Abstract
Objective
Patients with rheumatoid arthritis (RA) have increased risk of atherosclerotic cardiovascular disease (ASCVD) that is underestimated by the Framingham risk score (FRS). We hypothesized that the 2013 ACC/AHA 10-year risk score would perform better than the FRS and the Reynolds risk score (RRS) in identifying RA patients known to have elevated cardiovascular risk based on high coronary artery calcification (CAC) scores.
Methods
Among 98 RA patients eligible for risk stratification using the ACC/AHA score we identified 34 patients with high CAC (≥ 300 Agatston units or ≥75th percentile) and compared the ability of the 10-year FRS, RRS and the ACC/AHA risk scores to correctly assign these patients to an elevated risk category.
Results
All three risk scores were higher in patients with high CAC (P values <0.05). The percentage of patients with high CAC correctly assigned to the elevated risk category was similar among the three scores (FRS 32%, RRS 32%, ACC/AHA 41%) (P=0.233). The c-statistics for the FRS, RRS and ACC/AHA risk scores predicting the presence of high CAC were 0.65, 0.66, and 0.65, respectively.
Conclusions
The ACC/AHA 10-year risk score does not offer any advantage compared to the traditional FRS and RRS in the identification of RA patients with elevated risk as determined by high CAC. The ACC/AHA risk score assigned almost 60% of patients with high CAC into a low risk category. Risk scores and standard risk prediction models used in the general population do not adequately identify many RA patients with elevated cardiovascular risk.
Keywords: RA, cardiovascular risk, atherosclerosis
Introduction
Patients with rheumatoid arthritis (RA) have increased risk of atherosclerotic cardiovascular disease (ASCVD) compared to the general population (1,2), but it is difficult to identify those individuals who are at increased risk. In the general population, the 10-year Framingham risk score (FRS) has been widely used to predict cardiovascular risk and to identify individuals for interventions such as lipid lowering treatment. However, the FRS underestimates cardiovascular risk in women and young people (3) and in RA patients (4).
Recognizing the limitations of the FRS, there have been several approaches to improve ASCVD risk prediction, including the addition of C-reactive protein (CRP) to the model, as in the Reynolds risk score (RRS) (5,6). Also, the American College of Cardiology (ACC) and the American Heart Association (AHA) released recently a 10-year cardiovascular risk score (7). This new ACC/AHA cardiovascular risk score seeks to stratify risk in people aged 40–75 years, without diabetes or clinical ASCVD, who have a low density lipoprotein (LDL) cholesterol concentration <190 mg/dL. A predicted 10-year ASCVD risk ≥7.5% obtained using the AHA/ACC model identifies those who would benefit from lipid lowering therapy (7,8).
In the general population the amount of coronary artery calcium (CAC) detected correlates with the amount of subclinical coronary artery atherosclerosis and predicts ASCVD independent of traditional risk factors (9). We and others have previously shown that RA patients have increased CAC compared to control subjects (1,10). We have also shown that the majority of RA patients with CAC would be assigned to a low CV risk category by the FRS (11) and thus would not be thought to warrant lipid lowering therapy. In addition to the FRS, several other risk prediction models have been studied in RA using surrogates of global atherosclerosis (e.g., CAC, carotid intima media thickness (cIMT)) and hard cardiovascular events. Generally, these studies found that these risk scores underestimate cardiovascular risk in RA (4,11–13). In RA, cIMT and presence of carotid plaque may predict coronary events (14,15); however, the new ACC/AHA guidelines do not recommend cIMT for routine risk assessment (7).
Current guidelines for prevention of ASCVD in the general population support the measurement of CAC if there is uncertainty about CV risk categorization after standard risk assessment and suggest that the risk assessment be revised upwards in patients with high CAC scores (defined as ≥300 Agatston units or ≥75th percentile for age, sex and ethnicity) (7).
The presence of RA may represent a clinical situation in which there is uncertainty about CV risk categorization after standard risk assessment; thus, measurement of CAC could be considered. However, measurement of CAC carries with the expense of the test and the risk from exposure to radiation. The relationship between the new ACC/AHA risk score and CAC in RA patients is not known. If the ACC/AHA risk score detects most RA patients who have high CAC scores, then measurement of CAC would be less useful as it would reallocate fewer patients into the elevated risk group. Therefore, we compared the ability of the FRS, the RRS, and the ACC/AHA 10-year risk score to correctly identify RA patients with elevated ASCVD risk as indicated by a high CAC score.
