Abstract
Background & Aims
Reliable estimates of adenoma detection rates (ADRs) are needed to inform colonoscopy quality standards, yet little is known about the contributions of patient demographics to variation in ADR. We evaluated the effects of adjusting for patient age, race/ethnicity, and family history of colorectal cancer on variations in ADRs and the relative rank order of physicians.
Methods
In a retrospective cohort study, we collected data from Kaiser Permanente Northern California members ≥50 years old who received colonoscopies from 2006 through 2008. We evaluated ADRs (before and after adjustment for age, sex, race/ethnicity, and family history of colorectal cancer) for 102 endoscopists who performed 108,662 total colonoscopies and 20,792 screening colonoscopies. Adenomas were identified from the pathology database and cancers were detected using the Kaiser Permanente Northern California cancer registry.
Results
About two-thirds of examiners had unadjusted ADRs for screening exams that met gastroenterology society guidelines (>25% for men and >15% for women), although rates of detection varied widely (7.7%–61.5% for male patients and 1.7%–45.6% for female patients). Adjusting for case mix reduced the variation in detection rates (from 8-fold to 3-fold for male patients and from 27-fold to 5-fold for female patients), but the median change in physician order by detection rate was just 2 ranks, and few physicians changed quartiles of detection. For example, only 3/102 endoscopists moved into (and 3 out of) the lowest quartile of ADR.
Conclusions
In a community-based setting, most endoscopists met the ADR standards, although there was wide variation in ADRs—similar to that reported from academic and referral settings. Case-mix adjustment reduced variability, but had only small effects on differences in ADRs between physicians, and only a small percentage of physicians changed quartiles of detection. Adjustments to ADRs are therefore likely only needed in settings in which physicians have very different patient demographics, such as in sex or age. Moderate differences in patient demographics between physicians is unlikely to substantially change rates of adenoma detection.
Keywords: colon cancer, neoplasm, polyp, endoscopy
Introduction
Colonoscopy is a commonly used primary or follow-up screening test to detect colorectal cancer (CRC),1–3 the second leading cause of death from cancer in the United States.4,5 Colonoscopy can reduce the risk of CRC mortality through detection of tumors at an earlier, more treatable stage and through removal of precursor adenomatous polyps.1,2 Conversely, failure to detect adenomas during colonoscopy may increase the subsequent risk of CRC.
Physician adenoma detection rate (ADR), the percentage of screening colonoscopies performed by a physician that detect at least one adenoma or adenocarcinoma, has been recommended as a quality benchmark by specialty societies,6,7 and has been proposed by the Centers for Medicare and Medicaid Services as a reportable quality measure. Currently, professional societies recommend ADRs of 15% or higher for female patients and 25% or higher for male patients as indicators of adequate colonoscopy quality;7 however, reported detection rates have varied widely,8–20 and this variability predicts subsequent risk of CRC and mortality.21,22
Prior studies have examined physician factors related to ADR variability; however, patients differ substantially in the prevalence of adenomas based on their sex and age.23–25 To our knowledge no studies have examined the impact of adjusting for differences between physicians’ patient populations (case mix) on ADRs. If such adjustment markedly influences detection rates, it may be required for accurate comparisons between physicians.
We evaluated ADR variability in a large group of endoscopists performing colonoscopies in a community-based setting and the impact of adjusting for differences in patient case mix.
Methods
Setting
The study was conducted among health plan members of Kaiser Permanente Northern California (KPNC), an integrated health services delivery organization serving approximately 3.3 million people across 21 medical centers and hospitals in urban, suburban, and semi-rural regions within a large geographic area. KPNC’s membership demographics closely approximate the diverse underlying population of Northern California, as compared with census demographics, including members with Medicare, Medicaid (low-income), and commercial insurance; thus, studies within this setting provide results that can be generalized to a large region.26,27 The study was approved by the KPNC institutional review board.
Use of Colonoscopy in Colorectal Cancer Screening
During the study time interval (2006–2008), KPNC utilized multi-modality CRC screening that included fecal blood testing, flexible sigmoidoscopy, and colonoscopy; the majority of patients were not screened by colonoscopy. For patients receiving colonoscopy, exams were performed at multiple medical centers throughout the region; most physicians performed their exams exclusively at one of these centers.
Colonoscopy Exposure Ascertainment
Colonoscopy records were retrieved from electronic databases using Current Procedural Terminology (CPT) codes.28 Patients were included if they had a colonoscopy between January 1, 2006 and December 31, 2008 and were 50 years or older at the time of the examination. If a patient had more than one colonoscopy during this period, only the first was included. We included only exams performed by physicians with at least 300 total and 75 screening exams during the study period and excluded a small number of exams performed by physicians at facilities outside their regular service area.
