Abstract
Activators of G-protein Signaling (AGS) are a family of accessory proteins that were discovered as modulators of heterotrimeric G-protein subunits. The primary aim of the present study was to localize Group I and II AGS proteins and determine the renal expression profile using immunohistochemistry and quantitative RT-PCR, respectively, during normal and injured states of the kidney. Group I AGS1 was found to be predominantly localized to the proximal tubule, Group II AGS3 and AGS5 were exclusively localized to the distal tubular segments, and Group II AGS6 was ubiquitously expressed in every nephron segment of the rodent kidney. In rat kidneys following ischemia-reperfusion injury (IRI), Group I AGS1 mRNA was dramatically increased after 24 hours by 5-fold (P<0.05), whereas Group II AGS3 and AGS4 mRNA was significantly decreased at the same time point (P<0.05). No significant change in the transcript levels were detected at other time points for any of the AGS genes between control and IRI groups. In polycystic diseased kidneys, mRNA levels for AGS3, AGS4 and AGS6 was significantly increased (P<0.05) by 75–80% in PCK rat kidneys. The identification of Group I and II AGS mRNA and protein in the kidney may provide insight into the potential mechanism of action during normal and varying states of renal disease or injury.
Keywords: Activator of G-protein Signaling, kidney, acute kidney injury, polycystic kidney disease, nephrectomy, reverse transcription-polymerase chain reaction, immunohistochemistry
Introduction
Heterotrimeric G-proteins are comprised of distinct Gα and Gβγ subunits that are well documented to regulate a vast number of diverse biological functions in the kidney through the activation of cell surface G-protein coupled receptors (GPCRs) (Filardo and Thomas 2012, Ni, Chen 2013, Rieg, Tang 2013, Spiegel 2007, Vallon, Stockand 2012). Using a yeast-based system, a seminal discovery was made to identify new G-protein regulators known as Activators of G-protein Signaling (AGS) (Cismowski, Takesono 1999, Takesono, Cismowski 1999), which selectively interact with either Gα or Gβγ subunits to control their function independent of GPCR activation (Blumer and Lanier 2014). To date, there are currently 13 AGS proteins that have been directly identified using this approach, which are classified into four distinct groups depending on the type of interaction with the G-protein subunits and their protein structure (Blumer and Lanier 2014, Blumer, Smrcka 2007, Sato, Blumer 2006).
In the kidney, the best characterized AGS protein has been the Group II AGS3 protein also known as G-protein Signaling Modulator 1 (GPSM1). In normal adult kidneys from mice, rat and humans, the expression of AGS3/GPSM1 is very weak to undetectable (Blumer, Chandler 2002, De Vries, Fischer 2000, Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011), which may be attributed to its localization to the distal tubular epithelial cells, particularly the collecting ducts (Nadella, Blumer 2010, Regner, Nozu 2011). Following acute kidney injury, AGS3/GPSM1 expression is preferentially induced in the outer medullary proximal tubules (Regner, Nozu 2011). Abnormally high levels of AGS3/GPSM1 were also detected in the collecting duct epithelial cells from kidneys with a genetic mutation leading to polycystic kidney disease (Kwon, Pavlov 2012, Nadella, Blumer 2010). In these studies, the basal expression of AGS5/GPSM2, a close homolog to AGS3, did not differ between the normal and injured renal conditions. The site of renal AGS5/GPSM2 was not determined in these previous studies (Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011). Although other AGS proteins have been detected using various methods to analyze whole kidney mRNA or protein, the localization and biological role of these proteins remains largely undescribed in the kidney.
Because of the emerging interest in the function of accessory proteins during organ dysregulation, the aim of the present study was designed to perform immunohistochemical localization and mRNA transcript profiling in normal and pathologically injured (i.e., acute and chronically damaged) rodent kidneys to evaluate the spatial and temporal expression for Group I (AGS1) and II (AGS3, 4, 5, and 6) AGS family members. By elucidating the renal site(s) and expression profiles of Group I and II AGS, we will potentially provide new information on the putative biological function of Group I and II AGS in the kidney.
Materials and Methods
Animals and surgical procedures
All protocols used in this study with mice and rats were approved by the appropriate Institutional Animal Care and Use Committee at the Medical College of Wisconsin (Milwaukee, WI) and University of Tennessee Health Sciences Center (Memphis, TN). In this study, rodent models of acute and chronic renal injury were examined to investigate the expression and localization of Group I and II AGS proteins in the kidney. In the first type of renal injury, we performed bilateral ischemia-reperfusion to simulate acute kidney injury, which is associated with a proliferative phase during the recovery of the kidney. In the second type of renal injury, we examined two different genetic models of polycystic kidney disease (PKD), polycystic kidney (PCK) rat and the Pkd1V/V mouse, and a surgical model of unilateral nephrectomy, which can be considered models that will progress towards chronic kidney disease. PKD models progress towards end-stage renal disease with a proliferative phenotype, whereas unilateral nephrectomy exhibits compensatory hypertrophic response in the remnant kidney. Each of these genetic and surgically rodent models are described below
Bilateral ischemia-reperfusion injury (IRI)
Male Sprague Dawley rats (250–300 g) were obtained from Taconic Farms (Oxnard, CA) for the bilateral IRI experiments. All rats were allowed ad libitum access to food and water during the course of this experiment. Rats underwent sham (n=15) or 30 min bilateral renal ischemia surgeries (n=15) as previously described by our lab (Regner, Nozu 2011, White, North 2014). Time-control sham surgeries were performed in parallel in which the renal pedicles were not clamped. Upon reperfusion of the kidneys, the rats were allowed to recover for either 1, 3, or 7 days (n=5 rats/time point), at which point the rats were euthanized for organ collection. Sham and IRI rat kidneys were snap-frozen in liquid nitrogen and stored at −80°C until RNA preparation, or fixed in neutral buffered formalin for paraffin-embedding.
Polycystic kidney disease
Male polycystic kidney disease (PCK) rats were produced from breeder pairs in our lab, and control Sprague Dawley rats were obtained from Charles River (Portage, MI). PCK rats are an orthologous rat model of human autosomal recessive polycystic kidney disease with a two-base pair mutation in the polycystic kidney and hepatic disease 1 (PKHD1) gene (Lager, Qian 2001). Kidneys were harvested at post-natal week 16 (n=4 animals/strain), weighed, fixed in neutral buffered formalin, and paraffin-embedded.
Male Pkd1+/+ and Pkd1V/V progeny were produced from Pkd1V/+ breeders in our lab, and allowed to survive with their parents until post-natal day 19. The Pkd1V/+ mouse is an orthologous model of autosomal dominant polycystic kidney disease (ADPKD) as previously described (Kwon, Pavlov 2012, Yu, Hackmann 2007). At day 19, the kidneys were harvested (n=4), weighed and fixed in neutral buffer formalin for paraffin-embedding.
Unilateral nephrectomy
Male PCK rats (4 weeks of age; n=17) were anesthetized with pentobarbital (80 mg/kg IP) and a flank incision was made to isolate the left kidney. The left kidney was removed, weighed and cut into two halves. One half was frozen on dry ice for isolation of protein lysates, and the other half was fixed in neutral buffer formalin for paraffin-embedding. The unilateral nephrectomized rats were euthanized after 3, 7 and 28 days (n=4/time point). Kidney weights harvested from normal PCK rats at 4 (n=5), 8 (n=10) and 12 weeks of age (n=5) were analyzed to compare with the hypertrophied kidneys from the nephrectomized 8 week old PCK rats.
To determine the role of the Gpsm1 gene in renal hypertrophy, unilateral nephrectomy was performed in wild-type and Gpsm1-deficient mice (8–12 weeks of age). Gpsm1+/− mice were previously generated by Blumer et al. (Blumer, Lord 2008) and maintained in our lab to examine the role of AGS3/GPSM1 in the kidney (Regner, Nozu 2011). Following nephrectomy, the remaining kidneys were harvested after 21 days, weighed and fixed in neutral buffered formalin. Kidney-to-body weight ratios were calculated at the end of the experimental period.
Chemicals and antibodies
Monoclonal β-actin antibody (Cat. #A5441) were purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies were obtained either from commercial vendors or other scientific researchers as follows: RasD1/AGS1 (1:75 dilution; Merck/Millipore cat #AB15794), AGS3/GPSM1 [1:300 dilution; (Kwon, Pavlov 2012, Regner, Nozu 2011), AGS5/GPSM2 [1:300; generously provided by Dr. S. M. Lanier and published in (Blumer, Chandler 2002)], and AGS6/RGS12 (1:150 dilution; Millipore Cat. #07–1368). Secondary goat anti-rabbit (Cat. #7074) and horse anti-mouse IgG (Cat. #7076) conjugated to horseradish peroxidase (HRP) were purchased from Cell Signaling Technology (Danvers, MA).
