Abstract
Female reproductive tract pathologies arise largely from dysregulation of estrogen and progesterone receptor signaling leading to aberrant cell proliferation, survival and differentiation. The signaling pathways orchestrated by these nuclear receptors are complex, require the participation of many nuclear proteins serving as key binding partners or targets and involve a range of paracrine and autocrine regulatory circuits. Members of the Krüppel-like family of transcription factors are ubiquitously expressed in reproductive tissues and have been increasingly implicated as critical co-regulators and integrators of steroid hormone actions. Here we explore the involvement of KLF family members in uterine pathology, describe their currently known molecular mechanisms and discuss their potential as targets for therapeutic intervention.
Keywords: KLF, endometrial pathologies, progesterone, Notch, Wnt
Introduction
The human uterus has a unique role in the successful transmission of germ line DNA to guarantee the propagation of the human species. Biologically, it is destined to provide the fertilized egg with a ‘nurturing’ environment for its development and maturation into a complex entity with unique capabilities to eventually function on its own. Defects in the proper development and function of the uterus present a major hurdle to reproduction. Moreover, various uterine-related pathologies including endometrial and cervical carcinoma, endometriosis, and leiomyoma may arise post-puberty to further contribute to infertility. The steroid hormones estrogen (E) and progesterone (P), working through their cognate nuclear receptors [estrogen receptor (ESR) 1 and ESR2; progesterone receptor (PGR) -A and PGR-B isoforms] are major regulators of uterine development and function (Hamilton et al., 2014, Kim et al., 2013). Their multi-faceted transcriptional pathways involve interactions with numerous nuclear co-regulators (Sangupta & O’Malley, 2014) and result in altered levels of signaling molecules that act through paracrine and autocrine circuits. The underlying mechanism(s) for the autonomous and collective behavior of the multiple cell types of the uterus to maintain function, however, continues to be a work-in-progress, given recent discoveries of new participants and targets.
In this review, we highlight emerging evidence documenting the participation of the multi-member Krüppel-like factor (KLF) family of transcription factors and the dynamics of their transcriptional networks and roles in cellular communication in select uterine pathologies. The association of KLFs in ovarian carcinoma is similarly presented since the ovary is the major source of the nuclear receptor ligands E and P and because ovarian-related infertility is a major problem in reproductive medicine. Disentangling the various mechanistic points of action of KLFs in these pathologies may aid in the identification of key parameters for optimal reproductive function and contribute to the development of novel treatment strategies and clinical applications to address reproductive disorders.
Kruppel-like Factors
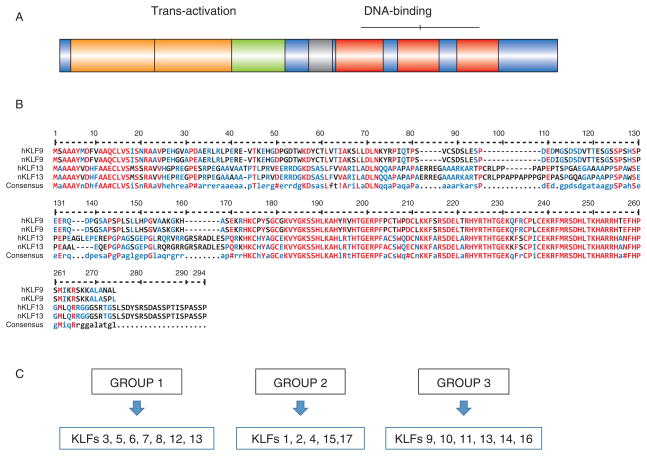
The Specificity Protein (SP)-related Krüppel-like factors (KLFs) is a 17-member family of DNA-binding transcriptional regulators of cellular proliferation, survival, differentiation, pluripotency and epithelial-mesenchymal interactions (Suske et al., 2005). We refer the reader to recent excellent reviews on this family (Tetrault et al., 2013; Knoedler & Denver, 2014; Limame et al., 2014), which now also includes multiple biologically active KLF splice isoforms (Camacho-Venegas et al., 2013) and the related gene KLF18 that is present in the sequenced genomes of most placental mammals (Pei & Grishin, 2013). KLF proteins are characterized by a conserved DNA-binding domain with three tandem C2H2-type zinc finger motifs at the carboxy-terminus and which recognizes the GT/GC box or CACCC element sites in promoter/5′ regulatory and enhancer regions (Fig. 1A). In contrast to the carboxy-termini, the amino-terminal regions of member proteins are highly variable in length and sequence and contain domains (including acidic transactivation domains, Sin-3 interacting repressor domains and CtBP2 interacting repressor domains) that interact with specific co-activators and co-repressors (Kaczynski et al., 2003); the diversity in this region is thought to confer unique functions to each family member. Figure 1B illustrates the sequence homologies between the two highly-related family members KLF9 and KLF13, where their respective C-terminal domains display highest similarities for both mouse and human proteins. Based on their phylogenetic relationships (Limame et al., 2014), KLF members can be categorized into three sub-groups (Fig. 1C). Interestingly, proteins within the same categories do not typically exhibit similar functions and tissue expression (discussed below), reflecting their distinct regulation, transcriptional activator or repressor roles, and the likely diversity of their interacting proteins under tissue-specific contexts.