Methods
Study participants
We previously recruited a cohort of patients who met the American College of Rheumatology classification for RA and have studied factors contributing to cardiovascular risk (1). In the present analysis we included those patients in whom the 2013 AHA/ACC risk prediction model would be applicable: aged 40–75 years, no history of a previous cardiovascular event or procedure, not on a statin, not diabetic, and LDL cholesterol <190 mg/dl. A standardized clinical interview and physical examination were performed as described elsewhere (1). Hypertension was defined as any the following: systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or taking antihypertensive medication. The study was approved by the Institutional Review Board at Vanderbilt University and all subjects provided written informed consent.
Coronary artery calcium assessment
Coronary calcium was measured by performing an electron beam tomography with an Imatron C-150 scanner (GE Imatron, South San Francisco, CA) as described before (1). Scans were scored as described by Agatston et al. (16) by a single experienced investigator (PR) who was unaware of the subjects’ clinical status. The estimated percentile for each CAC score was calculated using an online calculator (www.mesa-nhlbi.org/CACReference.aspx) (7). We used a CAC threshold of ≥300 Agatston units or ≥75th percentile for age, sex and ethnicity to identify those patients with high CAC (7).
Laboratory tests
We measured total cholesterol, high-density (HDL) and low-density lipoprotein cholesterol (LDL), and triglyceride concentrations in the hospital clinical laboratory on fasting blood samples. C-reactive protein (CRP) was measured by the hospital clinical laboratory or ELISA (Millipore).
Cardiovascular risk scores
We calculated the 10-year cardiovascular FRS (17), the RRS (5,6), and the ACC/AHA risk for developing a first ACSVD event (7). Because the highest 10-year risk assigned by the FRS is 30%, we assigned the same value for any patient that achieved or exceeded this risk level in the RRS and ACC/AHA risk scores. To compare risk scores, we categorized patients as having either low or elevated 10-year CV risk using the 10% threshold for the FRS and RRS and the 7.5% threshold for the ACC/AHA risk score, as recommended (5–7,17).
Statistical Analysis
Demographic and clinical characteristics were described as frequencies and proportions for categorical variables, or median with interquartile ranges (IQR) for continuous variables. Continuous variables were compared using Wilcoxon rank sum tests and categorical variables with Pearson Chi-square test. The area under the receiver operator characteristic (ROC) curve (or c-statistic) and 95% confidence interval (95%CI) were calculated to determine the ability of the three risk scores to discriminate between patients with and without high CAC. Cochran’s Q test was used to compare the frequency distribution of patients with high CAC classified as having elevated risk among the three risk scores. Statistical analysis was performed using IBM SPSS v.21 and a two-sided 5% significance level was considered significant.
Results
Clinical characteristics
Of the 98 RA patients who would be candidates for CV risk categorization by the ACC/AHA score, 34 (35%) had high CAC. Patients with high CAC had more cardiovascular risk factors (male, smokers, higher total and LDL cholesterol, and higher CRP levels) and a trend towards longer disease duration than those without high CAC (Table 1).
Table 1.
Demographic and clinical characteristics ofpatients with rheumatoid arthritis with and without high coronary artery calcium atherosclerosis
| Characteristics | High Coronary Artery Calcium* | ||
|---|---|---|---|
| No (n=64) |
Yes (n=34) |
P¶ | |
| Demographics | |||
| Age (years) | 52 (46, 62) | 54 (51, 63) | 0.259 |
| Female (%) | 53 (82.8) | 21 (61.8) | 0.021 |
| Caucasian (%) | 58 (90.6) | 30 (88.2) | 0.710 |
| Lipid profile | |||
| Total cholesterol (mg/dL) | 184 (157, 205) | 198 (165, 219) | 0.079 |
| High-density lipoprotein cholesterol (mg/dL) | 46 (39, 58) | 45 (38, 54) | 0.714 |
| Low-density lipoprotein cholesterol (mg/dL) | 114 (86, 133) | 122 (106, 148) | 0.032 |
| Triglycerides (mg/dL) | 106 (77, 142) | 111 (78, 136) | 0.988 |
| Other risk factors | |||
| Systolic blood pressure (mmHg) | 134 (117, 146) | 131 (119, 140) | 0.695 |
| Diastolic blood pressure (mmHg) | 73 (69, 80) | 76 (66, 85) | 0.506 |
| Body mass index (kg/m2) | 27.6 (23.6, 32.5) | 27.6 (24.2, 29.5) | 0.649 |
| Hypertension§ | 29 (45.3) | 19 (55.9) | 0.319 |
| Current smoking status | 11 (17.2) | 16 (47.1) | 0.002 |
| Family history of coronary heart disease | 18 (28.1) | 11 (32.4) | 0.816 |
| C-reactive protein (mg/L) | 3.6 (1.0, 10.5) | 9.5 (3.0, 20.0) | 0.016 |
| Disease duration (years) | 3 (2.0, 16.5) | 13.0 (2.0, 21.0) | 0.055 |
Only White and African-American patients who were 40–75 years old at enrollment were included. Patients with diabetes, or previous cardiovascular event (myocardial infarction, angina, stroke) or procedure (coronary artery bypass or graft), with low density lipoprotein cholesterol≥ 190mg/dL, or using statins were excluded.