Immunohistochemistry of AGS proteins in the kidney
Mouse and rat kidneys were formalin fixed, paraffin embedded, and sectioned (4 µm) for immunostaining with AGS primary antibodies using previously described protocols by Kwon et al. (Kwon, Pavlov 2012). In brief, heat-induced epitope retrieval was performed either by heating (90°C) in antigen retrieval solution (IHC World, Woodstock, MD) or trypsin digestion, subsequently treated with peroxidase, and then the appropriate animal serum was added prior to the incubation with the primary antibody against the specific AGS proteins listed above. In some sections, a collecting duct lectin, Dolichol Bifluorus Agglutinin (DBA), or a proximal tubule lectin, Phaseolus vulgaris erthyroagglutinin (PVE-A), were used to determine specific nephron segments. As a negative control, sections were treated with no primary antibody or when available, primary antibody pre-absorbed with the immunizing peptide. Subsequently, the appropriate secondary antibody conjugated with HRP was used to visualize the binding by detecting the DAB precipitation on the sections. Between each of the steps, the slides were rinsed multiple times in Tris-buffered saline (TBS). All kidney sections were subsequently counterstained with Mayer’s hematoxylin solution, cover-slipped, and digitized by light microscopy or a Hamamatsu NanoZoomer HT digital slide scanner.
RNA extraction and reverse transcription (RT)
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) from the control and experimental animal kidneys. The quantity and integrity of the isolated total RNA was analyzed using a NanoDrop® ND-1000 spectrophotometer (Wilmington, DE). Purity of the RNA samples must have exhibited a 260/280 absorbance ratio ≥ 1.9 for use in the reverse transcription (RT) assay. cDNA synthesis was performed using 2 µg total RNA and SuperScript® III Reverse Transcriptase (Life Technologies, Carlsbad, CA).
Real-time quantitative PCR (qPCR)
Real-time PCR analysis was performed using fluorogenic TaqMan® primers from Life Technologies and was listed in Table 1. The following 13 AGS genes were amplified: AGS1/Rasd1, AGS3/Gpsm1, AGS4/Gpsm3, AGS5/Gpsm2, and AGS6/Rgs12. All PCR product amplification was normalized to 18S RNA house-keeping gene (HKG). All real-time PCR reactions were performed using 100 ng cDNA sample with (+) and (−) primers at 900 nM each, and 6-FAM™ dye-labeled TaqMan® MGB probe (250 nM). PCR cycling conditions included an initial melting time of 10 min at 95°C followed by 40 two-step cycles at 95°C for 15 sec and 60°C for 60 sec. Each rat kidney sample was assayed in duplicate or triplicate per assay, and each assay was performed at three different times to demonstrate the reproducibility of the findings. As a negative control, no template controls were added to each plate for each gene to ensure the absence of any spurious DNA amplification by the primer combinations. The comparative CT (ΔΔCT) method was applied for calculating relative quantitation of gene expression (Livak and Schmittgen 2001).
Table 1.
Localization of AGS proteins in normal mouse and rat kidneys
| Normal kidney | PCT | TAL | DT | Glm | BV |
|---|---|---|---|---|---|
| Group I | |||||
| AGS1/RasD1 | + | − | − | − | − |
| Group II | |||||
| AGS3/Gpsm1 | − | − | + | − | − |
| AGS4/Gpsm3 | U/D | U/D | U/D | U/D | U/D |
| AGS5/Gpsm2 | − | + | + | − | − |
| AGS6/RGS12 | + | + | + | − | + |
Table 1. PCT = proximal convoluted tubules; TAL = thick ascending limb of Henle; DT = distal tubules, including distal convoluted tubules and collecting ducts; Glm = glomerulus; and BV = blood vessels. + (present) or – (absent) in the cell types; U/D = unable to detect by immunohistochemistry.
Protein isolation and immunoblot analysis
Brain and kidney tissue from the nephrectomized PCK rats were homogenized in 1X RIPA buffer (Millipore, Billerica, MA) with protease and phosphatase Inhibitors (Pierce Biotechnology), and protein lysates were isolated following differential centrifugation. Immunoblot analysis for AGS3/GPSM1 was performed as previous published (Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011, Akbulut, Regner 2009). GAPDH was used as a loading control. The bands were detected on film following incubation in chemiluminescent solution (GE Healthcare, Piscataway, NJ).
Statistical analysis
RT-PCR results are shown as mean +/− SD. Statistical analyses were performed using Sigma Plot ver. 11.0 (Systat Software Inc., San Jose, CA, USA). For gene expression analyses, significant differences between control and experimental groups were determined using unpaired Student’s t-test or Mann-Whitney U-test after checking the distribution of the ΔCT values by Shapiro-Wilk normality test. If P<0.05, this was considered significantly different between time-matched sham and IRI-treated animals. For the kidney measurements, one-way ANOVA was performed in the comparison of kidney-to-body weight ratios between sham and unilateral nephrectomized PCK rats over different time points. If P<0.05 was calculated, then Tukey’s post-hoc test was performed to confirm the significant difference between groups.
Results
Localization of Group I and II AGS proteins in the normal mouse and rat kidneys
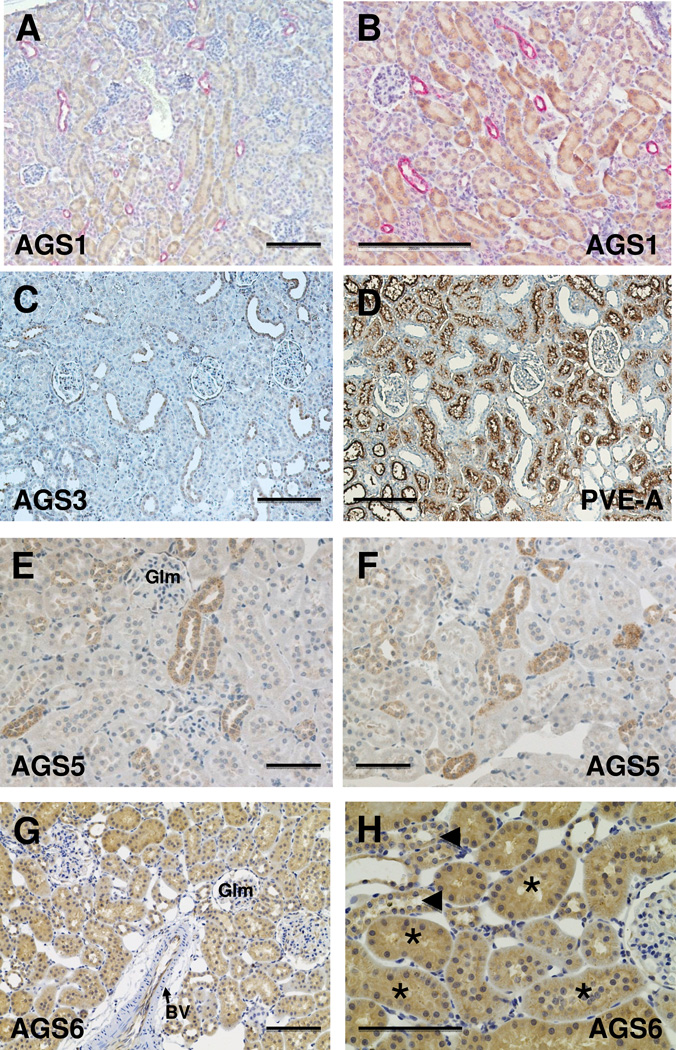
As shown in Figure 1 and summarized in Table 1, we detected the localization of Group I AGS1/RasD1 primarily in the proximal convoluted tubular epithelial cells within the renal cortex and outer medulla. Expression of AGS1/RasD1 was not observed in DBA-positive collecting duct epithelial cells (Figure 1B).
Figure 1.