Exam indication (screening vs. non-screening) was assigned using an algorithm that utilized information from referral, clinical, laboratory, pathology, radiologic, and diagnostic databases. Similar to other large screening studies,29,30 exams performed on patients with a family history of CRC were classified as screening. Examinations were considered non-screening if any of the above-mentioned sources included: evidence in the preceding 6 months of a diagnostic indication (e.g., abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, overt blood in stools, unexplained weight loss, change in bowel habits, abnormal abdominal imaging, or diverticulitis); a prior colorectal adenoma or colon polyp; a prior history of CRC; an inflammatory bowel disease diagnosis within the previous 10 years; a colonoscopy within the previous 10 years; a sigmoidoscopy within the previous 5 years; or a positive test for stool hemoglobin within the previous 1 year. All other exams were assigned a screening indication.
Outcome Ascertainment
Adenomas were identified from the pathology database and cancers were detected using the KPNC cancer registry which reports to the Surveillance, Epidemiology and End Results (SEER) registry; these results were linked to colonoscopy exams. Since few patients had cancers, detection of adenomas and cancers are collectively referred to as “adenoma” detection.
Patient Demographics
Patient age, sex, race/ethnicity, and family history of CRC were obtained from electronic medical records.
Validation Studies
Validation studies were performed to evaluate the accuracy of the electronic data capture methods compared with results from manual chart abstractions of progress notes, pathology reports, and colonoscopy procedure reports. These evaluations confirmed a high level of agreement and/or sensitivity for: capture of colonoscopy exam performance compared with manual procedure log books (99%); assignment of a screening vs. non-screening exam indication (98%); assignment of adenoma status (yes/no) (100%); and assignment of polyp histology for each container in patients with multiple containers (e.g., any adenoma vs. no adenomas).
Statistical Analyses
Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC) and Stata version 10.1 (StataCorp, College Station, TX), and P<0.05 was considered statistically significant. Pearson correlation tests were used to evaluate the consistency of physician ADRs by exam indication and patient sex. Detection rates were adjusted for patient age (50–54, 55–59, 60–64, 65–69, 70–74, and 75 years and older), race/ethnicity (non-Hispanic White, Hispanic, Black, Asian/Pacific Islander, Native American, mixed/other, and unknown), and family history of CRC (any biological relative diagnosed with CRC) using multilevel logistic regression with clustering on physician. Covariates in the model were selected a priori based on reports that sex and age are primary factors associated with adenoma prevalence,23 with race/ethnicity less of a factor, and the fact that individuals with a family history of CRC may be at increased risk of having an adenoma. Given that adjusted ADRs cannot be compared directly to unadjusted detection rates or to rates recommended by gastroenterology societies, we evaluated the effect of case-mix adjustment on changes in the rank order of physician ADRs. For this analysis, physicians were first ranked from 1 to 102 based on their unadjusted detection rates; we then contrasted physician rank order after adjustment. Reported results were stratified by sex or also adjusted for patient sex where the full patient population is reported. We also evaluated the impact of case-mix adjustment by comparing the standard deviations of the adjusted and unadjusted detection rates, calculating the median change in the absolute value of the difference in ranking resulting from adjusting detection rates, and determining the correlation of adjusted and unadjusted detection rates and rankings. The patient demographics of physicians whose order changed by five or more ranks after adjustment were compared to physicians whose order changed by less than five ranks using a two-sample t-test. We also compared the precision in detection rate estimates (standard deviations) between sex-stratified and pooled detection rates, adjusted and unadjusted.
Results
Patient Characteristics
We identified 125,462 total colonoscopy exams performed by 108 physicians among health plan members 50 years of age and older. After eligibility criteria were applied, the analytic sample consisted of 108,662 total exams (87,870 non-screening and 20,792 screening exams) performed by 102 physicians (Table 1).
Table 1.
