Staging TDP-43 pathology in Alzheimer's disease. Acta neuropathologica. 2014;127:441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta neuropathologica. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature genetics. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 114.Kalkonde YV, Jawaid A, Qureshi SU, Shirani P, Wheaton M, Pinto-Patarroyo GP, Schulz PE. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012;8:204–210. doi: 10.1016/j.jalz.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 115.Kamada M, Izumi Y, Ayaki T, Nakamura M, Kagawa S, Kudo E, Sako W, Maruyama H, Nishida Y, Kawakami H, et al. Clinicopathologic features of autosomal recessive amyotrophic lateral sclerosis associated with optineurin mutation. Neuropathology : official journal of the Japanese Society of Neuropathology. 2014;34:64–70. doi: 10.1111/neup.12051. [DOI] [PubMed] [Google Scholar]
- 116.Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-beta(1-42) and tau proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, Karydas AM, Wu T, Ketelle RS, Baker MC, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. Journal of neurology, neurosurgery, and psychiatry. 2012;83:358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kipps CM, Hodges JR, Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the 'bvFTD phenocopy syndrome'. Current opinion in neurology. 2010;23:628–632. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- 119.Kirby J, Highley JR, Cox L, Goodall EF, Hewitt C, Hartley JA, Hollinger HC, Fox M, Ince PG, McDermott CJ, et al. Lack of unique neuropathology in amyotrophic lateral sclerosis associated with p.K54E angiogenin (ANG) mutation. Neuropathology and applied neurobiology. 2013;39:562–571. doi: 10.1111/nan.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of neurology. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 121.Knopman DS. Overview of dementia lacking distinctive histology: pathological designation of a progressive dementia. Dementia. 1993;4:132–136. doi: 10.1159/000107354. [DOI] [PubMed] [Google Scholar]
- 122.Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. Journal of molecular neuroscience : MN. 2011;45:330–335. doi: 10.1007/s12031-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 124.Kwon I, Xiang S, Kato M, Wu L, Theodoropoulos P, Wang T, Kim J, Yun J, Xie Y, McKnight SL. Poly-dipeptides encoded by the C9orf72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science. 2014;345:1139–1145. doi: 10.1126/science.1254917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kwong LK, Irwin DJ, Walker AK, Xu Y, Riddle DM, Trojanowski JQ, Lee VM. Novel monoclonal antibodies to normal and pathologically altered human TDP-43 proteins. Acta neuropathologica communications. 2014;2:33. doi: 10.1186/2051-5960-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lashley T, Rohrer JD, Bandopadhyay R, Fry C, Ahmed Z, Isaacs AM, Brelstaff JH, Borroni B, Warren JD, Troakes C, et al. A comparative clinical, pathological, biochemical and genetic study of fused in sarcoma proteinopathies. Brain : a journal of neurology. 2011;134:2548–2564. doi: 10.1093/brain/awr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Le Ber I, Camuzat A, Guillot-Noel L, Hannequin D, Lacomblez L, Golfier V, Puel M, Martinaud O, Deramecourt V, Rivaud-Pechoux S, et al. C9ORF72 repeat expansions in the frontotemporal dementias spectrum of diseases: a flow-chart for genetic testing. Journal of Alzheimer's disease : JAD. 2013;34:485–499. doi: 10.3233/JAD-121456. [DOI] [PubMed] [Google Scholar]
- 128.Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain : a journal of neurology. 2008;131:732–746. doi: 10.1093/brain/awn012. [DOI] [PubMed] [Google Scholar]
- 129.Ledesma MD, Perez M, Colaco C, Avila J. Tau glycation is involved in aggregation of the protein but not in the formation of filaments. Cellular and molecular biology. 1998;44:1111–1116. [PubMed] [Google Scholar]