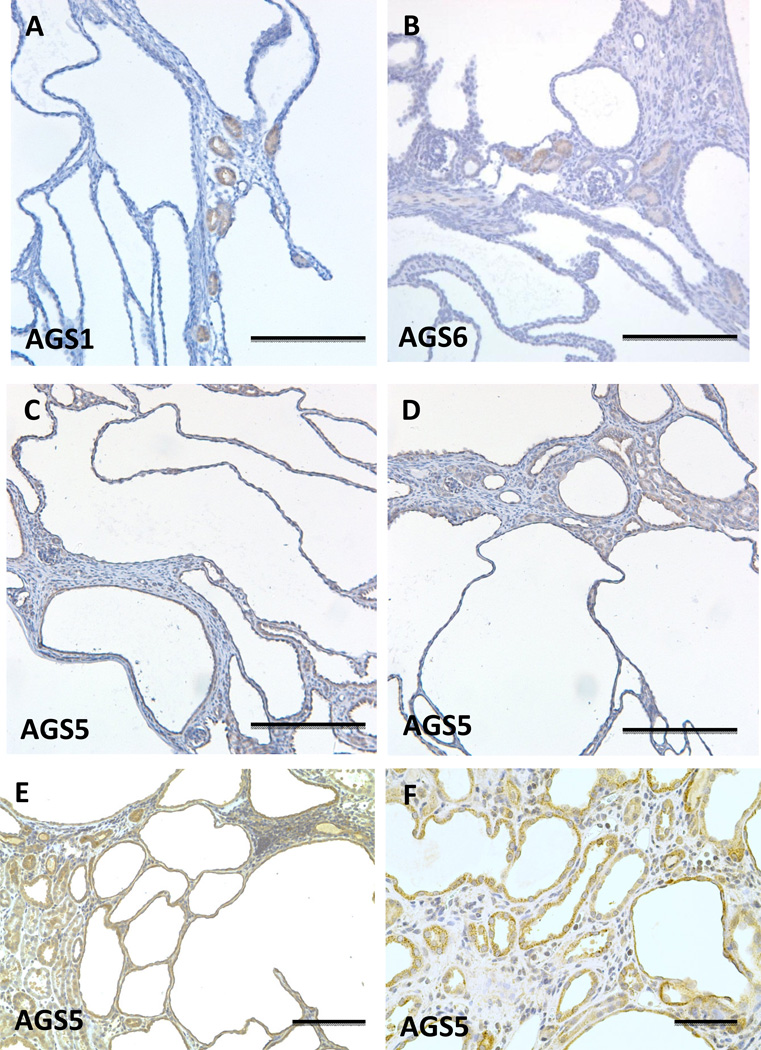
Localization of Group I and II AGS proteins in normal mouse or rat kidneys. Immunohistochemistry was performed using antigen-specific antibodies to Group I (AGS1/RasD1) and Group II (AGS3/GPSM1, AGS4/GPSM3. AGS5/GPSM2, and AGS6/RGS12) in paraffin-embedded mouse or rat kidneys. All AGS proteins were observed by the brown DAB staining in each respective section. AGS1 antibody (A, B) was used to immunostain mouse kidneys, and antibodies targeted to AGS3 (C), AGS5 (E, F), and AGS6 (G, H) were used to immunostain Sprague Dawley rat kidneys. For AGS1, the sections were co-labeled with Dolichos biflorus agglutinin (DBA), which is a selective marker for collecting ducts. Serial sections were performed using AGS3 (Fig. 1C) and PVE-A (Fig. 1D), a marker of proximal tubules, to demonstrate the selectivity of the AGS3-positive tubules were not proximal tubules, but distal tubular in its expression sites. Glm = glomerulus, BV = blood vessel, arrowhead points to collecting duct tubules (in H); and * indicates proximal tubules (in H). Scale bar = 200 um (A–D); 100 um (E–H).
The Group II AGS3-6 proteins were more diverse in their localization within the kidney. AGS3/GPSM1 and AGS5/LGN were localized to the distal tubular epithelial cells. The localization of AGS3 in the cortical to outer medullary collecting duct epithelial cells was consistent with previous studies in our lab (Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011). Under normal conditions, there was no AGS3/GPSM1 protein expression in LTA-stained tubular segments (Fig. 1C and 1D). In addition to the distal tubules, AGS5/LGN was also expressed in the thick ascending limbs of Henle (Fig. 1E and 1F). Neither AGS3/GPSM1 nor AGS5/LGN was detected in the proximal tubules or the thin limbs of Henle. AGS6/RGS12 was relatively ubiquitous in the epithelial cells in the kidney from the proximal tubules to the collecting ducts, and liminal cells in the blood vessels (Fig. 1G).
AGS4/GPSM3 was not reliably detected in the rat or mouse kidneys using commercially available antibodies (data not shown). Since we were able to detect mRNA changes for AGS4/GPSM3 in the kidney, the lack of protein detection may be associated with the inability of the antibody to bind properly to the antigen using frozenor paraffin-embedded tissue samples. Alternatively, the lack of AGS4/GPSM3 expression could be related to a low copy number for the AGS4/GPSM3 mRNA in the kidney tissue, so that it was insufficient for renal tubular protein expression. Immunoblotting of AGS4/GPSM3 in kidney tissue resulted in bands that could not be confirmed with positive control cell lines transfected with AGS4-expressing plasmid (data not shown).
Other than AGS6/RGS12, none of the other Group I and II AGS protein expression was observed in the glomerulus or any vascular structures, including blood vessels in the renal cortex and vasa recta capillaries in the renal medulla. AGS6/RGS12 was intensely stained in the luminal endothelial cells, but not in the smooth muscle layers in the vasculature and minimally in the glomeruli (Figure 1G).
Temporal mRNA expression profile of Group I and II AGS genes from normal kidneys or kidneys following acute kidney injury
Sprague Dawley rat kidneys under normal (n=5) or following IRI (n=5) were harvested and total RNA was extracted for analysis by quantitative RT-PCR. The specific primers used to amplify each specific AGS gene are listed in Table 2. The changes in the gene expression profile for each gene was normalized to 18S rRNA and the relative change in gene expression levels was calculated using the ΔΔCT method (Livak and Schmittgen 2001).
Table 2.
AGS gene primers used in quantitative real-time PCR
| Gene symbol |
Common gene aliases | Accession No. | ABI assay ID |
|---|---|---|---|
| Rasd1 | AGS1, Dexras1, | NM_009026 | Mm00842185_g1 |
| Gpsm1 | AGS3 | NM_001199146 | Mm00463033_m1 |
| NM_001199147 | |||
| NM_153410 | |||
| Gpsm3 | AGS4, G18, NG1 | NM_001003974 | Rn01525971_g1 |
| Gpsm2 | AGS5/LGN | NM_001191962 | Rn01522141_m1 |
| Rgs12 | AGS6 | NM_019339 | Rn01449045_m1 |
| Rn18s | 18S ribosomal RNA | NR_003278 | Mm03928990_g1 |
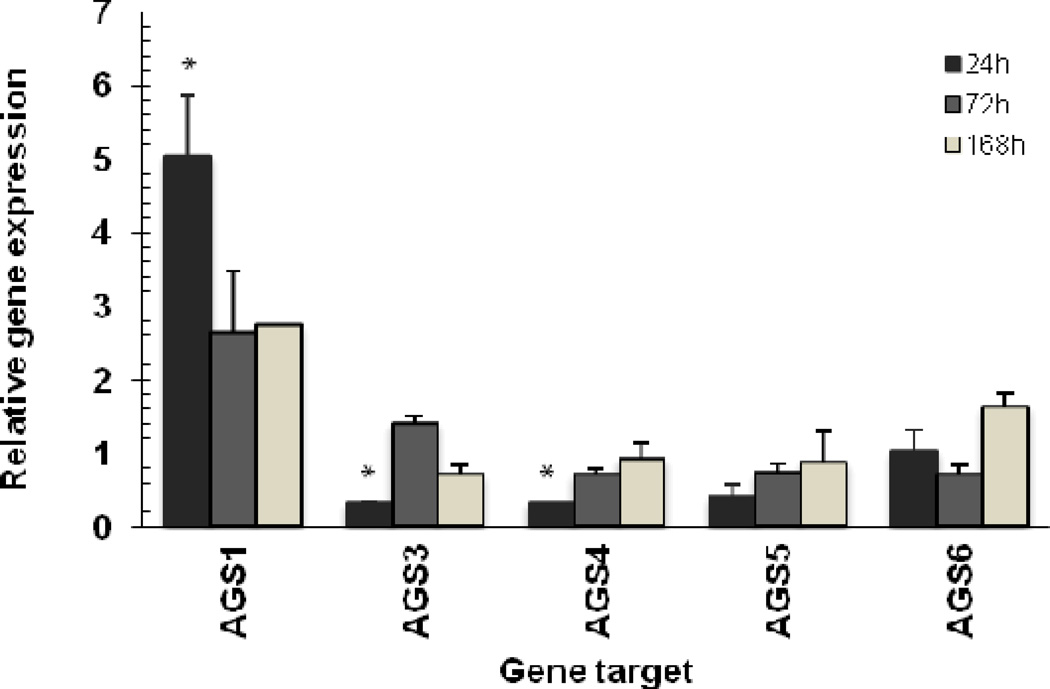
Using this approach, we determined that there was a significant increase of 5-fold (P<0.01) in the Group I AGS gene expression for AGS1/RasD1 following IRI for 24 hours. The steady-state levels of AGS1 mRNA remained elevated at a lower plateau level for the duration of the 7 day experimental period (Figure 2).