Patient Characteristics by Exam Type
| All Exams | Screening Exams | |||||
|---|---|---|---|---|---|---|
| Male n (%) |
Female n (%) |
Total n (%) |
Male n (%) |
Female n (%) |
Total n (%) |
|
| Total | 50,891 (47) | 57,771 (53) | 108,662 (100) | 8,786 (42) | 12,006 (58) | 20,792 (100) |
| Exam indication | ||||||
| Screening | 8,786 (17) | 12,006 (21) | 20,792 (19) | |||
| Race/ethnicity | ||||||
| Non-Hispanic White | 31,100 (61) | 36,674 (63) | 67,774 (62) | 5,389 (61) | 7,877 (66) | 13,266 (64) |
| Hispanic | 4,240(8) | 5,371 (9) | 9,611(9) | 544 (6) | 817 (7) | 1,361 (7) |
| Black | 2,718(5) | 3,537 (6) | 6,255(6) | 349 (4) | 543 (5) | 892 (4) |
| Asian/PI | 5,883(12) | 6,691 (12) | 12,574 (12) | 862 (10) | 1,290 (11) | 2,152 (10) |
| Native American | 204(0) | 233 (0) | 437(0) | 33 (0) | 31 (0) | 64 (0) |
| Mixed/Other | 981(2) | 1,244 (2) | 2,225 (2) | 105 (1) | 185 (2) | 290 (1) |
| Unknown | 5,765 (11) | 4,021 (7) | 9,786 (9) | 1,504 (17) | 1,263 (11) | 2,767 (13) |
| Age (years) | ||||||
| 50–54 | 9,378 (18) | 11,000 (19) | 20,378 (19) | 2,398 (27) | 3,195 (27) | 5,593 (27) |
| 55–59 | 9,526 (19) | 10,681 (18) | 20,207 (19) | 1,894 (22) | 2,599 (22) | 4,493 (22) |
| 60–64 | 9,705 (19) | 10,972 (19) | 20,677 (19) | 1,873 (21) | 2,718 (23) | 4,591 (22) |
| 65–69 | 7,930 (16) | 8,582 (15) | 16,512 (15) | 1,238 (14) | 1,665 (14) | 2,903 (14) |
| 70–74 | 6,395 (13) | 6,975 (12) | 13,370 (12) | 755 (9) | 1,052 (9) | 1,807 (9) |
| 75+ | 7,957 (16) | 9,561 (17) | 17,518 (16) | 628 (7) | 777 (6) | 1,405 (7) |
| Family history of CRC | ||||||
| Yes | 2,467 (5) | 4,209 (7) | 6,676 (6) | 1,263 (14) | 1,977 (16) | 3,240 (16) |
n, number; PI, Pacific Islander; CRC, colorectal cancer
For patients undergoing screening exams, 58% were female, 64% were non-Hispanic whites, 52% were 60 years or older, and 16% had a family history of CRC (Table 1). A total of 317 (0.3%) patients had one or more diagnosis codes for very-high-risk family history of CRC (i.e., hereditary nonpolyposis CRC, familial adenomatous polyposis, MYH-associated polyposis, or Peutz-Jeghers syndrome). The distribution of patient characteristics by physician ADR quartile is shown in Table 2.
Table 2.
Distribution of Patient Characteristics by Physician Adenoma Detection Rate Quartile for Screening Colonoscopies*
|
|
|||||
|---|---|---|---|---|---|
| Quartile 1 % (SD) |
Quartile 2 % (SD) |
Quartile 3 % (SD) |
Quartile 4 % (SD) |
Total % (SD) |
|
|
|
|||||
| Sex | |||||
| Female | 54 (3) | 54 (3) | 52 (3) | 53 (4) | 53 (3) |
| Male | 46 (3) | 46 (3) | 48 (3) | 47 (4) | 47 (3) |
| Exam Indication | |||||
| Screening | 21 (11) | 20 (5) | 17 (5) | 19 (5) | 19 (7) |
| Race/Ethnicity | |||||
| White | 64 (13) | 65 (10) | 61 (10) | 59 (12) | 62 (12) |
| Hispanic | 7 (4) | 9 (4) | 11 (5) | 9 (4) | 9 (4) |
| Black | 8 (9) | 4 (3) | 6 (3) | 7 (6) | 6 (6) |
| Asian/PI | 8 (5) | 10 (6) | 11 (7) | 15 (9) | 11 (7) |
| Native American | 0.4 (0.3) | 0.4 (0.2) | 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.3) |
| Unknown | 10 (5) | 11 (6) | 8 (3) | 9 (3) | 9 (4) |
| Age (years) | |||||
| 50–54 | 19 (3) | 19 (3) | 19 (3) | 19 (3) | 19 (3) |
| 55–59 | 19 (2) | 19 (2) | 18 (2) | 19 (2) | 19 (2) |
| 60–64 | 20 (2) | 19 (2) | 19 (1) | 19 (2) | 19 (2) |
| 65–69 | 15 (2) | 15 (2) | 15 (1) | 15 (1) | 15 (2) |
| 70–74 | 12 (2) | 12 (2) | 13 (2) | 12 (1) | 12 (2) |
| 75+ | 16 (3) | 16 (3) | 17 (3) | 16 (3) | 16 (3) |
| Mean age | 64 (1) | 64 (1) | 64 (1) | 64 (1) | 64 (1) |
| Family History of CRC | |||||
| Yes | 7 (3) | 7 (3) | 6 (3) | 6 (2) | 6 (3) |
SD, standard deviation; PI, Pacific Islander; CRC, colorectal cancer.
