Abstract
Diffuse gliomas are up till now graded based upon morphology. Recent findings indicate that isocitrate dehydrogenase (IDH) mutation status defines biologically distinct groups of tumors. The role of tumor grade and mitotic index in patient outcome has not been evaluated following stratification by IDH mutation status. To address this, we interrogated 558 WHO grade II–III diffuse gliomas for IDH1/2 mutations and investigated the prognostic impact of WHO grade within IDH-mutant and wild-type tumor subsets independently. The prognostic impact of grade was modest in IDH-mutant [hazard ratio (HR)=1.21, 95% confidence interval (CI)=0.91–1.61] compared to IDH-wild type tumors (HR=1.74, 95% CI=0.95–3.16). Using a dichotomized mitotic index cut-off of 4/1000 tumor cells, we found that while mitotic index was significantly associated with outcome in IDH-wild type tumors (log-rank p<0.0001, HR=4.41, 95% CI=2.55–7.63), it was not associated with outcome in IDH-mutant tumors (log-rank p=0.5157, HR=1.10, 95% CI=0.80–1.51), and could demonstrate a statistical interaction (p<0.0001) between IDH mutation and mitotic index (i.e. suggesting that the effect of mitotic index on patient outcome is dependent on IDH mutation status). Patient age, an established prognostic factor in diffuse glioma, was significantly associated with outcome only in the IDH-wild type subset, and consistent with prior data, 1p/19q co-deletion conferred improved outcome in the IDH-mutant cohort. These findings suggest that stratification of grade II–III gliomas into subsets defined by the presence or absence of IDH mutation leads to subgroups with distinct prognostic characteristics. Further evaluation of grading criteria and prognostic markers is warranted within IDH-mutant versus IDH-wild type diffuse grade II–III gliomas as independent entities.
Keywords: diffuse glioma, IDH, 1p/19q, outcome, WHO grade, pHH3
Introduction
Diffuse gliomas are common and variably aggressive primary central nervous system tumors, currently stratified by the World Health Organization (WHO) into three malignancy grades: II, III and IV. Glioblastoma (WHO grade IV) is generally classified as a diffuse glioma of astrocytic morphology with microvascular proliferation and/or necrosis. For WHO grades II and III diffuse gliomas classification is based on morphologic determination of cell type (i.e. astrocytic, oligodendroglial, mixed morphology). Determination of grade (II versus III), is made primarily on the basis of tumor proliferative activity (mitotic activity), along with additional criteria of increased cellularity and nuclear atypia. Additional factors, such as microvascular proliferation and tumor necrosis play a role in the grading of oligodendroglial and oligoastrocytic tumors [41]. Regarding proliferative activity in diffuse glioma, there is flexibility in the interpretation of the proposed WHO grading criteria, without strict criteria for delineation of mitotic figure cut-offs to distinguish grade II from grade III tumors. This can result in a great deal of interobserver variability in the determination of grade [62]. This consideration has become relevant for clinical practice because current treatment guidelines [46] and ongoing clinical trials [62] are primarily stratified by glioma morphology and WHO grade.
Isocitrate dehydrogenase (IDH) mutations and 1p/19q co-deletion have proven significant prognostic and predictive (1p/19q co-deletion) biomarkers that greatly impacted the field of neuro-oncology [29,7,8,63]. IDH mutations precede 1p/19q co-deletion [67], are associated with younger age, and are prognostic in most WHO grade III and IV diffuse gliomas [21,26,72,69,59,68,65,4] but their prognostic impact in low-grade diffuse glioma (WHO grade II) has not been completely clarified [24,45,34,44,1,50,28,20]. Additional signature lesions within the IDH-mutated grade II–III gliomas include mutations in ATRX (common in 1p/19q non-co-deleted tumors) and TERT promoter (common in 1p/19q co-deleted tumors) [32,33,31,70]. There is evidence that grade II and III diffuse gliomas are comprised of molecularly distinct subgroups based on the presence or absence of IDH mutations [17,73]. On the other hand assessment of IDH mutational and 1p/19q co-deletion status in diffuse glioma is becoming routine in clinical practice and efforts are made to find a way to integrate molecular and morphological information [42]. From a clinical stand-point and despite investigation of molecular signatures in diffuse glioma, the impact of the conventional WHO grade and mitotic index in low grade and anaplastic diffuse glioma following IDH molecular stratification is not established, and further efforts to integrate IDH mutational and 1p/19q status in a clinical context may improve stratification of patient risk groups.
The main purpose of this study was to evaluate the role of tumor grading in predicting patient outcome in grade II and III diffuse gliomas stratified by IDH mutation status. To accomplish this we interrogated a large cohort of low grade and anaplastic diffuse gliomas to ensure that molecularly-defined subsets could be represented in a robust fashion. Since tumor proliferative activity is a key component of WHO grading criteria to distinguish grade II from grade III gliomas, we also investigated the prognostic role of mitotic index [13] within IDH-mutant and wild-type tumor subsets.
Materials and Methods
Data collection
The study protocol was approved and carried out in accordance with institutional review board guidelines. Formalin-fixed paraffin embedded tissues from primary (non-treated) diffuse gliomas that were WHO grades II and III were retrospectively identified and collected from the files of our institutions:485/558 cases were obtained from the pathology files at MD Anderson Cancer Center and 73/558 cases were obtained from the VU University Medical Center. Some of the cases from VU University Medical Center/The Netherlands have been previously published in another context [66]. The cases were selected based on sufficient tissue availability (i.e. to provide adequate material for immunohistochemical and molecular testing) and availability of clinical information (i.e. duration of follow-up). Patients with gliomatosis cerebri were not included. Patients with stereotactic biopsies were not included. Slides stained with hematoxylin and eosin (H&E) were reviewed and the diagnosis was confirmed for all cases.
The following data were collected: age (at initial histopathological diagnosis), date of initial surgery, histological subtype of diffuse glioma (astrocytoma, oligodendroglioma, mixed morphology), WHO grade (as per original pathology report confirmed with H&E central review per current 2007 WHO grading criteria), survival status (alive or dead), and date of death or date of last follow-up. Overall survival (OS) was calculated by extracting the date of initial surgery (tissue diagnosis) from the date of last follow-up or death. Data sets are provided in Online resource 1.
Immunohistochemistry
As an initial step for IDH mutational assessment, immunohistochemistry with the anti-IDH1-R132H mutation specific antibody was utilized for sample interrogation. Immunohistochemistry was manually performed with anti-IDH1-R132H mouse monoclonal antibody (Dianova, Hamburg, Germany, clone H09, dilution 1:200) and anti-pHH3 (Ser 10) rabbit polyclonal antibody (Cell Signaling Technology, MA, USA, catalog#9701L, dilution 1:100) as previously described [51]. All controls were appropriate.