Figure 2.
Quantitative RT-PCR analysis of Group I and II AGS gene expression in normal and ischemia-reperfusion injured rat kidneys. Relative gene expression was determined by the AACT method using 18S RNA level for normalization. Data is shown as mean ± STD. Statistical analysis was performed on ACT values to compare gene expression between injured (n=5) and sham (controls) groups (n=5). * P<0.05 was considered significant.
The Group II AGS gene expression, specifically AGS3, 4 and 5, were calculated to have a significant reduction by ~70% (P<0.05) in the mRNA levels after 24 hours following IRI. After 72 hours, AGS3 mRNA levels demonstrated a dramatic increase of ~40% in the mRNA levels, whereas AGS4/GPSM3 and AGS5/LGN mRNA remained 20–30% lower than normal levels. By day 7, AGS3, 4 and 5 mRNA levels were similar to the sham-operated kidneys. AGS6/RGS12 mRNA levels were not significantly different at any of the time points analyzed in this assay.
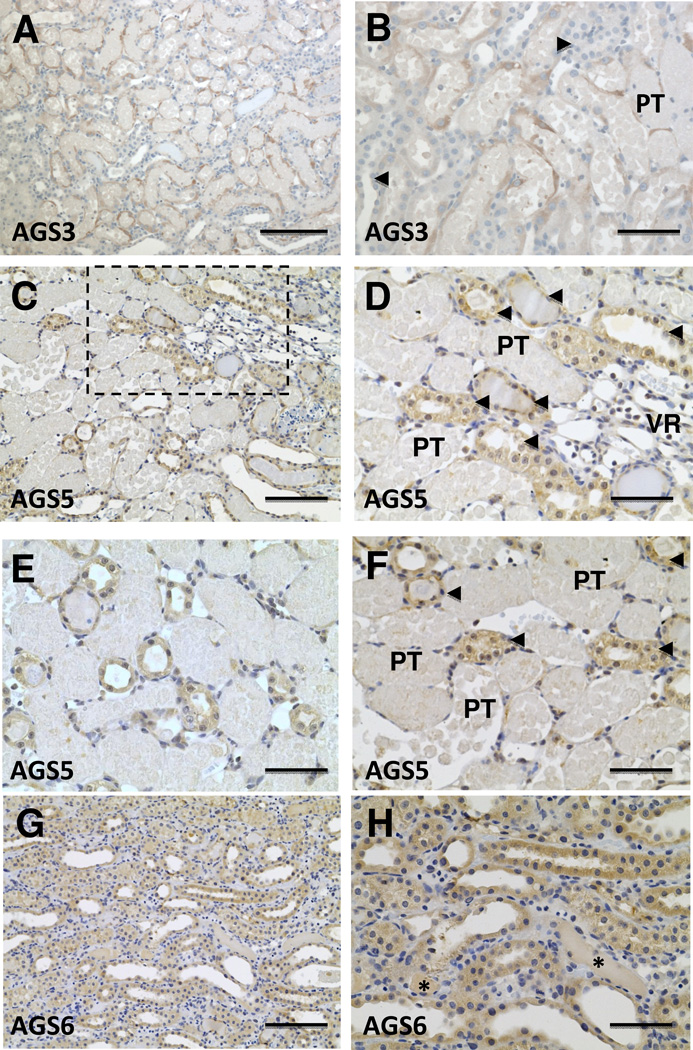
Renal sites of expression for AGS3/GPSM1 and AGS5/LGN following ischemia-reperfusion injury
Consistent with previous studies in our lab (Regner et al.), AGS3/GPSM1 expression was largely expressed in renal tubular epithelial cells of the collecting ducts (Fig. 3A and 3B). The intensity of the AGS3/GPSM1 signal was higher in the cortex than the medulla. AGS5/LGN was expressed in the distal tubular epithelial cells, including thick ascending limbs of Henle and collecting ducts. At 24 hours following IRI, the AGS5/LGN protein expression remained in the distal tubular epithelial cells (Fig. 3C–3F). Unlike AGS3/GPSM1, the induction of AGS5/LGN protein was not observed in damaged proximal tubular structures. Moreover, neither AGS3/GPSM1 nor AGS5/LGN expression was detected in vascular structures, like vasa recta bundles, or glomeruli following IRI.
Figure 3.
Immunolocalization of AGS3/GPSM1 and AGS5/LGN in the kidney following ischemia-reperfusion injury. Sprague Dawley rat kidneys were harvested after 24 hours following a 30 minute bilateral ischemic period. The kidneys were cut in half, fixed in neutral buffered formalin and paraffin embedded for immunostaining with primary antibodies targeted to AGS3/GPSM1 (Fig. 3A and 3B), AGS5/LGN (Fig. 3C–3F), and AGS6/RGS12 (Fig. 3G–3H). Figure 3D is a higher magnification of the dashed box in Fig. 3C. PT = proximal tubules containing casts, arrowheads = distal tubular epithelial cells, including thick ascending limb of Henle, distal convoluted tubules and collecting ducts; VR = vasa recta bundle. In Fig. 3H, * indicates the lack of GS6 staining in cast-positive tubules. Scale bar = 50 µm (B, D, E, F and H), 100 µm (C and G), and 200 µm (A).
AGS6/RGS12 expression was throughout the renal tubular epithelial cells following IRI (Fig. 3G and 3H), but there was no distinct change in the intensity other than the tubular structures that contained casts did not express AGS6/RGS12 protein unlike AGS3/GPSM1.
Changes in the gene expression levels for Group I and II AGS genes from non-cystic and polycystic kidney diseased kidneys
To investigate whether chronic renal diseases with a proliferative phenotype have altered AGS gene expression, we examined polycystic kidney (PCK) rat kidneys and their non-cystic Sprague Dawley (SD) controls at post-natal week 16 using the same quantitative RT-PCR methods described for the IRI experiment (Figure 4).
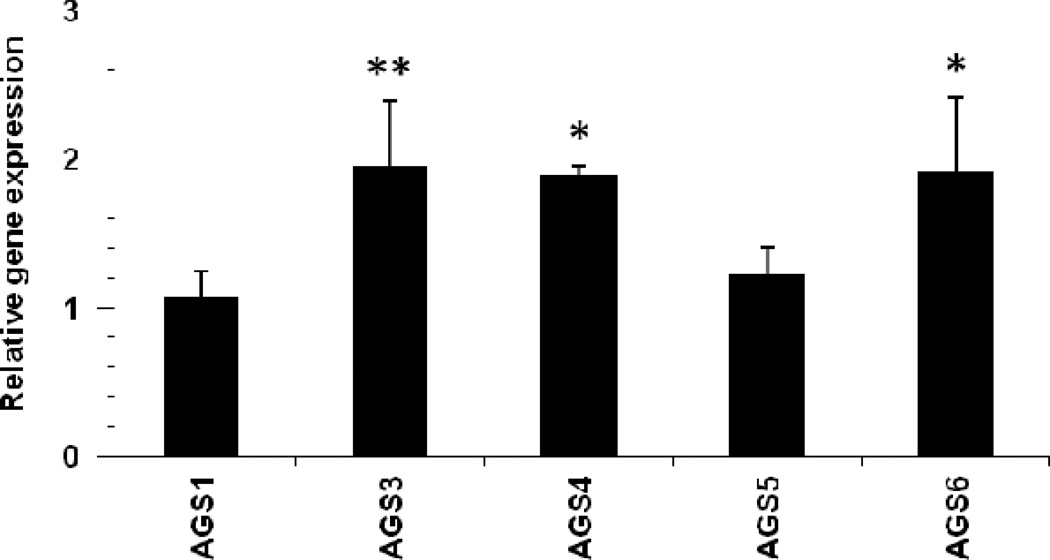
Figure 4.