Quartiles are based on adenoma detection rates for screening exams in male and female patients combined. The exam indication variable reflects the proportion of exams that had a screening indication.
Unadjusted Physician Adenoma Detection Rates
For screening exams, the ADR was 30.2% for male patients, 19.7% for female patients, and 24.1% overall (Table 3). Of the 102 examiners, 68 (67%) had unadjusted detection rates for screening exams that met the guideline of 25% or more for male patients; 69 (68%) met the guideline of 15% or more for female patients. Unadjusted ADRs varied about 8-fold in men (7.7% to 61.5%), about 27-fold in women (1.7% to 45.6%), and over 7-fold for both sexes combined (6.6% to 48.1%).
Table 3.
Physician Adenoma Detection Rates*
| n | Mean (95% CI) (%) | Median (%) | Min (%) | Max (%) | |
|---|---|---|---|---|---|
| Male patients | |||||
| All exams, unadjusted | 102 | 40.5 (38.8, 42.1) | 40.4 | 18.2 | 65.1 |
| All exams, adjusted | 102 | 42.0 (40.4, 43.5) | 41.7 | 21.1. | 63.9 |
| Screening exams, unadjusted | 102 | 30.2 (28.1, 32.4) | 29.5 | 7.7 | 61.5 |
| Screening exams, adjusted | 102 | 32.7 (30.9, 34.4) | 31.8 | 16.8 | 53.7 |
| Female patients | |||||
| All exams, unadjusted | 102 | 26.9 (25.5, 28.4) | 26.3 | 11.7 | 47.3 |
| All exams, adjusted | 102 | 28.2 (26.8, 29.5) | 27.3 | 13.9 | 49.0 |
| Screening exams, unadjusted | 102 | 19.7 (18.0, 21.4) | 18.4 | 1.7 | 45.6 |
| Screening exams, adjusted | 102 | 21.6 (20.2, 23.1) | 20.2 | 8.8 | 44.9 |
| Total patients | |||||
| All exams, unadjusted | 102 | 33.2 (31.7, 34.7) | 32.4 | 14.8 | 53.7 |
| All exams, adjusted | 102 | 34.5 (33.0, 36.0) | 33.9 | 16.2 | 55.2 |
| Screening exams, unadjusted | 102 | 24.1 (22.4, 25.9) | 22.6 | 6.6 | 48.1 |
| Screening exams, adjusted | 102 | 26.1 (24.5, 27.8) | 24.7 | 10.9 | 47.9 |
n, number; CI, confidence interval; min, minimum; max, maximum
Adenoma detection rates were adjusted for age, race/ethnicity, and family history of colorectal cancer using multilevel logistic regression with clustering on physician. For total patients, rates were also adjusted for patient sex. Adjusted values can be compared to each other, but their magnitude cannot be directly compared to unadjusted values.
Physicians with high ADRs for male patients were also likely to have high detection rates for female patients (r=0.90, P<0.001 for all exams; r=0.71, P<0.001 for screening exams). Similarly, physicians with high detection rates for screening exams were likely to have high detection rates for non-screening exams (r=0.84, P<0.001 for male patients; and r=0.87, P<0.001 for female patients).