Data are presented as median (interquartile range) for continuous variables, count (percentage) for categorical variables.
High coronary artery calcium (CAC) is defined as ≥300 Agatston units or CAC≥75th percentile expected for age, sex and race.
Hypertension defined as systolic blood pressure≥140mmHg, or diastolic blood pressure ≥90mmHg, or taking antihypertensive medication
Wilcoxon’s rank sum test was used for comparing continuous variables, and percentages were compared using the chi-square test.
Comparison of risk scores in patients with and without high CAC
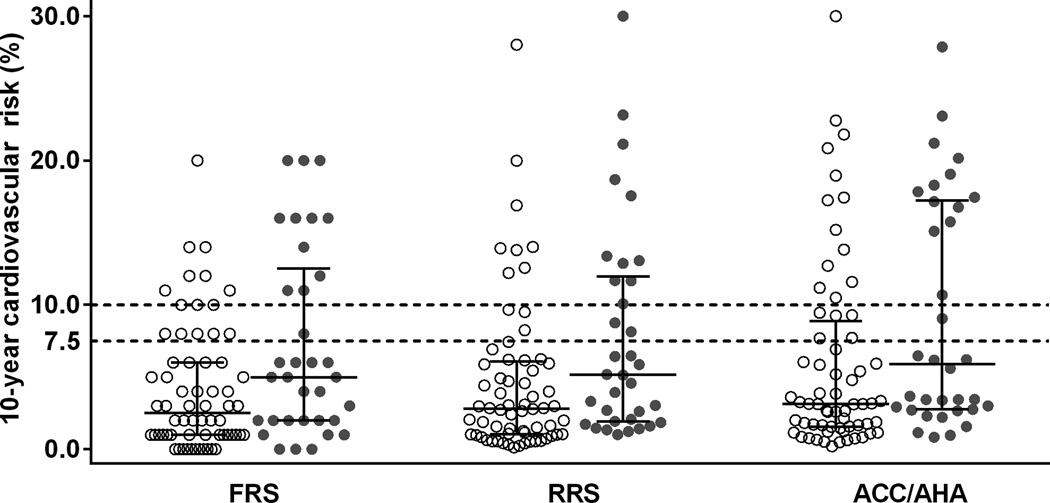
The 10-year risk score was higher in patients with high CAC than those without for the FRS (median 5.0%, [IQR 2.0, 12.0] vs. 2.5%, [1.0, 6.0]; P=0.015), the RRS (5.2%, [1.9, 11.7] vs. 2.8%, [1.0, 6.0]; P=0.008), and the ACC/AHA risk score (5.9%, [2.8, 17.2] vs. 3.1%, [1.6, 8.5]; P=0.017) (Figure 1). Both the FRS and the RRS were more likely to assign patients with high CAC into the elevated risk category than patients without high CAC (p=0.055 and p=0.018, respectively); however, both scores assigned only 32% of patients with high CAC into the elevated risk category. The ACC/AHA risk score assigned 41% of patients with high CAC and 28% without high CAC into the elevated risk categories, respectively (P=0.190) (Table 2). The c-statistic for the FRS, RRS and ACC/AHA risk scores predicting the presence of high CAC were 0.65 (95%CI 0.53, 0.76), 0.66 (95%CI 0.55, 0.77), and 0.65 (95%CI 0.55, 076), respectively.