Renal AGS gene expression profile from the Polycystic kidney (PCK) rat kidneys. Relative gene expression was determined by the ΔΔCT method using 45S RNA level for normalization. The bar graphs represent the mean ± STD (n = 5) from 3 separate RT-PCR runs. Statistical analysis was performed on ΔCT values to compare gene expression between injured and sham age-matched (controls) groups. * P<0.05 was considered significant from sham-operated controls; ** P< 0.01 was considered significant from sham-operated controls.
Using this approach, we were unable to detect any significant change in the AGS1/RasD1 and AGS5/LGN mRNA levels between non-cystic SD and PCK rat kidneys. On the other hand, AGS3/GPSM1, AGS4/GPSM3, and AGS6/RGS12 were calculated to have a significant increase with 83% (P<0.01), 89% (P<0.05), and 76% (P<0.05), respectively, in the cystic PCK rat kidneys compared to non-cystic controls.
Localization of AGS proteins in rodent kidneys affected by polycystic kidney disease
AGS1/RasD1 (Fig. 5A) and AGS6/RGS12 (Fig. 5B) were observed in proximal tubular epithelial cells from Pkd1V/V mouse kidneys. No observeable staining for AGS1 or AGS6 was detected in the cystic epithelial cells.
Figure 5.
Localization of Group I and II AGS3 proteins in polycystic kidney disease. Immunohistochemical staining was performed in mouse Pkd1V/V (A–D) and rat (E–F) PCK kidney models of polycystic kidney disease (PKD). AGS1, AGS5, and AGS6 were immunostained in kidneys sectioned from the Pkd1V/V mouse at post-natal day 19. In the PCK rat kidneys at 26 weeks of age, AGS5 immunostaining was performed and shown in (E) and (F). Scale bars = 200 µm (A–E), 50 µm (F).
On the other hand, AGS5/LGN was observed in the cystic epithelial cells in both the Pkd1V/V mouse kidneys (Fig. 5C and 5D) and PCK rat kidneys (Fig. 5E and 5F).
Role of AGS3/GPSM1 in the process of renal hypertrophy following unilateral nephrectomy
Since AGS3/GPSM1 mRNA and/or protein is induced or increased under pathological states of acute or chronic renal proliferative injuries, we hypothesized whether AGS3/GPSM1 may play a role during other renal processes that may be activated to regulate cell size, i.e., renal hypertrophy.
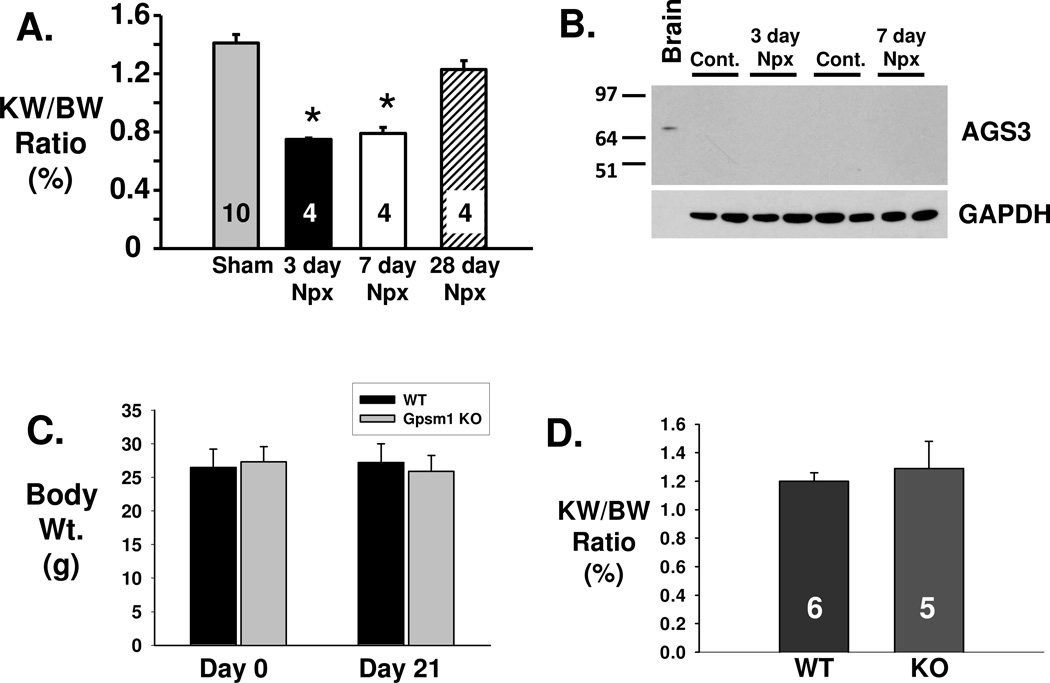
As a model of renal hypertrophy, we performed unilateral nephrectomy in PCK rats at post-natal 8 weeks of age. After 3 (n=4), 7 (n=4) and 28 days (n=4) after unilateral nephrectomy, the rats were euthanized and the kidneys were removed and weighed. As shown in Figure 6A, there was a significant reduction of 47% (P<0.05) in the KW/BW ratio at day 3 following nephrectomy. Afterwards, there was a trend for the KW/BW ratio to increase back towards sham-treated PCK rats (n=10) demonstrating that the remaining contralateral kidney in the nephrectomized PCK rat was capable of increasing its size. The two-kidney weight in 4 week old PCK rats was measured to be 1.28 ± 0.2 g (n=5). At 8 weeks of age, the kidneys increased in size and averaged 3.45 ± 0.53 g (n=10) in PCK rats. In our study, unilateral nephrectomy accelerated the size of the remaining kidney to weigh nearly the same (3.04 ± 0.15 g; n=4) as two kidneys from naïve 8 week old PCK rats or a single kidney from a 12 week old normal PCK rat (3.31 ± 0.14 g; n=5). These findings would suggest that kidney growth can be accelerated in the PCK rat using surgical nephrectomy.
Figure 6.
AGS3 does not play a role in the hypertrophic response of the kidney after unilateral nephrectomy. Male PCK rats (8 week of age) were either sham- or unilaterally nephrectomized and euthanized for kidney collection after 3, 7 or 28 days (n=4 for each time point). A) Kidney-to-body weight ratio is an index of the kidney size and were calculated at each time point. * P<0.05 significant difference between the sham-operated PCK rat kidney control group. B) Western blot analysis was performed using the kidney lysates (50 µg/lane) isolated from the PCK rats with (Npx) and without unilateral nephrectomy (Cont.). GAPDH was used as a loading control. Brain (1 µg) was used as a positive control for the detection of AGS3. Npx = unilateral nephrectomy. (C) and (D) Wild-type and Gpsm1-deficient mice were unilateral nephrectomized at day 0, and the remaining kidney was harvested 21 days later to measure kidney weight. Body weight was measured at multiple time points throughout the experimental period (C), including at the time of euthanization to calculate the kidney-to-body weight (KW/BW) ratio, which is an index of renal hypertrophy (D). The number of animals used in this experiment are shown in the bars.
To determine whether the expression of AGS3/GPSM1 was altered during the hypertrophic process, whole kidneys were isolated after 3 and 7 days after the nephrectomy for immunoblot analysis (Fig. 6B). AGS3/GPSM1 protein expression was not readily detectable at this early time points, which differs from our previous IRI rat and mouse studies where robust AGS3/GPSM1 expression was detected within 1–3 days after the biological insult (Regner, Nozu 2011).
To confirm whether AGS3/GPSM1 played a role in renal hypertrophy, wild-type Gpsm1+/+ (n=6) and genetically deficient Gpsm1−/− mice (n=5) were compared for their ability to undergo hypertrophy following surgery to remove a single kidney. The hypertrophied kidney was evaluated 21 days after the nephrectomy by measuring their kidney-to-body weight ratio, which would be an index of the growth of the kidney. As shown in Figure 6C and 6D, the KW/BW ratio was not different between Gpsm1−/− (n=5) versus wild-type Gpsm1+/+ littermates (n=6), which would suggest that the increased kidney growth is not dependent upon the expression of full-length AGS3/GPSM1 protein.
Discussion
Activator of G-protein Signaling (AGS) is a group of accessory proteins that were identified using a genetically modified yeast strain deficient of the pheromone receptor and expressing a mutant yeast-human Gα subunit (Cismowski, Takesono 1999, Takesono, Cismowski 1999, Nielsen, DiGiovanni 1993, Sato, Hiraoka 2011). At present, AGS proteins are classified into four distinct groups depending upon their protein structure and type of interaction with either α or βγ subunits of the heterotrimeric G-protein (Blumer and Lanier 2014).