- 130.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nature reviews Neuroscience. 2012;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee EB, Russ J, Jung H, Elman LB, Chahine LM, Kremens D, Miller BL, Branch Coslett H, Trojanowski JQ, Van Deerlin VM, et al. Topography of FUS pathology distinguishes late-onset BIBD from aFTLD-U. Acta neuropathologica communications. 2013;1:1–11. doi: 10.1186/2051-5960-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, Takada LT, Karydas AM, Block NR, Coppola G, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain : a journal of neurology. 2014;137:3047–3060. doi: 10.1093/brain/awu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY, et al. Clinicopathological correlations in corticobasal degeneration. Annals of neurology. 2011;70:327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee V, Brunden K, Hutton M, Trojanoswki JQ. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. In: Selkoe D, Holtzman DM, Mandelkow E, editors. The Biology of Alzheimer Disease 1 edn. Cold Spring Harbor Labratory Press, City; 2012. pp. 416–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 136.Liu EY, Russ J, Wu K, Neal D, Suh E, McNally AG, Irwin DJ, Van Deerlin VM, Lee EB. C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta neuropathologica. 2014;128:525–541. doi: 10.1007/s00401-014-1286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luk C, Compta Y, Magdalinou N, Marti MJ, Hondhamuni G, Zetterberg H, Blennow K, Constantinescu R, Pijnenburg Y, Mollenhauer B, et al. Development and assessment of sensitive immuno-PCR assays for the quantification of cerebrospinal fluid three- and four-repeat tau isoforms in tauopathies. Journal of neurochemistry. 2012;123:396–405. doi: 10.1111/j.1471-4159.2012.07911.x. [DOI] [PubMed] [Google Scholar]
- 138.Mackenzie IR, Arzberger T, Kremmer E, Troost D, Lorenzl S, Mori K, Weng SM, Haass C, Kretzschmar HA, Edbauer D, et al. Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta neuropathologica. 2013;126:859–879. doi: 10.1007/s00401-013-1181-y. [DOI] [PubMed] [Google Scholar]
- 139.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta neuropathologica. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta neuropathologica. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain : a journal of neurology. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, Troncoso JC, Hardy J, Singleton AB, Traynor BJ. Repeat expansion in C9ORF72 in Alzheimer's disease. The New England journal of medicine. 2012;366:283–284. doi: 10.1056/NEJMc1113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Chio A, Restagno G, Nicolaou N, Simon-Sanchez J, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet neurology. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mann DM, Rollinson S, Robinson A, Bennion Callister J, Thompson JC, Snowden JS, Gendron T, Petrucelli L, Masuda-Suzukake M, Hasegawa M, et al. Dipeptide repeat proteins are present in the p62 positive inclusions in patients with frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta neuropathologica communications. 2013;1:68. doi: 10.1186/2051-5960-1-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martinez-Lage M, Molina-Porcel L, Falcone D, McCluskey L, Lee VM, Van Deerlin VM, Trojanowski JQ. TDP-43 pathology in a case of hereditary spastic paraplegia with a NIPA1/SPG6 mutation. Acta neuropathologica. 2012;124:285–291. doi: 10.1007/s00401-012-0947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, Zhang MR, Trojanowski JQ, Lee VM, Ono M, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, Bocchio-Chiavetto L, Blankenstein MA, Carrillo MC, Chalbot S, et al. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:386–395. e386. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCluskey L, Vandriel S, Elman L, Van Deerlin VM, Powers J, Boller A, Wood EM, Woo J, McMillan CT, Rascovsky K, et al. ALS-Plus syndrome: non-pyramidal features in a large ALS cohort. Journal of the neurological sciences. 2014;345:118–124. doi: 10.1016/j.jns.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McMillan C, Irwin D, Avants B, Powers J, Cook PA, McCarty Wood E, Van Deerlin V, Lee V, Trojanoswki JQ, Grossman M. White Matter Imaging Helps Dissociate Tau from TDP-43 in Frontotemporal Lobar Degeneration. Journal of neurology, neurosurgery, and psychiatry under review. 2013 doi: 10.1136/jnnp-2012-304418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McMillan CT, Avants B, Irwin DJ, Toledo JB, Wolk DA, Van Deerlin VM, Shaw LM, Trojanoswki JQ, Grossman M. Can MRI screen for CSF biomarkers in neurodegenerative disease? Neurology. 2012 doi: 10.1212/WNL.0b013e31827b9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McMillan CT, Avants BB, Cook P, Ungar L, Trojanowski JQ, Grossman M. The power of neuroimaging biomarkers for screening frontotemporal dementia. Human brain mapping. 2014;35:4827–4840. doi: 10.1002/hbm.22515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McMillan CT, Brun C, Siddiqui S, Churgin M, Libon D, Yushkevich P, Zhang H, Boller A, Gee J, Grossman M. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology. 2012;78:1761–1768. doi: 10.1212/WNL.0b013e31825830bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McMillan CT, Toledo JB, Avants BB, Cook PA, Wood EM, Suh E, Irwin DJ, Powers J, Olm C, Elman L, et al. Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiology of aging. 2014;35:1473–1482. doi: 10.1016/j.neurobiolaging.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain : a journal of neurology. 2014;137:1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nature cell biology. 2012;14:117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 156.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mizielinska S, Gronke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, Moens T, Norona FE, Woollacott IO, Pietrzyk J, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345:1192–1194. doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mizielinska S, Lashley T, Norona FE, Clayton EL, Ridler CE, Fratta P, Isaacs AM. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta neuropathologica. 2013;126:845–857. doi: 10.1007/s00401-013-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta neuropathologica. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Montpetit V, Clapin DF, Guberman A. Substructure of 20 nm filaments of progressive supranuclear palsy. Acta neuropathologica. 1985;68:311–318. doi: 10.1007/BF00690834. [DOI] [PubMed] [Google Scholar]
- 161.Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 162.Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JS, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, et al. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Annals of neurology. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Munoz-Garcia D, Ludwin SK. Classic and generalized variants of Pick's disease: a clinicopathological, ultrastructural, and immunocytochemical comparative study. Annals of neurology. 1984;16:467–480. doi: 10.1002/ana.410160408. [DOI] [PubMed] [Google Scholar]
- 164.Munoz DG, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, Kuroda S, Mackenzie IR. FUS pathology in basophilic inclusion body disease. Acta neuropathologica. 2009;118:617–627. doi: 10.1007/s00401-009-0598-9. [DOI] [PubMed] [Google Scholar]
- 165.Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, Miller BL, Johnson JK, Clark CM, Hurtig HI, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 166.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta neuropathologica. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 167.Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain : a journal of neurology. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Neumann M. Frontotemporal lobar degeneration and amyotrophic lateral sclerosis: molecular similarities and differences. Revue neurologique. 2013;169:793–798. doi: 10.1016/j.neurol.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 169.Neumann M, Bentmann E, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, Roeber S, Kretzschmar HA, Munoz DG, Kusaka H, et al. FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain : a journal of neurology. 2011;134:2595–2609. doi: 10.1093/brain/awr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta neuropathologica. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IR. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta neuropathologica. 2009;118:605–616. doi: 10.1007/s00401-009-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 173.Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, Yoshida M, Murayama S, Mann DM, Akiyama H, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell reports. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 174.Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25:130–137. doi: 10.3109/09540261.2013.776523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. Journal of virology. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Proudfoot M, Gutowski NJ, Edbauer D, Hilton DA, Stephens M, Rankin J, Mackenzie IR. Early dipeptide repeat pathology in a frontotemporal dementia kindred with C9ORF72 mutation and intellectual disability. Acta neuropathologica. 2014;127:451–458. doi: 10.1007/s00401-014-1245-7. [DOI] [PubMed] [Google Scholar]