Figure 1. 10-year cardiovascular risk estimates (%) in patients with rheumatoid arthritis.
Footnote: Patients were considered to have high coronary artery calcification (CAC) if CAC ≥ 300 Agatston units or CAC ≥75th percentile of expected coronary artery calcium for their age, sex and race. Patients with and without high CAC are represented in filled and empty circles, respectively.
Risk scores are represented as the 25th, 50th and 75th percentile. Dotted lines represent the threshold that classified patients into low and elevated risk: 10% for Framingham risk score (FRS) and the Reynolds risk score (RRS), and 7.5% for 10-year American College of Cardiology/American Heart Association (ACC/AHA) risk score.
Table 2.
Cardiovascular risk estimates in patients with rheumatoid arthritis with and without high coronary artery calcium*
| 10-year cardiovascular risk scores | CAC<300 Agatston units or CAC<75th percentile (n=64) |
CAC≥300 Agatston units or CAC≥75th percentile (n=34) |
P values¶ | |
|---|---|---|---|---|
| Framingham risk score† | Low risk category | 54 (84) | 23 (68) | 0.055 |
| Elevated risk category | 10 (16) | 11 (32) | ||
| Reynolds risk score† | Low risk category | 56 (87) | 23 (68) | 0.018 |
| Elevated risk category | 8 (13) | 11 (32) | ||
| ACC/AHA risk score§ | Low risk category | 46 (72) | 20 (59) | 0.190 |
| Elevated risk category | 18 (28) | 14 (41) | ||
Data are presented as frequency and percentages.
High coronary calcium (CAC) was defined as CAC≥300 Agatston units or CAC≥75th percentile expected for age, sex and ethnicity.
P values were determined using Pearson Chi-square test to compare the proportions of patients with and without high CAC assigned into low and elevated risk category.
10% cut-off for low vs. elevated risk;
7.5% cut-off for low vs. elevated risk
ACC/AHA: American College of Cardiology/American Heart Association
Cardiovascular risk reclassification
In addition to comparing ROC curves, the clinical utility of predictive models can be compared by their ability to reclassify patients into a different risk category. Compared to the FRS, the ACC/AHA risk score reclassified 13% (13/98) of RA patients into a different risk category; all except one shifted from low to high risk. The ACC/AHA risk score primarily reclassified patients without high CAC into the elevated risk category and shifted only 3/23 (13%) patients with high CAC and low FRS risk to the elevated risk category (Figure 1 and Supplement Table S1). Similar results were found when contrasting the ACC/AHA risk score with the RRS (Figure 1 and Supplement Table S1). The ability of the three risk scores to allocate patients with high CAC into the elevated risk category did not differ significantly (Cochran’s Q =3.0, P=0.223).
Using a lower threshold for the ACC/AHA risk score
The ACC/AHA risk score correctly identified only 14 of 34 patients with high CAC as falling into the elevated risk category (Figure 1). To explore whether using a lower risk score threshold would improve detection of patients with high CAC, we examined the effect of reducing the threshold for definition of the elevated risk category from 7.5% to 5%, as suggested by the guidelines as being appropriate for patients with unusually elevated risk such as primary and genetic hyperlipidemias, family history of premature ASCVD, CRP≥2mg/L, CAC score ≥300 Agatston units or ≥ 75th percentile, ankle-brachial index <0.9 and elevated lifetime risk of ASCVD (8). Changing the threshold to 5% did indeed result in more patients with high CAC (53%) being correctly placed in the elevated risk category, but it also increased the number of patients without high CAC (38%) assigned to this category.
Discussion
The major finding of this study was that the ACC/AHA 10-year risk score failed to correctly categorize almost 60% of RA patients with high CAC into the elevated risk category.
Overall, the predicted ACC/AHA 10-year risk was higher than the FRS and RRS, and thus allocated more patients with high CAC into the elevated risk category. For example, the ACC/AHA risk score increased the identification of RA patients with high CAC eligible for lipid lowering therapy by almost 10% compared to the FRS (41% vs. 32%). Although this difference was not statistically significant in this small sample, it may be clinically important. Nevertheless, despite concerns that the ACC/AHA score overestimates risk in the general population (18), 20/34 RA patients with CAC high enough to elevate their CV risk categorization were not classified as having elevated risk. There is little information about the ability of the ACC/AHA score to identify patients in the general population with high CAC as having increased risk. However, since the CAC score improves risk prediction beyond that provided by traditional risk factors in the general population, it is possible that, as we found in RA, the ACC/AHA score may not identify many patients with high CAC. For example, among a group of individuals referred for CAC screening, the FRS only identified 46/115 (40%) individuals with a CAC ≥400 Agatston units as having moderately to high risk (≥10%). In that study 25 women had a CAC≥400 Agatston units, but only 8 had a FRS of 10–20% and none a FRS>20% (3).