Group I AGS proteins
AGS1 was the first protein isolated from the yeast screen by Cismowski et al. (Cismowski, Takesono 1999) and was classified as a Group I AGS protein. Group I AGS proteins function as a guanine nucleotide exchange factor (GEF), which can activate GTPases by facilitating the switch of a guanosine diphosphate (GDP) with a guanosine triphosphate (GTP). AGS1 has alternate names, RasD1 and Dexras1, and is a dexamethasone-inducible member of the Ras superfamily of small GTPases. AGS1/RasD1 mRNA was detected at lower abundance levels in the mouse (Kemppainen and Behrend 1998) and human kidneys (Kemppainen, Cox 2003, Tu and Wu 1999, Vaidyanathan, Cismowski 2004) compared to the skeletal muscle, heart and the brain. Kemppainen and Behrend (Kemppainen and Behrend 1998) showed AGS1/RasD1 mRNA induction in the kidney after 60 minutes following a bolus injection of dexamethasone. In our study, we observed a significant increase in the expression of AGS1/RasD1 mRNA following renal IRI in Sprague Dawley rats. Since AGS1/RasD1 is predominantly expressed in the proximal tubules in the renal cortex and outer medulla, which are the nephron sites that are most sensitive to ischemic injury, may play a role in the recovery process following IRI. On the other hand, AGS1/RasD1 was not detected in the cystic epithelial cells from the Pkd1V/V mouse, and only in proximal tubular cells, which would likely suggest that this protein has no pathological role in the progression of cystogenesis. Further studies are warranted to better describe the functional role of AGS1/RasD1 in the kidney.
More recently, three accessory proteins that were not originally isolated from the yeast screen have been included as a Group I AGS protein, because of their protein structure and proposed mechanism of action (Blumer and Lanier 2014): 1) Ras homolog enriched in striatum (Rhes), which is also known as tumor endothelial marker 2 (TEM2) or RasD2; 2) GIV/Girdin (Girders of actin filaments)/APE (Akt phosphorylation enhancer); and 3) Ric-8. To date, the expression of Rhes/RasD2 (Spano, Branchi 2004), GIV/Girdin (Anai, Shojima 2005), and Ric-8 (Miller, Emerson 2000, Tonissoo, Koks 2006, Tonissoo, Meier 2003) are minimally expressed in the normal kidney, but there is a recent study demonstrating that GIV/Girdin can be selectively induced following chemical injury (Wang, Misaki 2014). Upon induction, GIV/Girdin forms a complex with Gαi to protect the glomeruli by activating Akt signaling (Wang, Misaki 2014).
Group II AGS proteins
Group II AGS proteins are characterized by the presence of one or more GoLoco/G-protein regulatory (GPR) motifs at the C-terminal end, which plays a critical role in selective binding to Gαi2/i3 subunits. Group II AGS proteins can be categorized into three distinct types based upon their protein structure.
The first type of Group II AGS proteins contain N-terminal tetratricopeptide repeats and 4 C-terminal GPR domains separated by a linker region. AGS3/GPSM1 and AGS5/LGN/GPSM2 are mammalian homologs of the Drosophila Partner of Inscuteable (Pins) protein, and the primary members exhibiting this type of protein structure. AGS3/GPSM1 expression has been previously shown to be restricted to the distal tubular segments of the nephron in mature rodent kidneys (Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011), which is consistent with the findings in the present study. AGS5/LGN/GPSM2 was found to be exclusively expressed in distal tubule segments of the nephron, including the thick ascending limb of Henle and collecting ducts, in mature rodent kidneys.
In animal models of polycystic kidney disease, the expression of AGS3/GPSM1 remains elevated in the cystic epithelial cells in the collecting duct, which are known to be actively proliferative (Kwon, Pavlov 2012, Nadella, Blumer 2010). Similarly, there is marked induction of AGS3/GPSM1 protein expression during the regenerative phase of the proximal tubular epithelial cells following bilateral ischemia-reperfusion injury (Regner, Nozu 2011). Unlike AGS3/GPSM1, the protein expression of AGS5/LGN was unchanged regardless of the proliferative state of the epithelial cell (Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011). However, the intensity of the AGS5/LGN protein levels appeared higher than the endogenous expression of AGS3/GPSM1 (Nadella, Blumer 2010, Regner, Nozu 2011). In these previous studies, AGS3/GPSM1 was induced by biological stress on the tubular epithelia and may be involved as a compensatory protein to promote epithelial cell repair and recovery, particularly during the proliferative phase of the repair process. Although the biological role for AGS5/LGN in the kidney remains largely undescribed, there is evidence in vitro that AGS5/LGN may play a role during proliferative disease processes by controlling cyst formation in a renal epithelial cell system (Xiao, Wan 2012, Zheng, Zhu 2010). The in vitro effects of AGS5/LGN to promote cystogenesis may be consistent with the localization of this protein in the tubular epithelial cells that became cystic in multiple rodent models of ARPKD and ADPKD.
Since there is increasing evidence for Group II AGS proteins to modulate tubular epithelial cell recovery following biological injury associated with proliferation, we further examined whether AGS3/GPSM1 expression could be induced during other mechanisms leading to cellular growth, such as hypertrophy. Unlike IRI, there was no detectable change in the expression of AGS3/GPSM1 during the initial seven days following the unilateral nephrectomy procedure. Second, there was no significant difference in the kidney weight between wild-type Gpsm1+/+ and null Gpsm1−/− mice after 3 weeks following unilateral nephrectomy. These data would suggest that AGS3/GPSM1 may not play a crucial role in the hypertrophic response unlike its pivotal role during hyperplasia (Kwon, Pavlov 2012, Nadella, Blumer 2010, Regner, Nozu 2011). However, it is possible that other accessory proteins, which exhibits similar localization pattern in the kidney and overlapping function on G-protein regulation as AGS3/GPSM1, such as AGS5/LGN, or truncation forms of AGS3/GPSM1 normally observed in non-renal organs (Pizzinat, Takesono 2001) could have compensated for the genetic loss of AGS3/GPSM1 during hypertrophy allowing the kidney to maintain its ability to grow. Alternatively, AGS3/GPSM1 has been shown to function as a repair protein through the activation of Gβγ signaling (Kwon, Pavlov 2012, Regner, Nozu 2011, Nadella, Blumer 2010). Global blockade of Gβγ signaling in the kidney following IRI results in the inability of the damaged renal tubular epithelial cells to fully recover (White, North 2014), but it remains to be determined what role, if any, Gβγ signaling controls in the process of tubular epithelial cell hypertrophy.
The second type of Group II AGS proteins contains AGS6/RGS12, RGS14 and Rap1GAP, which are identified by the presence of a single GPR motif and other protein regulatory domains that function to accelerate Gα-GTP hydrolysis (Blumer and Lanier 2014). Previous studies detected human RGS12 mRNA in the whole kidney (Snow, Hall 1998), but not in the mouse using Northern blot analyses (Yang and Li 2007). Our study confirms the presence of AGS6/RGS12 in the rat kidney, but the expression is relatively weak to moderate throughout the nephron segments and cells lining the lumen of the vasculature. The GTPase activity of AGS6/RGS12 plays a major role in the function of AGS6/RGS12 (Snow, Hall 1998), while further studies are needed to characterize the GPR motif and elucidate the biological function for AGS6/RGS12 in the kidney.
In terms of RGS14 and Rap1GAP, neither were evaluated in this study due to their relatively restricted expression in adult mice. RGS14 is an accessory protein that interacts with Rap1/2 and Gαo subunits, and its mRNA expression was restricted to the spleen and select regions in the brain (Traver, Bidot 2000). Rap1GAP functions to inactivate RAP1 activity by accelerating the degradation of GTP-bound to Rap1 (Spilker and Kreutz 2010, Willard, Kimple 2004), and its expression in the kidney is limited to renal carcinoma cells (Kim, Gersey 2012) and podocytes within the renal glomeruli (Potla, Ni 2014).