Phospho-HH3 mitotic index (number of pHH3-positive mitotic figures per 1000 tumor cells) was calculated as previously described [13]. Briefly, we selected the best tissue section for each case as the one with the highest tumor density, the lowest amount of inflammation, and the lowest percentage of normal brain. If still present in selected best tissue sections, inflammation-rich areas were avoided for mitotic index assessment. Two experienced neuropathologists (AO, KDA) manually counted mitoses per 1000 tumor nuclei in the highest mitotically active foci. We only counted nuclei accompanied by chromatin condensation, the signature of a mitotic cycle. The uniformly stained positive nuclei and the finely speckled stained nuclei were not counted, as these findings are not part of the mitotic cycle [23]. When discrepant findings were observed [i.e. no mitoses in an anaplastic diffuse glioma which occurred in 21/296 (7%) or mitoses in a grade II diffuse glioma which occurred in 218/262 (83%)] the controls and the original H&E were reviewed and the mitotic index was confirmed.
IDH sequencing and 1p/19q analysis
All cases that were negative for the IDH1-R132H mutation by immunohistochemistry were interrogated by targeted sequencing for IDH1 exon 4 (codon 132) and IDH2 exon 4 (codons 172). Sanger sequencing was performed at the MD Anderson Cancer Center Genomics Core Facility. Some of the cases (those obtained via VU University Medical Center) had been previously analyzed and reported for 1p/19q analysis using low-pass whole genome sequencing [66]. Analysis for 1p/19q was performed on MD Anderson samples using either fluorescent in situ hybridization or quantitative microsatellite analysis as previously described [43,47,71].
Data mining and statistical analysis
To define the best mitotic index cut-off that best separates the data classification and regression tree analysis was performed (Statistica, v. 11, StatSoft, Inc., Tulsa, OK, USA). Survival analyses were performed using Kaplan-Meier, log-rank, and Cox proportional hazards methods (Statistica, v. 11, StatSoft, Inc., Tulsa, OK, USA; JMP Pro v. 11.2.0, Sas Institute Inc., NC, USA). Findings were considered statistically significant at p ≤ 0.05.
Results
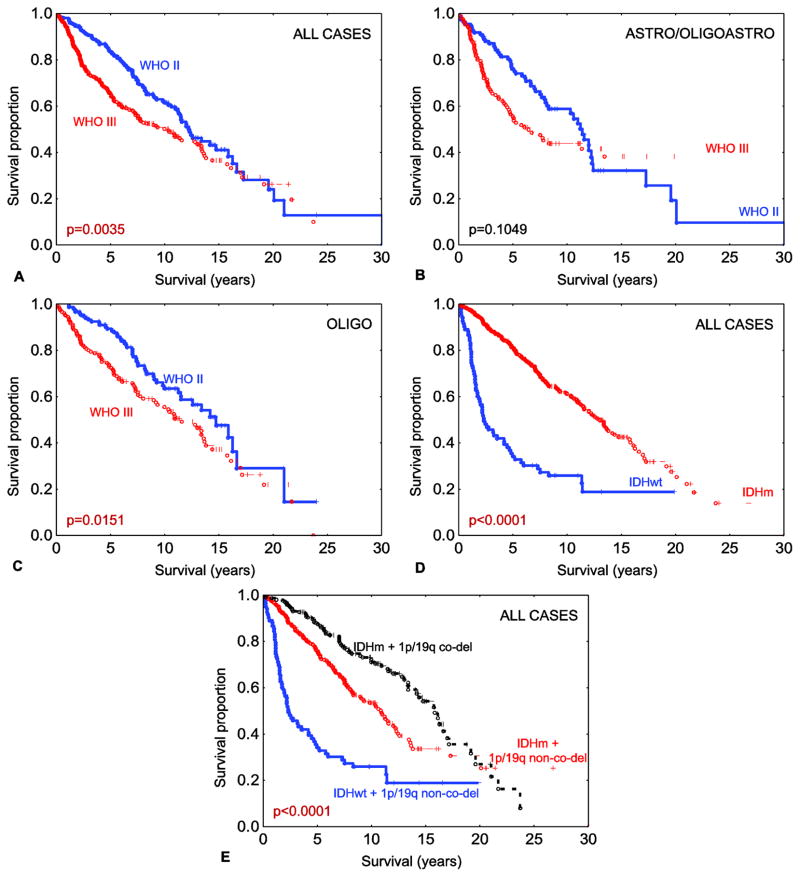
We included 558 patients with low grade (WHO grade II (n=262)) and anaplastic (WHO grade III (n=296)) diffuse gliomas. Patient age ranged from 17.4 to 78.4 years overall. Adjuvant treatment data was available for 439 patients, of which 281 received radiation and 236 received chemotherapy. One hundred and eighty seven patients received either radiation or chemotherapy, 165 patients received both, and 87 did not receive adjuvant treatment. Morphologically, after central review 235 tumors had astrocytic features, 11 had mixed oligoastrocytic, and 312 had oligodendroglial histology. As expected, in the whole group tumor grade was associated with patient outcome (Fig. 1a). This finding did not held true when the cases were stratified by astrocytic/mixed histology (Fig. 1b), but only when stratified by oligodendroglial histology (Fig. 1c). Using both IDH1-R132H-specific immunohistochemistry followed by targeted sequencing for IDH1 non-canonical (non IDH1-R132H) and IDH2 mutations, 475 tumors (85.12%) were IDH mutated, and in the remaining 83 cases (14.87%), an IDH mutation was not detected. A summary of the IDH mutation results is presented in Table 1. The age distribution in IDH-wild type tumors was between 19.0–78.4 years (median = 45) and in IDH mutated tumors was between 17.4–74.2 years (median = 37). As expected, IDH-mutant tumors were clinically less aggressive than IDH-wild type tumors (Fig. 1d).
Fig. 1.
Overall survival among all 558 grade II–III diffuse gliomas stratified by WHO grade (a). Overall survival among all 246 astrocytic gliomas and gliomas with mixed morphology stratified by WHO grade (b). Overall survival among all 312 oligodendroglial gliomas stratified by WHO grade (c). Overall survival among all 558 grade II–III diffuse gliomas stratified by IDH mutation status (d). Overall survival among all 558 grade II–III diffuse gliomas stratified by combined IDH mutation and 1p/19q co-deletion status (e). Legend: co-del – co-deleted; IDHm - IDH-mutant; IDHwt – IDH-wild type; non-co-del – non-co-deleted. Note: all reported p values are log-rank.
Table 1.