The inability of the ACC/AHA risk score to categorize most RA patients with high CAC as having elevated CV risk suggests that risk prediction models using standard CV risk factors derived in the general population may not accurately identify many RA patients with elevated CV risk.
The reduction in the threshold for risk stratification from ≥10% in the older FRS and RRS risk scores to ≥7.5% in the ACC/AHA risk score did not account for the majority of the additional RA patients with high CAC now identified as having elevated risk. Only one patient with high CAC who had a 10-year risk of 9% by ACC/AHA, 6% by FRS, and 5% by RRS was classified as having elevated risk based on the reduced (≥7.5%) threshold. Further reduction of the ACC/AHA threshold for elevated risk to ≥5%, as suggested by the new guidelines as a consideration for high risk patients (8), resulted in 53% of RA patients with high CAC being correctly allocated to the elevated risk category; however, it also increased the number of patients without high CAC classified as being at elevated risk.
The ACC/AHA risk score reclassified 12 (16%) patients in the FRS low risk category into the elevated risk category and shifted only 1 (5%) patient classified as having elevated risk by FRS into the low risk category. However, the majority of risk reclassification occurred among patients without high CAC (Supplement Table S1). Thus, although the ACC/AHA model classified more patients overall into the elevated risk group, it did not improve the accuracy of the identification of patients known to have elevated risk based on high CAC scores.
A limitation is that our study was cross-sectional and thus we are not able to determine the ability of risk scores to predict CV events. Related to this, we used a high CAC score as an indicator of high CV risk and thus increased risk of future CV events. The ability of CAC to predict cardiovascular events in RA has not been determined. In the general population high CAC scores are related to risk of future CV events (19). Nevertheless, not every patient with high CAC will have a CV event and thus CAC is not a gold standard. Therefore, studies to determine how well the ACC/AHA risk score predicts future cardiovascular outcomes in RA will be important
In conclusion, the new ACC/AHA 10-year risk score assigned almost 60% of patients with elevated risk as determined by a high CAC into a low risk category. New risk scores and standard risk prediction models used in the general population do not adequately identify many RA patients with elevated cardiovascular risk. RA may represent a clinical situation in which the use of CAC screening may be useful for CV risk stratification until better biomarkers or CV risk scores are developed.
Supplementary Material
Acknowledgments
Financial support: This study was supported by the National Institutes of Health grant 5P60AR56116, 3P01HL056693-17S1 (V.K.K.), K23AR064768 (C.P.C) grants and the Vanderbilt Physician Scientist Award (V.K.K. and C.P.C.)
Reference List
- 1.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 2.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 3.Nasir K, Michos ED, Blumenthal RS, Raggi P. Detection of high-risk young adults and women by coronary calcium and National Cholesterol Education Program Panel III guidelines. J Am Coll Cardiol. 2005;46:1931–1936. doi: 10.1016/j.jacc.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420–424. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 10.Giles JT, Szklo M, Post W, Petri M, Blumenthal RS, Lam G, et al. Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. 2009;11:R36. doi: 10.1186/ar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung CP, Oeser A, Avalos I, Gebretsadik T, Shintani A, Raggi P, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8:R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arts EE, Popa C, den Broeder AA, Semb AG, Toms T, Kitas GD, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204024. Published Online First [14 Jan 2014] [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Vaquero C, Corrales A, Zacarias A, Rueda-Gotor J, Blanco R, Gonzalez-Juanatey C, et al. SCORE and REGICOR function charts underestimate the cardiovascular risk in Spanish patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R91. doi: 10.1186/ar4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Juanatey C, Llorca J, Martin J, Gonzalez-Gay MA. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38:366–371. doi: 10.1016/j.semarthrit.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Evans MR, Escalante A, Battafarano DF, Freeman GL, O'Leary DH, del RI. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211–1220. doi: 10.1002/art.30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 18.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 19.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.