Impact of Adjusting for Case Mix on Physician Adenoma Detection Rates
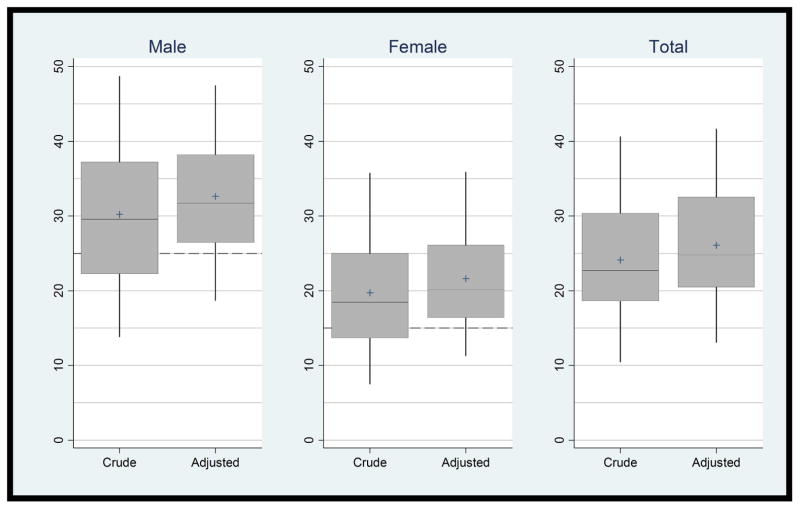
The distribution of ADRs for screening exams is shown in Figure 1. Adjustment decreased variability from 8-fold to 3-fold for males, from 27-fold to 5-fold for females, and from 7-fold to 4-fold for all patients (range in adjusted ADRs: 16.8%–53.7%, 8.8%–44.9%, and 10.9%–47.9%, respectively). Eliminating patients with a very-high-risk family history of CRC had no impact on ADR estimates.
Figure 1. Distribution of Physician Adenoma Detection Rates (Crude and Adjusted) for Screening Exams.
Vertical axes are physician adenoma detection rate (%). Dashed lines indicate guideline-recommended unadjusted adenoma detection rates, i.e., ≥25% for male patients and ≥15% for female patients. Rates are adjusted for patient age, race/ethnicity, and family history of colorectal cancer (and sex for Total) with a central line (median), a top box line (75th percentile) and lower box line (25th percentile), and a “+” symbol (mean). The two “whiskers” extend to the 5th and 95th percentiles. Values cannot be compared directly before and after adjustment.
Expressed as the change in standard deviation, case-mix adjustment reduced the ADR standard deviation 20.1% for male patients (from 10.9 to 8.7 percentage points), 14.5% for female patients (from 8.7 to 7.4 percentage points), and 6.5% for all patients combined (from 8.9 to 8.3 percentage points).
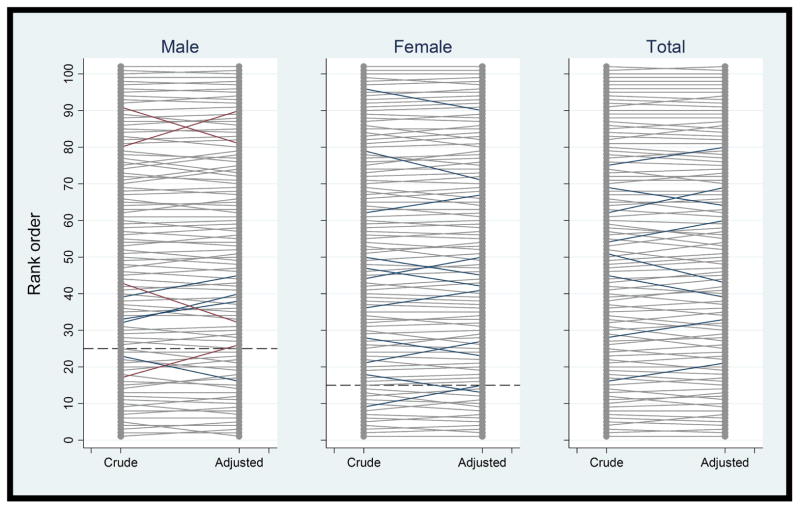
We also evaluated the impact of case-mix adjustment on changes in the screening exam ADR rank order for physicians, which, with 102 examiners, approximates percentile rankings. The median change in order (absolute value) resulting from case-mix adjustment was 2 ranks for male patients, 1 rank for female patients, and 2 ranks for males and female patients combined (Table 4). When male and female patients were combined, case-mix adjustment changed the order by 5 or more ranks for 8 physicians (8%); the largest increase was 7 ranks and the largest decrease was 8 ranks (Figure 2 and Table 4). Adjustment increased order by 5 or more ranks among 5 physicians whose patients were slightly younger (mean age: 62.9 vs. 63.9 years), and they had significantly higher proportions of patients who were in the 50–54 year age group (22% vs. 19%) and 55–59 year age group (21% vs. 19%). Conversely, adjustment decreased rank order among 3 physicians whose patients were slightly older (mean age: 65.1 vs. 63.9 years), and they had significantly higher proportions of patients who were in the 70–74 year age group (14% vs. 12%). Evaluated by quartile, adjustment resulted in 1 physician moving into (and 1 out of) the lowest 25th percentile for ADR for male patients, 2 moving into (and 2 out) for female patients, and 1 moving into (and 1 out) for male and female patients combined.
