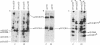Abstract
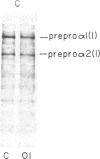
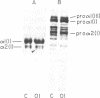
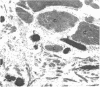
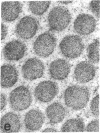
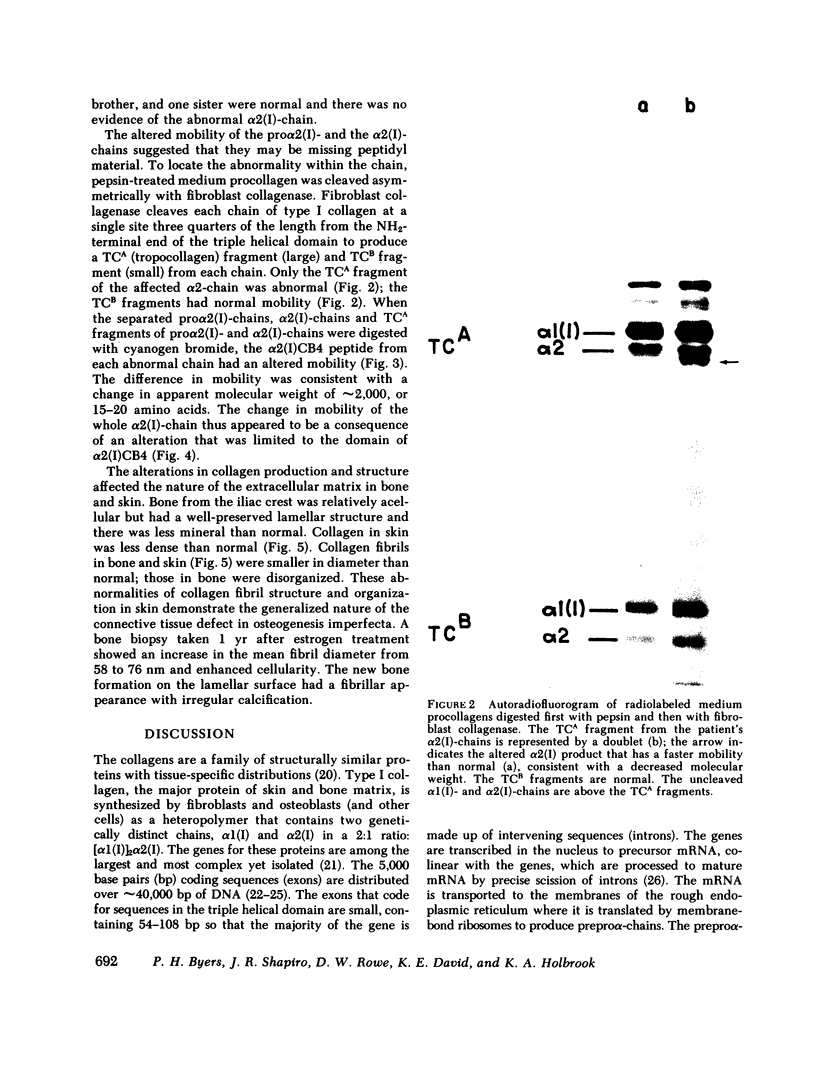
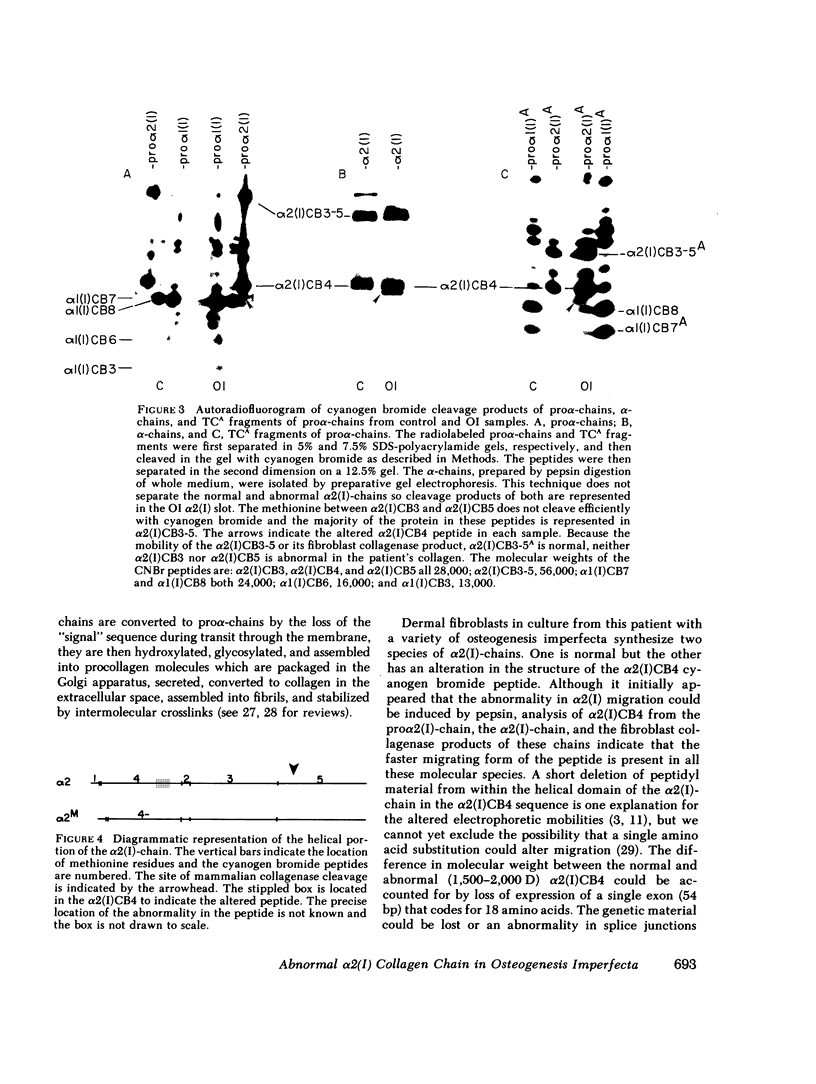
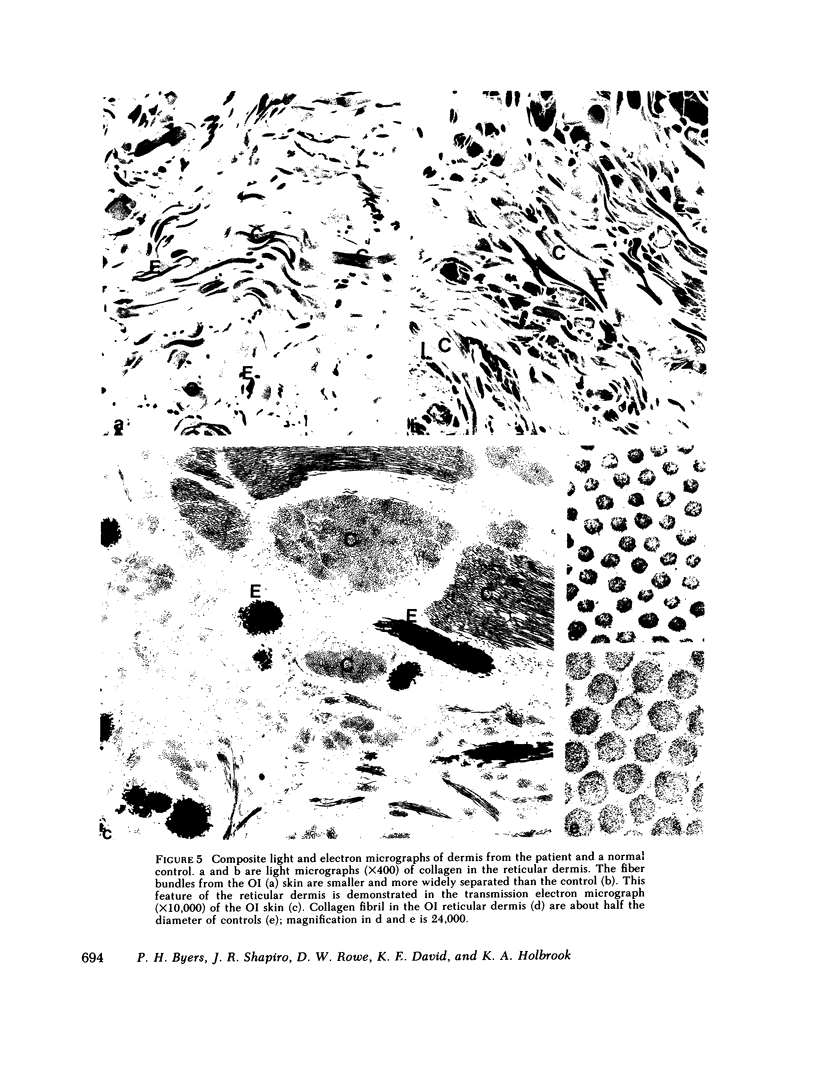
Dermal fibroblasts in culture from a woman with a mild to moderate form of osteogenesis imperfecta synthesize two species of the pro alpha 2-chain of type I procollagen. One chain is normal. The abnormal chain has a slightly faster mobility than normal during electrophoresis in sodium dodecyl sulfate polyacrylamide gels. Analysis of cyanogen bromide peptides of the pro alpha-chain, the alpha-chain, and of the mammalian collagenase cleavage products of the pro alpha- and alpha-chains indicates that the abnormality is confined to the alpha 2(I)CB4 fragment and is consistent with loss of a short triple-helical segment. Type I collagen production was decreased, perhaps because the molecules that contained the abnormal chain were unstable, with a resultant alteration in the ratio of type III to type I collagen secreted into culture medium. Collagen fibrils in bone and skin had a normal periodicity but their diameters were 50% of control; the bone matrix was undermineralized. The structural abnormality in the alpha 2(I)-chain in this patient may affect molecular stability, intermolecular interactions, and collagen-mineral relationships that act to decrease the collagen content of tissues and affect the mineralization of bone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avvedimento V. E., Vogeli G., Yamada Y., Maizel J. V., Jr, Pastan I., de Crombrugghe B. Correlation between splicing sites within an intron and their sequence complementarity with U1 RNA. Cell. 1980 Oct;21(3):689–696. doi: 10.1016/0092-8674(80)90432-8. [DOI] [PubMed] [Google Scholar]