Summary of IDH mutations identified.
| IDH1/IDH2 protein-coding change identified | # (% total) | # (%) in 1p/19q non-co-deleted tumors | # (%) in 1p/19q co-deleted tumors |
|---|---|---|---|
| IDH1 | |||
| p.R132H | 434 (77.77) | 243 (56.00) | 191 (44.00) |
| p.R132C | 14 (2.50) | 4 (28.58) | 10 (71.42) |
| p.R132G | 12 (2.15) | 12 (100.00) | 0 (0.00) |
| p.R132S | 9 (1.61) | 5 (55.55) | 4 (44.44) |
| IDH2 | |||
| p.R172K | 6 (1.07) | 0 (0.00) | 6 (100.00) |
number of events.
Two hundred and twelve tumors were 1p/19q co-deleted, representing a subset of the IDH-mutant tumors. Consistent with the literature, 1p/19q co-deletion was associated with improved outcome (Fig. 1e). When the two molecular signatures were combined, patients with IDH-mutant and 1p/19q co-deleted tumors performed best (median OS=15.86 years, range=0.02–23.98) compared to those with IDH-mutant and 1p/19q intact tumors (median OS=10.88 years, range=0.02–30.06) and to those with IDH-wild type and 1p/19q intact tumors (median OS=2.35 years, range=0.02–19.84) (log-rank p<0.0001). For distribution of IDH mutations and 1p/19q co-deletions among morphological subtypes see Online resource 2.
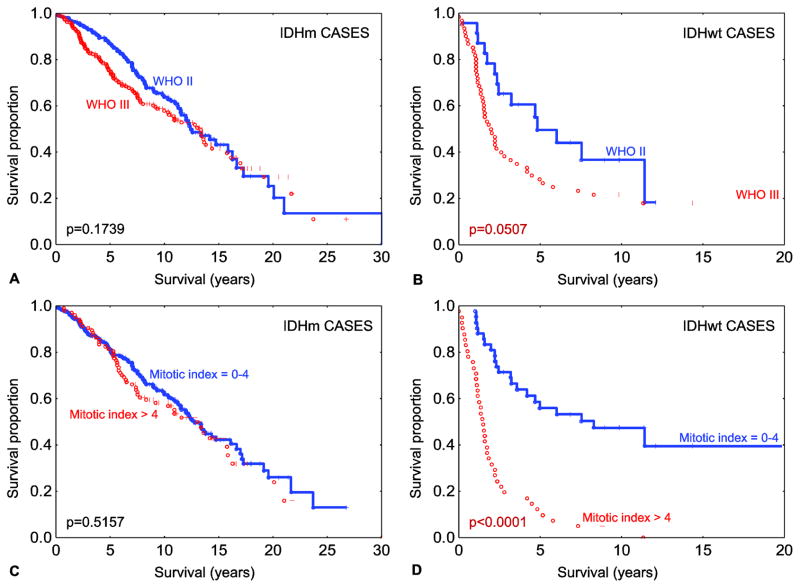
To date, the role of tumor grade/tumor proliferation on patient survival has not been well characterized in grade II–III gliomas after accounting for IDH mutation status. To evaluate the role of histologic grade/tumor proliferation rate within cases stratified by IDH status, the IDH-mutant (n=475) and IDH-wild type (n=83) tumors were considered separately. Among the 475 IDH-mutant tumors, comparison of outcome based on WHO grade (II versus III) showed no statistically significant difference on log-rank analysis (p=0.1739), with a small effect size of WHO grade on survival. Cox analysis revealed a hazard ratio (HR) of 1.21 (95% confidence interval (CI)=0.91–1.61) and median OS of 12.41 years (range=0.08–30.06) in the grade II tumors versus 13.35 years (range=0.02–26.77), in the grade III tumors (Fig. 2a). Survival analysis in the IDH-wild type cohort (Fig. 2b) showed a statistically significant difference in outcome based on WHO grade (p=0.0507), and with a larger effect size with a hazard ratio of 1.74 (95% CI = 0.95–3.16) and median OS of 4.82 years (range=0.24–12.07) for WHO grade II versus 1.97 years (range=0.02–19.84) for WHO grade III tumors.
Fig. 2.
Overall survival among IDH mutated diffuse gliomas (n=475) stratified by WHO grade (a). Overall survival amongst IDH-wild type diffuse gliomas (n=83) stratified by WHO grade (b). Overall survival amongst IDH mutated diffuse gliomas (n=475) stratified by mitotic index subgroups (c). Overall survival amongst IDH-wild type diffuse gliomas (n=83) stratified by mitotic index subgroups. Legend: IDHm - IDH-mutant; IDHwt – IDH-wild type. Note: all reported p values are log-rank.
To further characterize these results, we quantified the mitotic index (mitoses per 1000 tumor cells) using pHH3 immunostaining. We used a mitotic index cut-off of 4 as previously described [13] to distinguish low-proliferative (mitotic index = 0–4) from high-proliferative (mitotic index > 4) tumors. This cut-off value was also confirmed by classification and regression tree computational analysis (details in Online resource 3). With the use of the mitotic index, an overall finding similar to WHO grade was observed, with no statistical difference between high- versus low-proliferative tumors in the IDH-mutant subset (Fig. 2c) [HR = 1.10, 95% CI=0.80–1.51 and median OS 12.99 years (range=1.41–21.78) for high-proliferative versus 12.76 years (range=0.02–26.77) for low-proliferative tumors]. In contrast, the effect size of mitotic index on IDH-wild type tumors was more substantial (Fig. 2d), with a HR of 4.41 (95% CI = 2.55–7.63) and median OS of 1.49 years (range=0.24–11.34) for the high-proliferative tumors versus 8.28 years (range=1.01–19.84) in the low-proliferative tumors. To formally evaluate the prognostic effect of tumor proliferation and IDH mutation status on patient outcome, we tested for the presence of statistical interaction, which on Cox proportional hazards multivariate analysis, was present (Table 2).
Table 2.
Cox multivariate analysis to test for interaction between mitotic index and IDH mutation status on overall survival.
| Variable | p-value | 95% CI |
|---|---|---|
| Mitotic index high vs. low | <0.0001 | 3.58–10.32 |
| IDH status mut vs. wt | 0.0265 | 0.38–0.94 |
| Mitotic index-IDH interaction term | <0.0001 | 0.10–0.34 |
CI - confidence interval, mut – mutant, N/A-not applicable, wt – wild-type.