Table 4.
Impact of Adjusting for Provider Patient Characteristics on Ranking by Physician Adenoma Detection Rate
| Change in Rank Order with Adjustment* | Male Patients n (%) |
Female Patients n (%) |
Total Patients n (%) |
|---|---|---|---|
|
| |||
| Total number of physicians | 102 (100) | 102 (100) | 102 (100) |
| Any change in rank | 88 (86) | 90 (88) | 84 (82) |
| Median change in rank (absolute value) | 2 | 1 | 2 |
| Change of >1 rank | 55 (54) | 50 (49) | 60 (59) |
| Number who increased in rank | 45 (44) | 51 (50) | 42 (41) |
| Number who decreased in rank | 43 (42) | 39 (38) | 42 (41) |
| Change of ≥5 ranks | 8 (8) | 11 (11) | 8 (8) |
| Change of ≥10 ranks | 3 (3) | 0 (0) | 0 (0) |
| Changed out of the bottom 25th percentile | 1 (1) | 2 (2) | 1 (1) |
| Changed into the bottom 25th percentile | 1 (1) | 2 (2) | 1 (1) |
Change in rank order with adjustment for patient age, race/ethnicity, and family history of colorectal cancer compared to ranking based on unadjusted physician adenoma detection rate.
Figure 2. Change in Adenoma Detection Rate Rank Order for Each Physician after Adjustment for Patient Characteristics.
Vertical axes are physician adenoma detection rates in rank order. Changes in order of 0 to 4 ranks are shown in gray, 5 to 8 in blue, and >8 in red. Dashed lines indicate guideline-recommended unadjusted adenoma detection rates, i.e., ≥25% for male patients and ≥15% for female patients. Rates are adjusted for patient age, race/ethnicity, and family history of colorectal cancer (and sex for Total). Values cannot be compared directly before and after adjustment.
The correlations of the adjusted and unadjusted physician rankings for screening exams were r=0.99, P<0.001 for male patients, r=0.99, P<0.001 for female patients, and r=0.995, P<0.001 overall.
Precision in Sex-Stratified Versus Pooled Detection Rates
Pooled ADR estimates were more precise than specific estimates for men, but less precise than the estimates for women. The pooled adjusted estimate had a mean±standard deviation of 26.1±8.3%, whereas the sex-specific adjusted estimates were 32.7±8.7% and 21.6±7.4% for men and women, respectively. The pooled unadjusted estimate was 24.1±8.9%, whereas the sex-specific estimates were 30.2±10.9% and 19.7±8.7% for men and women, respectively.
Discussion
The success of CRC screening depends on the identification and removal of precancerous adenomas and early-stage cancers.2 We evaluated the influence of patient demographics on physician ADR estimates in a large group of endoscopists in a community-based setting. During this time interval, approximately two-thirds of the physicians had ADRs recommended by professional societies, but there was substantial variability in rates, similar to what has been previously reported.8–20
Variation in detection rates is associated with CRC outcomes. We recently reported a nearly linear decrease in the risk of interval CRC, advanced interval cancer, and interval CRC deaths across increasing quintiles of ADR in patients who were followed for up to 10 years after colonoscopy.21 A Polish study also reported an inverse association with interval CRC risk,22 and similar findings have been reported for studies evaluating polypectomy rates.31,32 Finally, a recent study of flexible sigmoidoscopy reported an inverse relationship between ADR and the risk of an interval distal cancer.33 Thus, a growing body of evidence suggests that physician ADR variability influences CRC outcomes.
The current study adds to knowledge regarding the causes of variation in ADRs. Prior publications have primarily explored physician factors (e.g., withdrawal time) related to detection rate variability; however, patient populations also vary in their prevalence of adenomas.23–25 A prior analysis of screening colonoscopies found that adenoma prevalence among women in their 70s was almost 70% higher than among women 50–55 years of age (26% vs. 15%); comparable prevalence estimates for men were 39% and 25%, respectively.23 These observations suggest that the variability in detection rates might be partially explained by differences between physicians in the prevalence of adenomas in their respective patients. A prior study among 43 gastroenterologists in community practice reported a 4-fold difference in physician ADRs (10%–39%) adjusted for patient age and sex, but the impact of adjusting for these factors was not evaluated.25
The detailed current analyses demonstrate that adjustment for case mix among KPNC physicians reduced the overall variability in detection rates (from 7 fold to 4 fold), but only modestly influenced the rank order (median change of 2 ranks). This suggests that in a widely dispersed medical group with numerous medical centers and local variation in patient demographics, some of the variation in detection rates is related to differences between physicians in case mix, but these differences don’t markedly influence the rank order of physicians by ADR. For those physicians whose order changed 5 or more ranks, differences in the age distributions of their patients appeared to be the driving factor.