- 177.Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O'Neil JP, Lal RA, Dronkers NF, Miller BL, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of neurology. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 180.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Robinson JL, Geser F, Stieber A, Umoh M, Kwong LK, Van Deerlin VM, Lee VM, Trojanowski JQ. TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta neuropathologica. 2013;125:121–131. doi: 10.1007/s00401-012-1055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, de Silva R, Warrington E, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain : a journal of neurology. 2011;134:2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rollinson S, Mead S, Snowden J, Richardson A, Rohrer J, Halliwell N, Usher S, Neary D, Mann D, Hardy J, et al. Frontotemporal lobar degeneration genome wide association study replication confirms a risk locus shared with amyotrophic lateral sclerosis. Neurobiology of aging. 2011;32:758, e751–e757. doi: 10.1016/j.neurobiolaging.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 184.Rosso SM, Landweer EJ, Houterman M, Donker Kaat L, van Duijn CM, van Swieten JC. Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case-control study. Journal of neurology, neurosurgery, and psychiatry. 2003;74:1574–1576. doi: 10.1136/jnnp.74.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Russ J, Liu EY, Wu K, Neal D, Suh E, Irwin DJ, McMillan CT, Harms MB, Cairns NJ, Wood EM, et al. Hypermethylation of repeat expanded C9orf72 is a clinical and molecular disease modifier. Acta neuropathologica. 2014 doi: 10.1007/s00401-014-1365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Yamanouchi H, Murayama S. Staging of argyrophilic grains: an age-associated tauopathy. Journal of neuropathology and experimental neurology. 2004;63:911–918. doi: 10.1093/jnen/63.9.911. [DOI] [PubMed] [Google Scholar]
- 187.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. The American journal of pathology. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Santa-Maria I, Haggiagi A, Liu X, Wasserscheid J, Nelson PT, Dewar K, Clark LN, Crary JF. The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta neuropathologica. 2012;124:693–704. doi: 10.1007/s00401-012-1017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Scherling CS, Hall T, Berisha F, Klepac K, Karydas A, Coppola G, Kramer JH, Rabinovici G, Ahlijanian M, Miller BL, et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Annals of neurology. 2014;75:116–126. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, Khan B, Karydas A, Baker MC, DeJesus-Hernandez M, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. 2012;79:1002–1011. doi: 10.1212/WNL.0b013e318268452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of neurology. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta neuropathologica. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Simon-Sanchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H, de Graaf JR, de Koning I, van Schoor NM, Deeg DJ, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain : a journal of neurology. 2012;135:723–735. doi: 10.1093/brain/awr353. [DOI] [PubMed] [Google Scholar]
- 194.Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, Kilander L, Religa D, Wimo A, Winblad B, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 195.Sleegers K, Brouwers N, Van Damme P, Engelborghs S, Gijselinck I, van der Zee J, Peeters K, Mattheijssens M, Cruts M, Vandenberghe R, et al. Serum biomarker for progranulin-associated frontotemporal lobar degeneration. Annals of neurology. 2009;65:603–609. doi: 10.1002/ana.21621. [DOI] [PubMed] [Google Scholar]
- 196.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta neuropathologica. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 197.Snowden JS, Hu Q, Rollinson S, Halliwell N, Robinson A, Davidson YS, Momeni P, Baborie A, Griffiths TD, Jaros E, et al. The most common type of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia but is not related to mutations in the FUS gene. Acta neuropathologica. 2011;122:99–110. doi: 10.1007/s00401-011-0816-0. [DOI] [PubMed] [Google Scholar]
- 198.Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, Jones M, Gerhard A, Davidson YS, Robinson A, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain : a journal of neurology. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Steinacker P, Hendrich C, Sperfeld AD, Jesse S, von Arnim CA, Lehnert S, Pabst A, Uttner I, Tumani H, Lee VM, et al. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Archives of neurology. 2008;65:1481–1487. doi: 10.1001/archneur.65.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2009;10:131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- 201.Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, Fostvedt E, Jansen-West K, Belzil VV, Desaro P, et al. Discovery of a Biomarker and Lead Small Molecules to Target RGGGGCC)-Associated Defects in c9FTD/ALS. Neuron. 2014;83:1043–1050. doi: 10.1016/j.neuron.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, Lee VM, Shaw LM, Trojanowski JQ. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta neuropathologica. 