- Baird M., Driscoll C., Schreiner H., Sciarratta G. V., Sansone G., Niazi G., Ramirez F., Bank A. A nucleotide change at a splice junction in the human beta-globin gene is associated with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4218–4221. doi: 10.1073/pnas.78.7.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Peterson K. E., Byers P. H. Peptide mapping of collagen chains using CNBr cleavage of proteins within polyacrylamide gels. Coll Relat Res. 1981 Nov;1(6):543–548. doi: 10.1016/s0174-173x(81)80035-0. [DOI] [PubMed] [Google Scholar]
- Bauze R. J., Smith R., Francis M. J. A new look at osteogenesis imperfecta. A clinical, radiological and biochemical study of forty-two patients. J Bone Joint Surg Br. 1975 Feb;57(1):2–12. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Boyd C. D., Tolstoshev P., Schafer M. P., Trapnell B. C., Coon H. C., Kretschmer P. J., Nienhuis A. W., Crystal R. G. Isolation and characterization of a 15-kilobase genomic sequence coding for part of the Pro alpha 2 chain of sheep type I collagen. J Biol Chem. 1980 Apr 10;255(7):3212–3220. [PubMed] [Google Scholar]
- Byers P. H., Siegel R. C., Peterson K. E., Rowe D. W., Holbrook K. A., Smith L. T., Chang Y. H., Fu J. C. Marfan syndrome: abnormal alpha 2 chain in type I collagen. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7745–7749. doi: 10.1073/pnas.78.12.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. Information contained in the amino acid sequence of the alpha1(I)-chain of collagen and its consequences upon the formation of the triple helix, of fibrils and crosslinks. Mol Cell Biochem. 1975 Sep 30;8(3):141–157. doi: 10.1007/BF01792765. [DOI] [PubMed] [Google Scholar]
- Fujimoto W. Y., Teague J., Williams R. H. Fibroblast monolayer cultures in scintillation counting vials: metabolic and growth experiments using radioisotopes and a microfluoremetric DNA assay. In Vitro. 1977 Apr;13(4):237–244. doi: 10.1007/BF02615081. [DOI] [PubMed] [Google Scholar]