Recent modeling studies have suggested that as many as 500 exams are needed to provide sufficiently precise confidence intervals for comparing physician ADRs to target rates.34,35 Thus, we evaluated whether a pooled detection rate estimate that increased the number of exams evaluated increased precision as compared to sex-stratified rates with fewer exams. The pooled estimate was more precise than the specific estimate for men, but less precise than the estimate for women. Thus, a pooled detection rate offers the advantage of being simpler to calculate for general comparisons, but doesn’t necessarily improve precision. Also, sex-specific ADRs are warranted in practice settings where physicians predominantly perform exams in patients of one sex (e.g., gastroenterologists whose patient populations consist primarily of women and physicians practicing in Veterans Administration hospitals whose patient populations are almost completely male).
There are potential limitations of the current study. We lacked information on the adequacy of bowel preparation and extent of exam (i.e., cecal intubation). However, evidence suggests that adequate bowel preparations are common and exam completion rates by trained physicians are high, making this lack of data unlikely to have markedly biased the results. Among 43 gastroenterologists in a previous study, for example, physician ADRs ranged from 10%–39%, despite a 99% frequency of adequate bowel preparation and 98% exam completion rate.25 Second, there may be other patient-level differences between physicians associated with ADR or the ability to complete an examination, such as body mass index and socioeconomic factors, which are not fully accounted for by the current analyses, although we have no reason to suspect such variability in this population. Third, case-mix adjustment may perform differently in other settings, e.g., where differences in case mix between endoscopists might be more pronounced or where fewer endoscopists are available for analysis. Also, while our population included a low percentage of patients with a very-high-risk family history of CRC, in other settings where endoscopists have large practices in patients with very-high-risk family histories, the impact of including these patients in the calculation of ADRs is uncertain. These patients might be expected to have higher rates of detection, or the frequency of examinations could result in a lower detection rate.
Strengths of the current study include its use of validated approaches to capture screening exams and pathology data in a defined, community-based population with comprehensive ascertainment of procedures; the manual validation of electronic codes and indications to confirm accuracy of electronic capture; pathology data to provide histological confirmation of adenomas; a large pool of experienced endoscopists; a wide distribution of patients by sex, age, and racial/ethnic group; numerous medical centers in urban, rural, and semi-rural areas with inherent local variation in practices and populations; and the ability to adjust risk estimates for demographic factors. The supplementation of referral indications with comprehensive laboratory, diagnostic, pathology, and radiologic records permitted the reliable differentiation of screening and non-screening exams. Also, the study’s community-based design is less subject to selection bias of subjects and examiners.
In conclusion, approximately two-thirds of physicians in our community-based setting met gastroenterology society recommendations for ADR; however, there was wide variability in rates. Variability was reduced after adjustment for differences between physicians in patient case mix, but rank order was not strongly affected. The findings raise the question of whether ADRs should routinely be adjusted for case mix. The need for adjustment will likely depend on the degree of variation between physicians in patient case mix and how rates are used as a performance metric. Adjusted rates would likely only be needed in settings where physicians had very different patient demographics relative to sex and age; in contrast, our study suggests that moderate variability in patient demographics is unlikely to substantially change the rank order of ADRs. Also, if rates are used for tracking colonoscopy quality improvement trends, unadjusted rates may suffice for measuring the relative changes in each physician’s detection rates over time or between physicians. However, if detection rates are to be used as the basis for rewarding or penalizing examiners, there will likely be a keen interest by each endoscopist in having as accurate an ADR measurement as possible, including taking into account differences in the patients evaluated.
Acknowledgments
Grant Support: The project was supported by grants from the Kaiser Permanente Community Benefits program, the National Cancer Institute (U54 CA163262 [PROSPR] and U24 CA171524), and the National Institute of Diabetes and Digestive and Kidney Diseases (T32DK007007).
Abbreviations
- ADR
adenoma detection rate
- CI
confidence interval
- CPT
Current Procedural Terminology
- CRC
colorectal cancer
- ICD
International Classification of Diseases
- KPNC
Kaiser Permanente Northern California
- min
minimum
- max
maximum
- n
number
- OR
odds ratio
- PI
Pacific Islander
- SNOMED
Systematized Nomenclature of Medicine
- SEER
Surveillance Epidemiology and End Results
Footnotes
Disclosures: No conflicts of interest exist for any of the authors.