2012;124:23–35. doi: 10.1007/s00401-012-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Urwin H, Josephs KA, Rohrer JD, Mackenzie IR, Neumann M, Authier A, Seelaar H, Van Swieten JC, Brown JM, Johannsen P, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta neuropathologica. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. Journal of neuropathology and experimental neurology. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Blitterswijk M, Mullen B, Wojtas A, Heckman MG, Diehl NN, Baker MC, DeJesus-Hernandez M, Brown PH, Murray ME, Hsiung GY, et al. Genetic modifiers in carriers of repeat expansions in the C9ORF72 gene. Molecular neurodegeneration. 2014;9:38. doi: 10.1186/1750-1326-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet neurology. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nature genetics. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van der Zee J, Van Langenhove T, Kleinberger G, Sleegers K, Engelborghs S, Vandenberghe R, Santens P, Van den Broeck M, Joris G, Brys J, et al. TMEM106B is associated with frontotemporal lobar degeneration in a clinically diagnosed patient cohort. Brain : a journal of neurology. 2011;134:808–815. doi: 10.1093/brain/awr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, Parnetti L, Perret-Liaudet A, Shaw LM, Teunissen C, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 211.Villela D, Kimura L, Schlesinger D, Goncalves A, Pearson PL, Suemoto CK, Pasqualucci C, Krepischi AC, Grinberg LT, Rosenberg C. Germline DNA copy number variation in individuals with Argyrophilic grain disease reveals CTNS as a plausible candidate gene. Genetics and molecular biology. 2013;36:498–501. doi: 10.1590/S1415-47572013000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta neuropathologica. 1994;87:545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- 213.Watts GD, Thomasova D, Ramdeen SK, Fulchiero EC, Mehta SG, Drachman DA, Weihl CC, Jamrozik Z, Kwiecinski H, Kaminska A, et al. Novel VCP mutations in inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Clinical genetics. 2007;72:420–426. doi: 10.1111/j.1399-0004.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 214.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nature genetics. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 215.Whitwell JL, Josephs KA. Neuroimaging in frontotemporal lobar degeneration--predicting molecular pathology. Nature reviews Neurology. 2011;8:131–142. doi: 10.1038/nrneurol.2012.7. [DOI] [PubMed] [Google Scholar]
- 216.Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA neurology. 2013;70:1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wood EM, Falcone D, Suh E, Irwin DJ, Chen-Plotkin AS, Lee EB, Xie SX, Van Deerlin VM, Grossman M. Development and Validation of Pedigree Classification Criteria for Frontotemporal Lobar Degeneration. JAMA neurology. 2013 doi: 10.1001/jamaneurol.2013.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yoshiyama Y, Lee VM, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. Journal of neurology, neurosurgery, and psychiatry. 2013;84:784–795. doi: 10.1136/jnnp-2012-303144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang YJ, Jansen-West K, Xu YF, Gendron TF, Bieniek KF, Lin WL, Sasaguri H, Caulfield T, Hubbard J, Daughrity L, et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta neuropathologica. 2014;128:505–524. doi: 10.1007/s00401-014-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhukareva V, Joyce S, Schuck T, Van Deerlin V, Hurtig H, Albin R, Gilman S, Chin S, Miller B, Trojanowski JQ, et al. Unexpected abundance of pathological tau in progressive supranuclear palsy white matter. Annals of neurology. 2006;60:335–345. doi: 10.1002/ana.20916. [DOI] [PubMed] [Google Scholar]
- 221.Zhukareva V, Mann D, Pickering-Brown S, Uryu K, Shuck T, Shah K, Grossman M, Miller BL, Hulette CM, Feinstein SC, et al. Sporadic Pick's disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Annals of neurology. 2002;51:730–739. doi: 10.1002/ana.10222. [DOI] [PubMed] [Google Scholar]
- 222.Zhukareva V, Shah K, Uryu K, Braak H, Del Tredici K, Sundarraj S, Clark C, Trojanowski JQ, Lee VM. Biochemical analysis of tau proteins in argyrophilic grain disease, Alzheimer's disease, and Pick's disease : a comparative study. The American journal of pathology. 2002;161:1135–1141. doi: 10.1016/s0002-9440(10)64390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhukareva V, Trojanowski JQ, Lee VM. Assessment of pathological tau proteins in frontotemporal dementias: qualitative and quantitative approaches. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2004;12:136–145. [PubMed] [Google Scholar]