- Holbrook K. A., Byers P. H. Ultrastructural characteristics of the skin in a form of the Ehlers-Danlos syndrome type IV. Storage in the rough endoplasmic reticulum. Lab Invest. 1981 Apr;44(4):342–350. [PubMed] [Google Scholar]
- Hollister D. W., Byers P. H., Holbrook K. A. Genetic disorders of collagen metabolism. Adv Hum Genet. 1982;12:1–87. doi: 10.1007/978-1-4615-8315-8_1. [DOI] [PubMed] [Google Scholar]
- Krieg T., Kirsch E., Matzen K., Müller P. K. Osteogenesis imperfecta: biochemical and clinical evaluation of 13 cases. Klin Wochenschr. 1981 Jan 15;59(2):91–93. doi: 10.1007/BF01477288. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Veis A. Studies on the structure and chemistry of dentin collagen-phosphophoryn covalent complexes. Calcif Tissue Int. 1980;31(2):123–134. doi: 10.1007/BF02407173. [DOI] [PubMed] [Google Scholar]
- Meigel W. N., Müller P. K., Pontz B. F., Sörensen N., Spranger J. A constitutional disorder of connective tissue suggesting a defect in collagen biosynthesis. Klin Wochenschr. 1974 Oct 1;52(19):906–912. doi: 10.1007/BF01468935. [DOI] [PubMed] [Google Scholar]
- Murdoch J. L., Walker B. A., McKusick V. A. Parental age effects on the occurrence of new mutations for the Marfan syndrome. Ann Hum Genet. 1972 Mar;35(3):331–336. doi: 10.1111/j.1469-1809.1957.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P. K., Lemmen C., Gay S., Meigel W. N. Disturbance in the regulation of the type of collagen synthesized in a form of osteogenesis imperfecta. Eur J Biochem. 1975 Nov 1;59(1):97–104. doi: 10.1111/j.1432-1033.1975.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Pope F. M., Schloon H. Biochemical heterogeneity of osteogenesis imperfecta: New variant. Lancet. 