Figure 1.
KLF members and their functional domains. (A) Schematic diagram of the highly variable amino-terminal (transactivation domain) and the highly conserved carboxy-terminal (DNA-binding domain) regions of KLF member proteins. (B) Sequence homology among human and mouse KLF9 and KLF13 proteins. Invariant (Red), conserved (blue) and variable (black) amino acid residues are depicted as single-letter codes. (C) KLFs are assigned to three sub-groups based on their phylogenetic relationships (Limame et al., 2014).
KLFs in Uterine and Ovarian Pathologies
Early studies suggested a potential role for KLFs in female reproductive tissues, with our laboratory’s initial report on the cloning and expression of KLF9 in the pregnant pig uterus (Wang et al.,1997). Subsequent investigations using Klf9 null mice showed that the global loss of KLF9 expression while non-embryo lethal, caused a subfertility phenotype characterized by reduced numbers of post-implantation embryos (Simmen et al., 2004) and which was associated with decreased proliferation and increased apoptosis (glandular and luminal epithelial, stromal) and partial P-resistance (stromal) of endometrial cells during the peri-implantation window (day post-coitum 2.5 to 3.5) when compared to wildtype counterparts (Velarde et al., 2005). By using WT and Klf9-null mice endometrial tissues and KLF9-siRNA targeting of a human endometrial stromal cell line HESC (Krikun et al., 2004), the mechanistic underpinnings for the aberrant proliferative and apoptotic status with KLF9 loss-of-expression were partly attributed to disruptions in the temporal patterns of expression of the Wnt signaling pathway component BMP2, PGR (specifically the PGR-B isoform), and IGFBP1 (Pabona et al., 2010). These collective findings provide robust support for the relevance of KLF9 and raise the likelihood for the participation of other KLFs, in uterine PGR and Wnt signaling, both of which are major regulators of cellular proliferation, survival and differentiation.
Table 1 lists uterine and ovarian pathologies that have now been linked to deregulated expression of KLF family members in women and in mouse models. It is worth noting that: 1) the attenuated expression of multiple KLFs (KLFs 2, 4, 5, 6, 9 and 11) with a few exceptions are relevant to ovarian, endometrial, cervical and/or myometrial pathologies; 2) the absence of several KLFs in distinct pathologies (e.g., KLF9 and KLF4 in endometrial cancer and endometriosis; KLF9 and KLF11 in endometriosis and leiomyoma) suggests roles for multiple KLFs in maintaining homeostasis in female reproductive tissues; 3) the loss of specific KLFs in various disease states occurs irrespective of their phylogenetic categories (e.g., KLF6 (Group 1), KLFs 2 and 4 (Group 2) and KLF9 (Group 3) in ovarian cancer; KLF4 (Group 2) and KLF9 (Group 3) in endometrial cancer and endometriosis), implicating distinct KLF-interacting proteins and gene targets to underlie common pathologies; and 4) KLF13 does not appear to be associated with any of the disorders attributed to KLF9, suggesting these proteins’ distinct molecular regulation and function. In this regard, KLF13 expression was not altered in endometrial tumors relative to adjacent non-tumor tissue in women with endometrial cancer (Simmons et al., 2011). Moreover, Klf13 null mice did not exhibit the subfertility and prolonged labor phenotypes found for Klf9 null mutants (Heard et al., 2012).