The third type of group II AGS proteins consists of 3 proteins, AGS4/GPSM3 (Cao, Cismowski 2004), a truncated form of AGS3 (AGS3-SHORT) (Pizzinat, Takesono 2001), and Pcp-2/L7/GPSM4 (Nordquist, Kozak 1988), which has multiple C-terminal GPR domains, but no other defined regulatory protein binding sites. In the present study, we detected AGS4/GPSM3 mRNA in the rat kidney similar to previously published studies (Cao, Cismowski 2004), but we were unable to confirm the protein translation or localization in the kidney. A recent study has shown that AGS4/GPSM3 can interact with intracellular proteins, such as 14-3-3, to reduce protein degradation (Giguere, Laroche 2012), which may be absent in the kidney. This could alter the rate of degradation of AGS4/GPSM3 protein and could be one contributing factor leading to the inability to detect this protein in the kidney sections. For this reason, AGS4/GPSM3 has been largely studied in other cell types, particularly immune cells, due to its relatively high expression levels (Cao, Cismowski 2004, Billard, Gall 2014). Functionally, there is evidence that AGS4/GPSM3 couples to Gαi subunits to modulate agonist-dependent G-protein coupled receptor signaling (Oner, Maher 2010).
The other type III proteins in Group II AGS were not examined for renal expression in this study. First, Pcp-2/L7/GPSM4 has been shown to have exclusive expression in the central nervous system and retinal bipolar neurons (Nordquist, Kozak 1988, Saito, Tsumura 2005). Second, the two truncated isoforms of AGS3/GPSM1, known as AGS3-SHORT1 and AGS3-SHORT2 (Pizzinat, Takesono 2001), are not able to be discriminated from full-length AGS3/GPSM1 in the kidney using the available antibodies. Both AGS3-SHORT isoforms contain only the 4 GPR motifs, and are devoid of the N-terminal tetratricopeptide region (TPR) and most of the linker region. Conceivably, these truncated isoforms will likely maintain their ability to interact with Gαi subunits, but their subcellular localization and G-protein subunit modulation may be distinct to full-length AG3/GPSM1, which can interact with other proteins in the N-terminal TPR or linker region (Blumer, Bernard 2003). It is important to note that these short forms of AGS3/GPSM1 are observed in the heart from Gpsm1−/− mice (Blumer, Lord 2008), but we have not detected these bands in the normal kidneys (unpublished observations).
Limitations in determining the role of AGS in the kidney
The identification of Group I and II AGS proteins at the level of mRNA and protein expression in the kidney provides molecular and cellular evidence that there may be a biological role for these accessory proteins in the kidney. However, the biological relevance of Group I and II AGS in the kidney requires further investigation in genetically modified animal models. At present, mouse models that are genetically deficient for AGS1 (Cha, Kim 2013, Chen, Khan 2013, Cheng, Obrietan 2004), AGS3 (Regner, Nozu 2011, Blumer, Lord 2008), AGS4 (Giguere, Billard 2013, Giguere, Gall 2014), AGS5 (Konno, Shioi 2008) and AGS6 (Yang, Li 2013) are available, so there is opportunity exists to determine whether changes in the AGS gene expression can play a crucial role during various pathologies in the kidney.
In summary, this study provides valuable new information regarding the expression and spatial localization of the Group I and II AGS proteins in normal and pathologically injured or diseased kidneys. AGS proteins have diverse biological function, and exhibit varied sites of expression within the nephron. Minimal to no expression of AGS proteins are detected outside of the renal tubular epithelial cell, except for AGS6/RGS12 in the endothelial cells. These findings would provide some insight into the biological function of AGS proteins by allowing us to determine their site of action within the nephron, which has distinct roles in fluid and electrolyte balance, pH regulation, and hormone production.
Acknowledgements
The authors would like to thank Dr. Dr. Joe B. Blumer (Medical University of South Carolina, Charleston, SC) for his helpful discussions regarding AGS proteins, and Dr. Stephen M. Lanier (Wayne State University, Detroit, MI) for providing the AGS5/LGN antibody. This work was funded by RO1 DK09123 (F.P., K.R.R) and institutional lab funds (F.P.)
Funding: This study was funded by NIH RO1 DK09123.
Footnotes
Disclosures
Conflict of Interest: The authors declare that there is no conflict of interest.
CITED LITERATURE
- 1.Filardo EJP, Thomas Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni H, Chen J, Pan M, Zhang M, Zhang J, et al. FTY720 prevents progression of renal fibrosis by inhibiting renal microvasculature endothelial dysfunction in a rat model of chronic kidney disease. J Mol Histol. 2013;44:693–703. doi: 10.1007/s10735-013-9521-8. [DOI] [PubMed] [Google Scholar]
- 3.Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, et al. Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol. 2013;182:96–106. doi: 10.1016/j.ajpath.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel AM. Inherited endocrine diseases involving G proteins and G protein-coupled receptors. Endocr Dev. 2007;11:133–144. doi: 10.1159/000111069. [DOI] [PubMed] [Google Scholar]
- 5.Vallon V, Stockand J, Rieg T. P2Y receptors and kidney function. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:731–742. doi: 10.1002/wmts.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, et al. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–883. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- 7.Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, et al. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 8.Blumer JB, Lanier SM. Activators of G protein signaling exhibit broad functionality and define a distinct core signaling triad. Mol Pharmacol. 2014;85:388–396. doi: 10.1124/mol.113.090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumer JB, Smrcka AV, Lanier SM. Mechanistic pathways and biological roles for receptor-independent activators of G-protein signaling. Pharmacol Ther. 2007;113:488–506. doi: 10.1016/j.pharmthera.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Blumer JB, Simon V, Lanier SM. Accessory proteins for G proteins: partners in signaling. Annu Rev Pharmacol Toxicol. 2006;46:151–187. doi: 10.1146/annurev.pharmtox.46.120604.141115. [DOI] [PubMed] [Google Scholar]
- 11.Blumer JB, Chandler LJ, Lanier SM. Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J Biol Chem. 2002;277:15897–15903. doi: 10.1074/jbc.M112185200. [DOI] [PubMed] [Google Scholar]
- 12.De Vries L, Fischer T, Tronchere H, Brothers GM, Strockbine B, et al. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc Natl Acad Sci U S A. 2000;97:14364–14369. doi: 10.1073/pnas.97.26.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon M, Pavlov TS, Nozu K, Rasmussen SA, Ilatovskaya DV, et al. G-protein signaling modulator 1 deficiency accelerates cystic disease in an orthologous mouse model of autosomal dominant polycystic kidney disease. Proc Natl Acad Sci U S A. 2012;109:21462–21467. doi: 10.1073/pnas.1216830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadella R, Blumer JB, Jia G, Kwon M, Akbulut T, et al. Activator of G protein signaling 3 promotes epithelial cell proliferation in PKD. J Am Soc Nephrol. 2010;21:1275–1280. doi: 10.1681/ASN.2009121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regner KR, Nozu K, Lanier SM, Blumer JB, Avner ED, et al. Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB J. 2011;25:1844–1855. doi: 10.1096/fj.10-169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White SM, North LM, Haines E, Goldberg M, Sullivan LM, et al. G-Protein betagamma Subunit Dimers Modulate Kidney Repair after Ischemia-Reperfusion Injury in Rats. Mol Pharmacol. 2014;86:369–377. doi: 10.1124/mol.114.092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126–136. doi: 10.1046/j.1523-1755.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Hackmann K, Gao J, He X, Piontek K, et al. Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc Natl Acad Sci U S A. 2007;104:18688–18693. doi: 10.1073/pnas.0708217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumer JB, Lord K, Saunders TL, Pacchioni A, Black C, et al. Activator of G protein signaling 3 null mice: I. Unexpected alterations in metabolic and cardiovascular function. Endocrinology. 2008;149:3842–3849. doi: 10.1210/en.2008-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, et al. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR-and c-Src-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F662–F670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Hiraoka M, Suzuki H, Bai Y, Kurotani R, et al. Identification of transcription factor E3 (TFE3) as a receptor-independent activator of Galpha16: gene regulation by nuclear Galpha subunit and its activator. J Biol Chem. 2011;286:17766–17776. doi: 10.1074/jbc.M111.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- 25.Kemppainen RJ, Cox E, Behrend EN, Brogan MD, Ammons JM. Identification of a glucocorticoid response element in the 3′-flanking region of the human Dexras1 gene. Biochim Biophys Acta. 2003;1627:85–89. doi: 10.1016/s0167-4781(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 26.Tu YC, Wu Cloning, expression and characterization of a novel human Ras-related protein that is regulated by glucocorticoid hormone. Biochim Biophys Acta. 1999;1489:452–456. doi: 10.1016/s0167-4781(99)00197-9. [DOI] [PubMed] [Google Scholar]