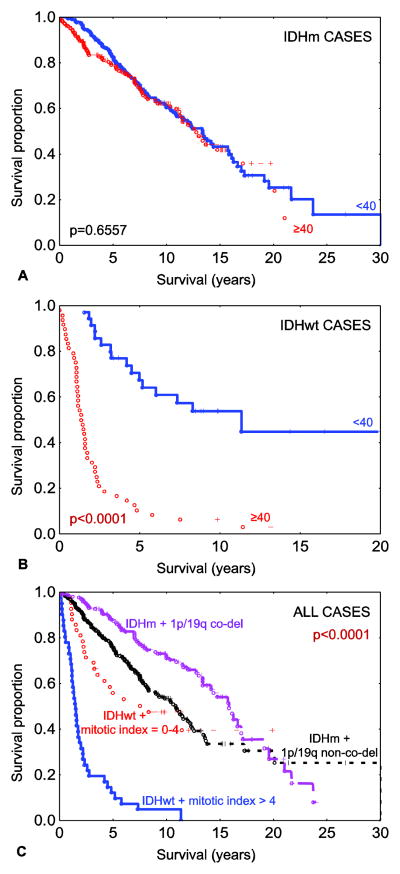
To further characterize relationships between relevant co-variates and patient outcome, Cox multivariate analysis was performed (for multivariate analyses with WHO grade and adjuvant treatment see Online resource 4). When the entire cohort was examined, IDH mutation status, 1p/19q co-deletion status, mitotic index, and age at initial diagnosis were all statistically significant (Table 3). This held true after adjustment for adjuvant treatment (Online resource 4, Table S4.1) The finding that older patient age is significantly correlated with poorer patient outcome is a consistent finding in glioma. To evaluate this in subsets defined by IDH mutation status, Cox multivariate analyses were performed independently in the IDH-mutant and wild-type cohorts. In the IDH-mutant cohort, mitotic index was not a significant predictor of outcome (p=0.3535) (Table 4) and only a borderline significant predictor of outcome after adjustment for treatment (p=0.0566) (Online resource 4, Table S4.2.A), while a much stronger predictor in the IDH-wild type cohort (p=0.0010) (Tables 5 and S4.3.A). Interestingly, patient age was not independently predictive of survival in the IDH-mutant cohort (Tables 4 and S4.2) but was significantly correlated with outcome in the IDH-wild type cohort (Tables 5 and S4.3). To illustrate this graphically, Kaplan-Meier curves demonstrated no statistical difference in older versus younger patients (using an age cut-off of 40) in IDH-mutant cases (Fig. 3a, median OS= 13.36 years (range=0.02–30.06) for younger patients and 12.76 years (range=0.02–23.98) for older patients). In contrast, IDH-wild type tumors showed a striking difference in OS (median OS=11.34 years (range=1.52–19.84) for younger patients and 1.35 years (range=0.02–13.18) for older patients (log-rank p<0.0001) (Fig. 3b). To further test these findings, similar analyses were performed on the subgroup of IDH-mutant and 1p/19q non-co-deleted cohort of tumors (n=264). In this subset, mitotic index, WHO grade, and age were not statistically correlated with patient survival (details in Online resource 5). Based on these findings along with mitotic index results in Tables 3–5, results show that the prognostic effect of mitotic index in the overall glioma cohort is primarily driven by the effect limited to the IDH-wild type cohort.
Table 3.
Cox multivariate analysis on all 558 diffuse glioma.
| Variable | p-value | Hazard Ratio | 95% CI |
|---|---|---|---|
| IDH status (mutvswt) | <0.0001 | 0.38 | 0.28–0.52 |
| 1p/19q status (codel vs non-codel) | <0.0001 | 0.53 | 0.40–0.71 |
| Mitotic index (high vs low) | <0.0001 | 1.70 | 1.31–2.19 |
| Age | <0.0001 | 1.03 | 1.02–1.04 |
CI: confidence interval, codel – co-deleted, mut mutant, wt – wild-type.
Table 4.
Cox multivariate analysis on all 475 IDH-mutant diffuse glioma.
| Variable | p-value | Hazard Ratio | 95% CI |
|---|---|---|---|
| 1p/19q status (codel vs. non-codel) | 0.0002 | 0.57 | 0.42–0.77 |
| Mitotic index (high vs. low) | 0.3535 | 1.16 | 0.85–1.59 |
| Age | 0.1243 | 1.01 | 0.99–1.03 |
CI: confidence interval, codel – co-deleted, mut – mutant, wt – wild-type.
Table 5.
Cox multivariate analysis on all 83 IDH-wild type diffuse glioma.
| Variable | p-value | Hazard Ratio | 95% CI |
|---|---|---|---|
| Mitotic index (high vs. low) | 0.0010 | 2.73 | 1.50–4.96 |
| Age | <0.0001 | 1.05 | 1.03–1.06 |
CI: confidence interval
Fig. 3.
Overall survival among IDH mutated diffuse gliomas (n=475) stratified by age groups (in years) (a) and overall survival amongst IDH-wild type diffuse gliomas (n=83) stratified by age groups (b). Overall survival among all 558 grade II-III diffuse gliomas stratified by combined IDH mutation, 1p/19q co-deletion status and mitotic index subgroups (c). Legend: co-del – co-deleted; IDHm - IDH-mutant; IDHwt – IDH-wild type; non-co-del – non-co-deleted. Note: all reported p values are log-rank.
Taking the findings together and in context, two clinical groups can be defined in the IDH-mutant cohort, based on the presence or absence of 1p/19q co-deletion. In the IDH-wild type cohort, clinical groups are defined by proliferative activity, here defined using a mitotic index cut-off of 4. These findings define 4 clinical-pathologic groups of low grade and anaplastic diffuse glioma, as shown in Fig. 3c.
Discussion
In this study we investigated the prognostic value of tumor proliferation and patient age in low grade and anaplastic diffuse glioma stratified by IDH mutation status. We evaluated patient outcome as related to initial tumor grade, and found that the effect of grade (II versus III) on patient outcome was small in the IDH-mutant subset of tumors as compared to the IDH-wild type subset of tumors. Recognizing that tumor grade is subject to interobserver variability, as well as the fact that, in large part, the distinction of grade II versus grade III diffuse gliomas is based primarily on mitotic activity, we further evaluated mitotic index as related to patient outcome.
We used a cut-off of 0–4 versus >4 immunohistochemistry-confirmed mitoses per 1000 tumor cells to define two groups, based on our prior experience [13] and confirmed this cut-off using classification data mining methods (Online resource 3).
Similar to the findings using WHO grade, the impact of mitotic index was small in the IDH-mutant cohort relative to the IDH-wild type cohort. A significant statistical interaction could be discerned suggesting that the prognostic impact of mitotic index groups, so defined, was conditional on IDH status. Verifying past literature, we found that 1p/19q co-deletion was restricted to a subset of the IDH-mutant tumors [36] and within this subset offers further prognostic value [10,16,20,24]. Interestingly, the impact of patient age, a long-known prognostic marker in glioma, also varied based on IDH mutation status, and was pronounced only in the IDH-wild type subset.