1979 Jun 2;1(8127):1193–1193. doi: 10.1016/s0140-6736(79)91872-5. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pope F. M., Nicholls A. C. Heterogeneity of osteogenesis imperfecta congenita. Lancet. 1980 Apr 12;1(8172):820–821. doi: 10.1016/s0140-6736(80)91313-6. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Shapiro J. R., Pikus A., Weiss G., Rowe D. W. Hearing and middle ear function in osteogenesis imperfecta. JAMA. 1982 Apr 16;247(15):2120–2126. [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. Osteogenesis imperfecta: an expanding panorama of variants. Clin Orthop Relat Res. 1981 Sep;(159):11–25. [PubMed] [Google Scholar]
- Smith R., Francis M. J., Bauze R. J. Osteogenesis imperfecta. A clinical and biochemical study of a generalized connective tissue disorder. Q J Med. 1975 Oct;44(176):555–573. [PubMed] [Google Scholar]
- Solomon E., Cheah K. S. Collagen evolution. Nature. 1981 Jun 11;291(5815):450–450. doi: 10.1038/291450a0. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin fibroblast collagenase: chemical properties of precursor and active forms. Biochemistry. 1978 Jun 13;17(12):2331–2337. doi: 10.1021/bi00605a012. [DOI] [PubMed] [Google Scholar]
- Sykes B., Francis M. J., Smith R. Altered relation of two collagen types in osteogenesis imperfecta. N Engl J Med. 1977 May 26;296(21):1200–1203. doi: 10.1056/NEJM197705262962104. [DOI] [PubMed] [Google Scholar]
- Vogeli G., Avvedimento E. V., Sullivan M., Maizel J. V., Jr, Lozano G., Adams S. L., Pastan I., de Crombrugghe B. Isolation and characterization of genomic DNA coding for alpha 2 type I collagen. Nucleic Acids Res. 1980 Apr 25;8(8):1823–1837. doi: 10.1093/nar/8.8.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Tate V., Boedtker H., Doty P. Structure of the pro alpha 2 (I) collagen gene. Nature. 1981 Nov 12;294(5837):129–135. doi: 10.1038/294129a0. [DOI] [PubMed] [Google Scholar]