Table 1.
Female Reproductive Dysfunctions and KLF Dysregulated Expression in Humans (h) and Mouse (m) Models
| PATHOLOGY | KLF (Species) | Over (↑)/Under (↓) Expression | References |
|---|---|---|---|
| Endometrial Cancer | KLF4 (h) KLF 9 (h) KLF17 (h) |
↓ ↓ ↑ |
Simmons et al. (2011) Simmen et al. (2008) Simmons et al. (2011) Korani et al. 2013 Dong et al. (2014) |
| Ovarian Cancer | KLF2 (h) KLF4 (h) KLF6 (h) KLF9 (h) |
↓ ↓ ↓ ↓/↑ |
Wang et al. (2005) Yoon & Roh (2012) DiFeo et al. 2006 Huang et al. (2014) Zhang et al. (2014) |
| Cervical Cancer | KLF4 (h) KLF5 (h) |
↓ ↑ |
Yang & Zhang (2014) Marrero-Rodrigues et al. (2014) |
| Endometriosis | KLF4 (h) KLF9 (h, m) KLF11 (h, m) |
↓ ↓ ↓ |
Adammek et al. (2013) Lee et al. (2008) Pabona et al. (2012) Heard et al. (2014) Daftary et al. (2013) |
| Leiomyoma | KLF9 (h) KLF11(h) |
↓ ↓ |
Rackow & Taylor (2010) Yin et al. (2010) |
| Implantation/Pregnancy | KLF5 (m) KLF9 (m) |
↓ ↓ |
Sun et al. (2012) Simmen et al. (2004) |
| Labor Dysfunction | KLF9 (h, m) | ↓ | Zeng et al. (2007) Pabona et al. (2014) |
Given the paucity of currently available mouse models and limited access to human tissues for studying KLF function in the uterus and ovary, human cell lines that model reproductive disease states have been used to dissect mechanisms of action of particular KLFs. These cell lines are summarized in Table 2. The human Ishikawa, endometrial endocarcinoma (EEC) and human endometrial carcinoma-1A (HEC-1A) cell lines have been investigated as models for endometrial carcinoma. The ovarian cancer cell lines OV202, SKOV3, OVCAR3 and to a limited extent, T80 have been employed to model ovarian cancer. Further, the human endometrial stromal cell line HESC, generated by overexpression of human telomerase and shown to be P-responsive (Krikun et al., 2004) is commonly used as a paradigm for human endometrial stromal cells during early pregnancy, due to their ability to decidualize in vitro after treatments with a cocktail of cAMP, E and P, and can be evaluated for poor decidual response upon specific KLF siRNA targeting (Pabona et al., 2010; Shen et al., 2013). To mimic the labor dysfunction observed with Klf9 null mice (Zeng et al., 2007), the response of a recently generated human uterine smooth muscle cell line HutSMC was tested in E+P-treated cells without or with KLF9 siRNAs (Pabona et al., 2014). While such studies have resulted in the identification of common and distinct pathways for KLFs (Fig. 2), there are acknowledged limitations to the use of cell lines for extending relevance to the whole organism, providing impetus for generating new and reproductive system-targeted mouse models to further understand the dynamics of KLF actions in vivo.
Table 2.