For some time the WHO grade and the morphological subtype (oligodendroglial vs. astrocytic) have been the principal pathologic prognostic factors for patients with grade II–III diffuse gliomas [41]. Additional clinical variables like patient’s age [53], Karnofsky score to assess patient’s performance status [3], tumor size [12,53], and the extent of surgical tumor resection [39,27,57,58], added prognostic value for these patients. To date, the WHO grade and the morphological subtype are the primary criteria for treatment stratification [46]; however the current diagnostic criteria for diffuse gliomas [41] can be subjective, significant interobserver variability having been reported in both mitotic figure assessment and call, and in morphological subtyping [14,62,35,2]. There is a critical need for more objective criteria to define prognosis and guide therapy for patients with II–III grade diffuse glioma.
Several molecular biomarkers have emerged to characterize subgroups of diffuse glioma. Since 1994, when combined loss of 1p and 19q was identified in oligodendrogliomas [54], the field has made significant progress. Today several mutually-exclusive molecular signatures have been well-characterized for groups of astrocytomas (mutations involving TP53 and ATRX) and for oligodendrogliomas (1p/19q co-deletion, mutations involving CIC and FUBP1) [5,26,30,70,56,32] in adults. Both molecular tumor categories have a common denominator: mutations involving IDH [26,70,30,36].
IDH mutations quickly emerged as powerful prognostic biomarkers, challenging current concepts by overcoming histology in certain instances [21,44]. They changed the view on gliomagenesis by highlighting the critical role of epigenetic regulation [25,61]. IDH mutations are responsible for the glioma-CpG island methylator phenotype (G-CIMP) associated with a proneural genetic signature [49]. They have been proven to be acquired early in tumor formation [67,28] and retained following treatment or tumor recurrence/progression [28,38], becoming the molecular signature for secondary glioblastomas [48].
Pathologic grading of diffuse gliomas is performed to stratify patients into clinical risk groups, as a means to tailor therapy based on estimated clinical aggressiveness. Mitotic activity is an important criterion to distinguish grade II from grade III diffuse gliomas. Microvascular proliferation and necrosis may play a role in distinguishing oligodendroglial and oligoastrocytic grade II versus grade III gliomas as well (according to the most recent, i.e. WHO 2007 classification the presence of necrosis in mixed gliomas even leading to a diagnosis of glioblastoma with oligodendroglioma component, WHO grade IV)[41]. The finding that the grade II and III gliomas are in fact composed of at least two distinct biologic subtypes, defined by IDH mutation status, raises questions regarding the role of conventional grading/tumor proliferation rate on patient outcome within IDH-mutant versus IDH-wild type tumors.
In this study we investigated the prognostic value of WHO grade, mitotic index, and patient age in grade II and III diffuse glioma stratified by IDH mutation status. We found, consistent with prior studies, that IDH-mutant tumors comprised the majority of the cohort and further, that the IDH-mutant subset had an improved outcome compared to IDH-wild type tumors. With that as a background, we compared the role of tumor grade and outcome in IDH-mutant vs. wild-type tumors and found a larger effect size of tumor grade on outcome among the IDH-wild type tumors, compared to IDH-mutant tumors. To further characterize this result and acknowledging that the distinction of grade II from grade III is based largely on mitotic activity, we used mitotic index and found an analogous result. Inspection of the Kaplan-Meir curves among the IDH-mutant tumors for tumor grade (Fig. 2a) and mitotic index (Fig. 2c) with the curves for the IDH-wild type tumors (Fig. 2b and 2d) shows that the relationship between tumor grade/proliferation and outcome is related to a substantial degree upon the IDH mutation status. This finding was confirmed by demonstrating statistical interaction between mitotic index and IDH status (Table 2) (i.e. the prognostic effect of tumor proliferation on outcome depends on IDH mutation status).
Morphologically identical gliomas have distinct molecular signatures, expression signatures, and prognostic features [18]. By molecular profiling of 101 grade II–III astrocytomas, Gorovets et al identified molecular subgroups of gliomas mainly stratified by IDH mutation status. Significant molecular differences were reported between IDH-mutant and IDH-wild type gliomas [17]. IDH-mutant gliomas were associated with TP53 mutations, PTEN promoter methylation, gains of 8q, and defined two distinct subgroups based on transcriptional profiling. These two distinct subgroups, called neuroblastic and early progenitor-like, showed mature neuronal signatures and developmental pathway signatures respectively and provided potential evidence of glioma cells originating from subventricular zone progenitors as previously shown [37,60]. The TP53 mutations associated to the early progenitor-like subtype, which also showed additional chromosomal copy number aberrations (7p, 15q gains; 13q, 9p23, 19q, 4q34.3, 11p, 12q21.33 losses). IDH-wild type gliomas exhibited EGFR amplification, PTEN loss accompanied by 7p gain, 9p and 10q loss, signatures characteristic of primary glioblastoma. Similar to primary glioblastomas, PI3K/AKT pathway was activated in IDH-wild type tumors [11,52,17]. This subset of IDH-wild type gliomas defined a third highly heterogeneous transcriptional subgroup, suggestively called pre-glioblastoma. Importantly the authors did not find survival differences among WHO grades within molecular subgroups and the latter were more predictive of OS compared to the WHO grade [17].
Yan et al, on a cohort of 225 diffuse gliomas of all WHO grades also identified three distinct prognostic molecular subclasses based on gene expression profiles. The subgroup that lacked IDH mutations, although enriched with primary glioblastomas (71), contained a substantial number (24) of grade II and III diffuse gliomas [73].
Our data show that IDH, followed by 1p/19q testing, can be reliably integrated as a first approach toward risk stratification (Fig. 1e) and this is consistent with data reported by others [16,20,10,24]. With the established predictive value for 1p19q for chemotherapeutic benefit [6,9,8,64,63], and potential predictive value for IDH mutations [10,15], this paradigm holds the potential to be extremely important for the management of patients with grade II and III diffuse gliomas.