Author Contributions: DAC participated in study concept and design, obtaining of funding, study supervision, acquisition and interpretation of data, statistical analysis, and critical revision of the manuscript for important intellectual content. CDJ, ARM, and WKZ participated in acquisition and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. TRL participated in study concept and design and critical revision of the manuscript for important intellectual content. CAD, JKL, VPQ, JES, NRG, AGZ, and JLS participated in critical revision of the manuscript for important intellectual content. BHF and CPQ participated in study concept and design, statistical analysis, and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008 May-Jun;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012 Feb 23;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004 Dec;127(6):1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002 Jun;97(6):1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointestinal Endoscopy. 2006 Apr;63(4 Suppl):S16–28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004 May;126(5):1247–1256. doi: 10.1053/j.gastro.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006 Dec 14;355(24):2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 10.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004 Aug;127(2):452–456. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Bretthauer M, Skovlund E, Grotmol T, et al. Inter-endoscopist variation in polyp and neoplasia pick-up rates in flexible sigmoidoscopy screening for colorectal cancer. Scand J Gastroenterol. 2003 Dec;38(12):1268–1274. doi: 10.1080/00365520310006513. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007 Apr;102(4):856–861. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 13.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011 Sep;74(3):656–665. doi: 10.1016/j.gie.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst. 1990 Nov 21;82(22):1769–1772. doi: 10.1093/jnci/82.22.1769. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa O, Shirasaki S, Kaizaki Y, Hayashi H, Douden K, Hattori M. Invasive colorectal cancer detected up to 3 years after a colonoscopy negative for cancer. Endoscopy. 2003 Jun;35(6):506–510. doi: 10.1055/s-2003-39665. [DOI] [PubMed] [Google Scholar]
- 16.Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004 Jun;36(6):499–503. doi: 10.1055/s-2004-814399. [DOI] [PubMed] [Google Scholar]
- 17.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004 Sep 7;141(5):352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997 Jan;112(1):24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez W, Harewood GC, Petersen BT. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol. 2004 Oct;99(10):1941–1945. doi: 10.1111/j.1572-0241.2004.40569.x. [DOI] [PubMed] [Google Scholar]
- 20.Schoen RE, Pinsky PF, Weissfeld JL, et al. Results of repeat sigmoidoscopy 3 years after a negative examination. JAMA. 2003 Jul 2;290(1):41–48. doi: 10.1001/jama.290.16.2123-b. [DOI] [PubMed] [Google Scholar]
- 21.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;307:1298–1306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010 May 13;362(19):1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 23.Corley DA, Jensen CD, Marks AR, et al. Variation of Adenoma Prevalence by Age, Sex, Race, and Colon Location in a Large Population: Implications for Screening and Quality Programs. Clin Gastroenterol Hepatol. 2012 Sep 14; doi: 10.1016/j.cgh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc. 2011 May 23; doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaukat A, Oancea C, Bond JH, Church TR, Allen JI. Variation in detection of adenomas and polyps by colonoscopy and change over time with a performance improvement program. Clin Gastroenterol Hepatol. 2009 Dec;7(12):1335–1340. doi: 10.1016/j.cgh.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992 May;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon NP. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? 2006 http://www.dor.kaiser.org/dor/mhsnet/public/kpnc_community.htm.
- 28.CPT 2011 Professional Edition. American Medical Association Press; 2010. [Google Scholar]
- 29.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000 Jul 20;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008 Sep 24;300(12):1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011 Jan;140(1):65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012 Jun 15;118(12):3044–3052. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogal SS, Pinsky PF, Schoen RE. Relationship between detection of adenomas by flexible sigmoidoscopy and interval distal colorectal cancer. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013 Jan;11(1):73–78. doi: 10.1016/j.cgh.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Do A, Weinberg J, Kakkar A, Jacobson BC. Reliability of adenoma detection rate is based on procedural volume. Gastrointestinal Endoscopy. 2013 Mar;77(3):376–380. doi: 10.1016/j.gie.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Saini SD, Schoenfeld P, Vijan S. Can the adenoma detection rate reliably identify low-performing endoscopists? Results of a modeling study. Digestive diseases and sciences. 2013 Jul;58(7):1856–1862. doi: 10.1007/s10620-013-2592-2. [DOI] [PubMed] [Google Scholar]