- 27.Vaidyanathan G, Cismowski MJ, Wang G, Vincent TS, Brown KD, et al. The Ras-related protein AGS1/RASD1 suppresses cell growth. Oncogene. 2004;23:5858–5863. doi: 10.1038/sj.onc.1207774. [DOI] [PubMed] [Google Scholar]
- 28.Spano D, Branchi I, Rosica A, Pirro MT, Riccio A, et al. Rhes is involved in striatal function. Mol Cell Biol. 2004;24:5788–5796. doi: 10.1128/MCB.24.13.5788-5796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anai M, Shojima N, Katagiri H, Ogihara T, Sakoda H, et al. A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J Biol Chem. 2005;280:18525–18535. doi: 10.1074/jbc.M500586200. [DOI] [PubMed] [Google Scholar]
- 30.Miller KG, Emerson MD, McManus JR, Rand JB. RIC-8 (Synembryn): a novel conserved protein that is required for G(q)alpha signaling in the C. elegans nervous system. Neuron. 2000;27:289–299. doi: 10.1016/s0896-6273(00)00037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonissoo T, Koks S, Meier R, Raud S, Plaas M, et al. Heterozygous mice with Ric-8 mutation exhibit impaired spatial memory and decreased anxiety. Behav Brain Res. 2006;167:42–48. doi: 10.1016/j.bbr.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Tonissoo T, Meier R, Talts K, Plaas M, Karis A. Expression of ric-8 (synembryn) gene in the nervous system of developing and adult mouse. Gene Expr Patterns. 2003;3:591–594. doi: 10.1016/s1567-133x(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Misaki T, Taupin V, Eguchi A, Ghosh P, et al. GIV/Girdin Links Vascular Endothelial Growth Factor Signaling to Akt Survival Signaling in Podocytes Independent of Nephrin. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao Z, Wan Q, Du Q, Zheng Z. Galpha/LGN-mediated asymmetric spindle positioning does not lead to unequal cleavage of the mother cell in 3-D cultured MDCK cells. Biochem Biophys Res Commun. 2012;420:888–894. doi: 10.1016/j.bbrc.2012.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Z, Zhu H, Wan Q, Liu J, Xiao Z, et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J Cell Biol. 2010;189:275–288. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizzinat N, Takesono A, Lanier SM. Identification of a truncated form of the G-protein regulator AGS3 in heart that lacks the tetratricopeptide repeat domains. J Biol Chem. 2001;276:16601–16610. doi: 10.1074/jbc.M007573200. [DOI] [PubMed] [Google Scholar]
- 37.Nadella R, Blumer JB, Jia G, Kwon M, Akbulut T, et al. Activator of G Protein Signaling 3 Promotes Epithelial Cell Proliferation in PKD. J Am Soc Nephrol. 2010:1275–1280. doi: 10.1681/ASN.2009121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snow BE, Hall RA, Krumins AM, Brothers GM, Bouchard D, et al. GTPase activating specificity of RGS12 and binding specificity of an alternatively spliced PDZ (PSD-95/Dlg/ZO-1) domain. J Biol Chem. 1998;273:17749–17755. doi: 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- 39.Yang SYP, Li RGS12 is essential for RANKL-evoked signaling for terminal differentiation of osteoclasts in vitro. J Bone Miner Res. 2007;22:45–54. doi: 10.1359/jbmr.061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traver S, Bidot C, Spassky N, Baltauss T, De Tand MF, et al. RGS14 is a novel Rap effector that preferentially regulates the GTPase activity of galphao. Biochem J 350 Pt. 2000;1:19–29. [PMC free article] [PubMed] [Google Scholar]
- 41.Spilker CMR, Kreutz RapGAPs in brain: multipurpose players in neuronal Rap signalling. Eur J Neurosci. 2010;32:1–9. doi: 10.1111/j.1460-9568.2010.07273.x. [DOI] [PubMed] [Google Scholar]
- 42.Willard FS, Kimple RJ, Siderovski DP. Return of the GDI: the GoLoco motif in cell division. Annu Rev Biochem. 2004;73:925–951. doi: 10.1146/annurev.biochem.73.011303.073756. [DOI] [PubMed] [Google Scholar]
- 43.Kim WJ, Gersey Z, Daaka Y. Rap1GAP regulates renal cell carcinoma invasion. Cancer Lett. 2012;320:65–71. doi: 10.1016/j.canlet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potla U, Ni J, Vadaparampil J, Yang G, Leventhal JS, et al. Podocyte-specific RAP1GAP expression contributes to focal segmental glomerulosclerosis -associated glomerular injury. J Clin Invest. 2014;124:1757–1769. doi: 10.1172/JCI67846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao X, Cismowski MJ, Sato M, Blumer JB, Lanier SM. Identification and characterization of AGS4: a protein containing three G-protein regulatory motifs that regulate the activation state of Gialpha. J Biol Chem. 2004;279:27567–27574. doi: 10.1074/jbc.M312786200. [DOI] [PubMed] [Google Scholar]
- 46.Nordquist DT, Kozak CA, Orr HT. cDNA cloning and characterization of three genes uniquely expressed in cerebellum by Purkinje neurons. J Neurosci. 1988;8:4780–4789. doi: 10.1523/JNEUROSCI.08-12-04780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giguere PM, Laroche G, Oestreich EA, Duncan JA, Siderovski DP. Regulation of the subcellular localization of the G-protein subunit regulator GPSM3 through direct association with 14-3-3 protein. J Biol Chem. 2012;287:31270–31279. doi: 10.1074/jbc.M112.394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Billard MJ, Gall BJ, Richards KL, Siderovski DP, Tarrant TK. G protein signaling modulator-3: a leukocyte regulator of inflammation in health and disease. Am J Clin Exp Immunol. 2014;3:97–106. [PMC free article] [PubMed] [Google Scholar]
- 49.Oner SS, Maher EM, Breton B, Bouvier M, Blumer JB. Receptor-regulated interaction of activator of G-protein signaling-4 and Galphai. J Biol Chem. 2010;285:20588–20594. doi: 10.1074/jbc.C109.088070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito H, Tsumura H, Otake S, Nishida A, Furukawa T, et al. L7/Pcp-2-specific expression of Cre recombinase using knock-in approach. Biochem Biophys Res Commun. 2005;331:1216–1221. doi: 10.1016/j.bbrc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 51.Blumer JB, Bernard ML, Peterson YK, Nezu J, Chung P, et al. Interaction of activator of G-protein signaling 3 (AGS3) with LKB1, a serine/threonine kinase involved in cell polarity and cell cycle progression: phosphorylation of the G-protein regulatory (GPR) motif as a regulatory mechanism for the interaction of GPR motifs with Gi alpha. J Biol Chem. 2003;278:23217–23220. doi: 10.1074/jbc.C200686200. [DOI] [PubMed] [Google Scholar]
- 52.Cha JY, Kim HJ, Yu JH, Xu J, Kim D, et al. Dexras1 mediates glucocorticoid-associated adipogenesis and diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:20575–20580. doi: 10.1073/pnas.1320454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Khan RS, Cwanger A, Song Y, Steenstra C, et al. Dexras1, a small GTPase, is required for glutamate-NMDA neurotoxicity. J Neurosci. 2013;33:3582–3587. doi: 10.1523/JNEUROSCI.1497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng HY, Obrietan K, Cain SW, Lee BY, Agostino PV, et al. Dexras1 potentiates photic and suppresses nonphotic responses of the circadian clock. Neuron. 2004;43:715–728. doi: 10.1016/j.neuron.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Giguere PM, Billard MJ, Laroche G, Buckley BK, Timoshchenko RG, et al. G-protein signaling modulator-3, a gene linked to autoimmune diseases, regulates monocyte function and its deficiency protects from inflammatory arthritis. Mol Immunol. 2013;54:193–198. doi: 10.1016/j.molimm.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giguere PM, Gall BJ, Ezekwe EA, Jr, Laroche G, Buckley BK, et al. G Protein Signaling Modulator-3 Inhibits the Inflammasome Activity of NLRP3. J Biol Chem. 2014;289:33245–33257. doi: 10.1074/jbc.M114.578393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- 58.Yang S, Li YP, Liu T, He X, Yuan X, et al. Mx1-cre mediated Rgs12 conditional knockout mice exhibit increased bone mass phenotype. Genesis. 2013;51:201–209. doi: 10.1002/dvg.22373. [DOI] [PMC free article] [PubMed] [Google Scholar]