In our study, following IDH stratification, only 1p/19q was prognostic in IDH mutated grade II–III gliomas after adjustment for tumor proliferation, age, and adjuvant treatment. Interestingly in our cohort tumor proliferation and age were prognostic only in IDH-wild type gliomas, but not in IDH-mutant gliomas, suggesting that the overall effect is attributed only to the IDH-wild type tumors. This effect seems relatively strong, given the minority of IDH-wild type tumor samples in our cohort (n=83) compared to IDH-mutant samples (n=475). In our cohort IDH-wild type tumors had the lowest median OS, of only 2.35 years and due to the lack of IDH mutations we speculate that these tumors share the biology of primary glioblastoma, with clinical behavior that is distinct from IDH-mutant glioma and approaches that of grade IV IDH-wild type tumors [52,48,25,55,26,17,73]. Further molecular characterization for commonly described molecular markers of primary glioblastoma [52,11] is needed to prove this suspicion. Also, it remains to be seen if other morphological components traditionally used in grading of diffuse gliomas (i.e. microvascular proliferation and necrosis) still carry additional prognostic information (besides mitotic activity) in the IDH-wild type, 1p/19q non-co-deleted subgroups of grade II and III diffuse gliomas. Additional markers are need to distinguish favorable from unfavorable grade II–III IDH-mutant gliomas, and in this regard, one clue comes from a recent study on grade II gliomas which suggests that distal loss of 10q is a late onset event and a marker for reduced overall survival [66].
Limitations of this study are its retrospective nature with potential selection bias due to tissue and clinical data availability; we lack data on the extent of resection and clinical performance status, both important predictors of outcome in diffuse glioma, but difficult to accurately assess retrospectively. In this study only a small percentage of mixed oligoastrocytomas is present [11/558 (2%)]; this diagnostic category was only sparingly used at MD Anderson, the major source for the cases; therefore we caution the reader for this additional selection bias to not draw conclusions on this particular morphological category but rather to integrate the molecular signatures in the histological context. An additional bias was possibly caused by the choice of only a single block per case for mitotic index, which can of course vary within the tumor. Even with these limitations, a comparison of IDH-mutant and IDH-wild type tumors, examined in similar fashion, yielded different relationships of grade and mitotic index on patient survival and at minimum, our results suggest that independent confirmation is warranted.
Although pHH3 immunohistochemistry raises practical issues, there are several advantages to assess mitotic activity. On top of the antibody’s known increased sensitivity and specificity for mitotic figure detection [23,22,19,13,40], the mitotic index (per 1000 glioma cells) is, in concept, favored over a per-10 high power fields assessment, since tumors vary widely in cellularity. At some institutions this method is part of routine practice, complementing the assessment of the MIB-1/Ki-67 index (also measured as a true index, rather than on a high power field basis). Additional experience is required to more fully evaluate its clinical utility in daily practice.
A major strength of the study is the cohort size (n=558), allowing us to examine the existence of at least two distinct biological molecular subgroups of grade II and III glioma: IDH mutated and IDH-wild type, each likely encompassing several distinct prognostic molecular subgroups [17]. Based on these findings we suggest that the concept of tumor grading, as a means of defining clinical risk groups, be further examined independently for IDH-wild type versus IDH-mutant grade II–III diffuse gliomas. In addition, consistent with ongoing efforts to integrate IDH mutation status within WHO tumor classification guidelines [42], it is hoped that this study will spur further effort into the identification of factors that control clinical behavior among diffuse gliomas, acknowledging that IDH-mutant and IDH-wild type low and anaplastic grade diffuse gliomas are biologically and clinically distinct.
Supplementary Material
Acknowledgments
Funding was provided by the National Institutes of Health/National Cancer Institute (SPORE Grant No. P50CA127001)(to KDA, EPS), and the Burroughs Wellcome Career Award, the Koch Institute-Dana Farber Harvard Cancer Center Bridge Foundation, and the National Institutes of Health/National Cancer Institute (SPORE Grant No. 5P50CA165962) (to DPC); the Dutch Cancer Society (KWF grant VU 2009-4470) to JCR, BY, the foundation ‘STOPHersentumoren’ to JCR, BY, the Edli foundation to BY, PW. AO was supported by the National Institutes of Health/National Cancer Institute (Training Grant No. 5T32CA163185). Material and clinical information obtained via the VU University Medical Center was kindly provided by the Elisabeth Hospital Tilburg, the Isala Klinieken Zwolle, the Radboud University Medical Center, Nijmegen, and the Academic Medical Center, Amsterdam, The Netherlands.
Footnotes
Conflict of Interest
The authors declare they have no conflict of interest.
References
- 1.Ahmadi R, Stockhammer F, Becker N, Hohlen K, Misch M, Christians A, Dictus C, Herold-Mende C, Capper D, Unterberg A, von Deimling A, Wick W, Hartmann C. No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neurooncol. 2012 doi: 10.1007/s11060-012-0863-y. [DOI] [PubMed] [Google Scholar]
- 2.Aldape K, Burger PC, Perry A. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med. 2007;131 (2):242–251. doi: 10.1043/1543-2165(2007)131[242:CAOQLA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Bauman G, Lote K, Larson D, Stalpers L, Leighton C, Fisher B, Wara W, MacDonald D, Stitt L, Cairncross JG. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45 (4):923–929. doi: 10.1016/s0360-3016(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 4.Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, Shonka N, Gilbert MR, Sawaya R, Prabhu SS, Weinberg J, Lang FF, Aldape KD, Sulman EP, Rao G, McCutcheon IE, Cahill DP. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-oncology. 2014;16 (1):81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai J, Yang P, Zhang C, Zhang W, Liu Y, Bao Z, Liu X, Du W, Wang H, Jiang T, Jiang C. ATRX mRNA expression combined with IDH1/2 mutational status and Ki-67 expression refines the molecular classification of astrocytic tumors: evidence from the whole transcriptome sequencing of 169 samples samples. Oncotarget. 2014 doi: 10.18632/oncotarget.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24 (18):2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 7.Cairncross G, Jenkins R. Gliomas with 1p/19q codeletion: a.k.a. oligodendroglioma. Cancer J. 2008;14 (6):352–357. doi: 10.1097/PPO.0b013e31818d8178. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31 (3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90 (19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 10.Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, Buckner JC, Fink KL, Souhami L, Laperriere NJ, Huse JT, Mehta MP, Curran WJ., Jr Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32 (8):783–790. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455 (7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, Barbaro NM, Parsa AT, Berger MS, McDermott MM. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109 (5):817–824. doi: 10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 13.Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, Fuller G, Langford L, Pelloski C, Aaron J, Burger P, Aldape K. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol. 2006;30 (5):657–664. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- 14.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79 (7):1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Erdem-Eraslan L, Gravendeel LA, de Rooi J, Eilers PH, Idbaih A, Spliet WG, den Dunnen WF, Teepen JL, Wesseling P, Sillevis Smitt PA, Kros JM, Gorlia T, van den Bent MJ, French PJ. Intrinsic molecular subtypes of glioma are prognostic and predict benefit from adjuvant procarbazine, lomustine, and vincristine chemotherapy in combination with other prognostic factors in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. J Clin Oncol. 2013;31 (3):328–336. doi: 10.1200/JCO.2012.44.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frenel JS, Leux C, Loussouarn D, Le Loupp AG, Leclair F, Aumont M, Mervoyer A, Martin S, Denis MG, Campone M. Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience. J Neurooncol. 2013;114 (1):85–91. doi: 10.1007/s11060-013-1152-0. [DOI] [PubMed] [Google Scholar]
- 17.Gorovets D, Kannan K, Shen R, Kastenhuber ER, Islamdoust N, Campos C, Pentsova E, Heguy A, Jhanwar SC, Mellinghoff IK, Chan TA, Huse JT. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18 (9):2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 18.Gravendeel LA, Kouwenhoven MC, Gevaert O, de Rooi JJ, Stubbs AP, Duijm JE, Daemen A, Bleeker FE, Bralten LB, Kloosterhof NK, De Moor B, Eilers PH, van der Spek PJ, Kros JM, Sillevis Smitt PA, van den Bent MJ, French PJ. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69 (23):9065–9072. doi: 10.1158/0008-5472.CAN-09-2307. [DOI] [PubMed] [Google Scholar]
- 19.Habberstad AH, Gulati S, Torp SH. Evaluation of the proliferation markers Ki-67/MIB-1, mitosin, survivin, pHH3, and DNA topoisomerase II alpha in human anaplastic astrocytomas--an immunohistochemical study. Diagnostic pathology. 2011;6:43. doi: 10.1186/1746-1596-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, Stein R, Reifenberger G, Pietsch T, von Deimling A, Loeffler M, Weller M. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17 (13):4588–4599. doi: 10.1158/1078-0432.CCR-10-3194. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120 (6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 22.Hendzel MJ, Nishioka WK, Raymond Y, Allis CD, Bazett-Jones DP, Th’ng JP. Chromatin condensation is not associated with apoptosis. J Biol Chem. 1998;273 (38):24470–24478. doi: 10.1074/jbc.273.38.24470. [DOI] [PubMed] [Google Scholar]
- 23.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106 (6):348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 24.Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75 (17):1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 25.Ichimura K. Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol. 2012 doi: 10.1007/s10014-012-0090-4. [DOI] [PubMed] [Google Scholar]
- 26.Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, Rosemberg S, Oba-Shinjo SM, Nagahashi Marie SK, Bettegowda C, Agrawal N, Lipp E, Pirozzi C, Lopez G, He Y, Friedman H, Friedman AH, Riggins GJ, Holdhoff M, Burger P, McLendon R, Bigner DD, Vogelstein B, Meeker AK, Kinzler KW, Papadopoulos N, Diaz LA, Yan H. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3 (7):709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juratli TA, Kirsch M, Geiger K, Klink B, Leipnitz E, Pinzer T, Soucek S, Schrock E, Schackert G, Krex D. The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol. 2012;110 (3):325–333. doi: 10.1007/s11060-012-0977-2. [DOI] [PubMed] [Google Scholar]
- 28.Juratli TA, Kirsch M, Robel K, Soucek S, Geiger K, von Kummer R, Schackert G, Krex D. IDH mutations as an early and consistent marker in low-grade astrocytomas WHO grade II and their consecutive secondary high-grade gliomas. J Neurooncol. 2012 doi: 10.1007/s11060-012-0844-1. [DOI] [PubMed] [Google Scholar]
- 29.Kaloshi G, Benouaich-Amiel A, Diakite F, Taillibert S, Lejeune J, Laigle-Donadey F, Renard MA, Iraqi W, Idbaih A, Paris S, Capelle L, Duffau H, Cornu P, Simon JM, Mokhtari K, Polivka M, Omuro A, Carpentier A, Sanson M, Delattre JY, Hoang-Xuan K. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68 (21):1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 30.Kannan K, Inagaki A, Silber J, Gorovets D, Zhang J, Kastenhuber ER, Heguy A, Petrini JH, Chan TA, Huse JT. Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3 (10):1194–1203. doi: 10.18632/oncotarget.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, Friedman H, Friedman AH, Keir ST, He J, He Y, McLendon RE, Herndon JE, 2nd, Yan H, Bigner DD. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5 (6):1515–1525. doi: 10.18632/oncotarget.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killela PJ, Pirozzi CJ, Reitman ZJ, Jones S, Rasheed BA, Lipp E, Friedman H, Friedman AH, He Y, McLendon RE, Bigner DD, Yan H. The genetic landscape of anaplastic astrocytoma. Oncotarget. 2013 doi: 10.18632/oncotarget.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih Ie M, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110 (15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YH, Nobusawa S, Mittelbronn M, Paulus W, Brokinkel B, Keyvani K, Sure U, Wrede K, Nakazato Y, Tanaka Y, Vital A, Mariani L, Stawski R, Watanabe T, De Girolami U, Kleihues P, Ohgaki H. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177 (6):2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kros JM, Gorlia T, Kouwenhoven MC, Zheng PP, Collins VP, Figarella-Branger D, Giangaspero F, Giannini C, Mokhtari K, Mork SJ, Paetau A, Reifenberger G, van den Bent MJ. Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. J Neuropathol Exp Neurol. 2007;66 (6):545–551. doi: 10.1097/01.jnen.0000263869.84188.72. [DOI] [PubMed] [Google Scholar]
- 36.Labussiere M, Idbaih A, Wang XW, Marie Y, Boisselier B, Falet C, Paris S, Laffaire J, Carpentier C, Criniere E, Ducray F, El Hallani S, Mokhtari K, Hoang-Xuan K, Delattre JY, Sanson M. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74 (23):1886–1890. doi: 10.1212/WNL.0b013e3181e1cf3a. [DOI] [PubMed] [Google Scholar]
- 37.Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F, Forrest WF, Pujara K, Carrillo JA, Pandita A, Ellingson BM, Bowers CW, Soriano RH, Schmidt NO, Mohan S, Yong WH, Seshagiri S, Modrusan Z, Jiang Z, Aldape KD, Mischel PS, Liau LM, Escovedo CJ, Chen W, Nghiemphu PL, James CD, Prados MD, Westphal M, Lamszus K, Cloughesy T, Phillips HS. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29 (34):4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lass U, Numann A, von Eckardstein K, Kiwit J, Stockhammer F, Horaczek JA, Veelken J, Herold-Mende C, Jeuken J, von Deimling A, Mueller W. Clonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1- mutation as common tumor initiating event. PloS one. 2012;7 (7):e41298. doi: 10.1371/journal.pone.0041298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leighton C, Fisher B, Bauman G, Depiero S, Stitt L, MacDonald D, Cairncross G. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15 (4):1294–1301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Chen N, Wang X, He Y, Chen X, Huang Y, Yin W, Zhou Q. Apoptosis and proliferation markers in diffusely infiltrating astrocytomas: profiling of 17 molecules. J Neuropathol Exp Neurol. 2006;65 (9):905–913. doi: 10.1097/01.jnen.0000235857.79502.c3. [DOI] [PubMed] [Google Scholar]
- 41.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of tumors of the central nervous system. 4. IARC; Lyon: 2007. (France) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, Aldape K, Brat D, Collins VP, Eberhart C, Figarella-Branger D, Fuller GN, Giangaspero F, Giannini C, Hawkins C, Kleihues P, Korshunov A, Kros JM, Beatriz Lopes M, Ng HK, Ohgaki H, Paulus W, Pietsch T, Rosenblum M, Rushing E, Soylemezoglu F, Wiestler O, Wesseling P. International Society of Neuropathology-Haarlem Consensus Guidelines for Nervous System Tumor Classification and Grading. Brain Pathol. 2014 doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald JM, See SJ, Tremont IW, Colman H, Gilbert MR, Groves M, Burger PC, Louis DN, Giannini C, Fuller G, Passe S, Blair H, Jenkins RB, Yang H, Ledoux A, Aaron J, Tipnis U, Zhang W, Hess K, Aldape K. The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer. 2005;104 (7):1468–1477. doi: 10.1002/cncr.21338. [DOI] [PubMed] [Google Scholar]
- 44.Metellus P, Coulibaly B, Colin C, de Paula AM, Vasiljevic A, Taieb D, Barlier A, Boisselier B, Mokhtari K, Wang XW, Loundou A, Chapon F, Pineau S, Ouafik L, Chinot O, Figarella-Branger D. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120 (6):719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 45.Mukasa A, Takayanagi S, Saito K, Shibahara J, Tabei Y, Furuya K, Ide T, Narita Y, Nishikawa R, Ueki K, Saito N. Significance of IDH mutations varies with tumor histology, grade, and genetics in Japanese glioma patients. Cancer science. 2012;103 (3):587–592. doi: 10.1111/j.1349-7006.2011.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NCCN. Clinical Practice Guidelines in Oncology. [Accessed 9/1/2014 2014];Central Nervous System Cancers Version 2. 2014 http://www.nccn.org.
- 47.Nigro JM, Takahashi MA, Ginzinger DG, Law M, Passe S, Jenkins RB, Aldape K. Detection of 1p and 19q loss in oligodendroglioma by quantitative microsatellite analysis, a real-time quantitative polymerase chain reaction assay. Am J Pathol. 2001;158 (4):1253–1262. doi: 10.1016/S0002-9440(10)64076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15 (19):6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 49.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RG, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, Neuvial P, Cope LM, Buckley J, Herman JG, Baylin SB, Laird PW, Aldape K. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer cell. 2010;17 (5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okita Y, Narita Y, Miyakita Y, Ohno M, Matsushita Y, Fukushima S, Sumi M, Ichimura K, Kayama T, Shibui S. IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol. 2012;41 (4):1325–1336. doi: 10.3892/ijo.2012.1564. [DOI] [PubMed] [Google Scholar]
- 51.Olar A, Wani KM, Sulman EP, Mansouri A, Zadeh G, Wilson CD, DeMonte F, Fuller GN, Aldape KD. Mitotic Index is an Independent Predictor of Recurrence-Free Survival in Meningioma. Brain Pathol. 2014 doi: 10.1111/bpa.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321 (5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C, Karim AB. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20 (8):2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 54.Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145 (5):1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 55.Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 2010;120 (5):567–584. doi: 10.1007/s00401-010-0736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sahm F, Reuss D, Koelsche C, Capper D, Schittenhelm J, Heim S, Jones DT, Pfister SM, Herold-Mende C, Wick W, Mueller W, Hartmann C, Paulus W, von Deimling A. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1326-7. [DOI] [PubMed] [Google Scholar]
- 57.Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics : the journal of the American Society for Experimental Neuro Therapeutics. 2009;6 (3):478–486. doi: 10.1016/j.nurt.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26 (8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 59.Sonoda Y, Kumabe T, Nakamura T, Saito R, Kanamori M, Yamashita Y, Suzuki H, Tominaga T. Analysis of IDH1 and IDH2 mutations in Japanese glioma patients. Cancer science. 2009;100 (10):1996–1998. doi: 10.1111/j.1349-7006.2009.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stockhammer F, Misch M, Helms HJ, Lengler U, Prall F, von Deimling A, Hartmann C. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21 (3):194–197. doi: 10.1016/j.seizure.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483 (7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010;120 (3):297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Enting RH, French PJ, Dinjens WN, Vecht CJ, Allgeier A, Lacombe D, Gorlia T, Hoang-Xuan K. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31 (3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 64.van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Haaxma-Reiche H, Kros JM, van Kouwenhoven MC, Vecht CJ, Allgeier A, Lacombe D, Gorlia T. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24 (18):2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 65.van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe D, Idbaih A, van Marion R, Kros JM, Dinjens WN, Gorlia T, Sanson M. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16 (5):1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 66.van Thuijl HF, Scheinin I, Sie D, Alentorn A, van Essen HF, Cordes M, Fleischeuer R, Gijtenbeek AM, Beute G, van den Brink WA, Meijer GA, Havenith M, Idbaih A, Hoang-Xuan K, Mokhtari K, Verhaak R, van der Valk P, van de Wiel MA, Heimans JJ, Aronica E, Reijneveld JC, Wesseling P, Ylstra B. Spatial and temporal evolution of distal 10q deletion, a prognostically unfavorable event in diffuse low-grade gliomas. Genome biology. 2014;15 (9):471. doi: 10.1186/PREACCEPT-1419175304135559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174 (4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, Heese O, Krex D, Nikkhah G, Pietsch T, Wiestler O, Reifenberger G, von Deimling A, Loeffler M. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27 (34):5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 69.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27 (35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 70.Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, Platten M, Weller M, Wick W. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126 (3):443–451. doi: 10.1007/s00401-013-1156-z. [DOI] [PubMed] [Google Scholar]
- 71.Wu A, Aldape K, Lang FF. High rate of deletion of chromosomes 1p and 19q in insular oligodendroglial tumors. J Neurooncol. 2010;99 (1):57–64. doi: 10.1007/s11060-009-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360 (8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan W, Zhang W, You G, Zhang J, Han L, Bao Z, Wang Y, Liu Y, Jiang C, Kang C, You Y, Jiang T. Molecular classification of gliomas based on whole genome gene expression: a systematic report of 225 samples from the Chinese Glioma Cooperative Group. Neuro-oncology. 2012;14 (12):1432–1440. doi: 10.1093/neuonc/nos263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.