Human Cell Lines Used to Model Reproductive Dysfunctions Associated with KLF Dysregulated Expression
| PATHOLOGY | Cell Line | KLF | References |
|---|---|---|---|
| Endometrial Cancer | EM/PR Ishikawa, EEC, Hec-1-A EEC |
KLF4 KLF9 KLF17 |
Shimizu et al. (2010) Simmen et al. (2008) Simmons et al.(2011) Dong et al. (2014) |
| Ovarian Cancer | OV202, SKOV3 SKOV3, OVCAR3 SKOV3 T80, SKOV3 SKOV3, OVCAR3 |
KLF2 KLF4 KLF5 KLF8 KLF9 |
Wang et al. (2005) Yoon & Roh (2012); Chen et al. (2014) Dong et al. (2013) Lu et al. 2014) Zhang et al. (2014) |
| Endometriosis | 12Z HESC |
KLF4 KLF9 |
Adammek et al. (2013) Pabona et al. (2012) |
| Implantation Defects | HESC HESC |
KLF9 KLF12 |
Pabona et al. (2010) Shen et al. (2013) |
| Labor Dysfunction | HutSMC | KLF9 | Pabona et al. (2014) |
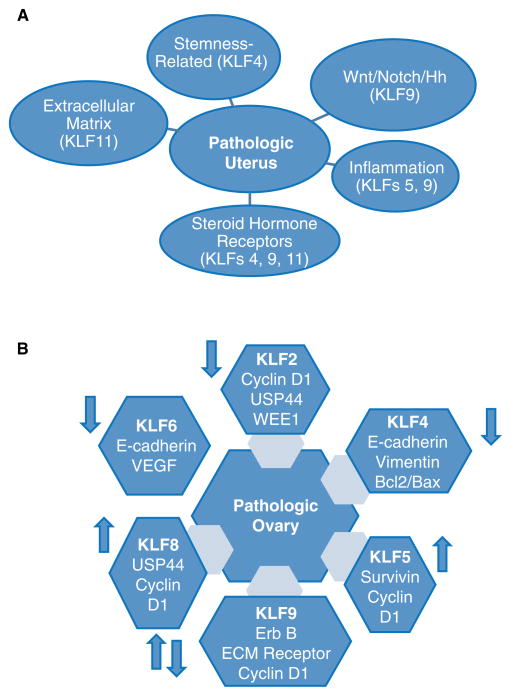
Figure 2.
Signaling pathways and gene targets associated with dysregulated expression of KLF family members in the pathologic uterus (A) and ovary (B). Arrows in panel B (↑ and ↓) signify up- and down-regulated expression of each KLF with ovarian carcinoma.
KLFs and Targeted Signaling Pathways in Uterine Pathologies
Since KLFs are known to regulate cell proliferation, survival and differentiation, it is quite expected that their reduced expression in many uterine diseases (Table 1) will be associated with perturbations in signaling pathways for PGR and ESR, Wnt, Notch, Hedgehog (Hh), immune activation, and epithelial-mesenchymal transitions, all of which are requisite for maintenance of uterine integrity and function (Fig. 2). Whether cross-talk between these pathways is mediated by KLF actions is not completely understood, albeit limited reports support this possibility for PGR and the Notch/Hh signaling pathways. In one study, ectopic lesions formed from Klf9 null endometrial tissues in a mouse model displayed activated Notch and Hh signaling and conversely, reduced PGR expression (Heard et al., 2014). Moreover, eutopic endometria of women with endometriosis, a disease state characterized by loss of P-sensitivity, display reduced KLF9 (Pabona et al., 2012) and enhanced Notch 3 (Tamaresis et al., 2014) expression. Reduced P-sensitivity with loss of KLF9 is due in part to KLF9’s role as a PGR-interacting protein (Zhang D et al., 2002; Zhang XL et al., 2003) and its promotion of E-dependent ESR1 down-regulation (Velarde et al, 2007). As regards to P/PGR and the Notch and Hh signaling pathways, studies have demonstrated their opposing and complementary associations in endometrial cells. For example, transcript levels of the Notch ligand, Delta-like 4 are reduced by medroxyprogesterone acetate in primary cultures of human endometrial glandular and stromal cells (Mazella et al., 2008), Moreover, the Hh ligand Indian Hedgehog is a negatively-regulated downstream target of P/PGR (Simon et al., 2009). On the other hand, Notch 1 has been shown to mediate P-dependent uterine stromal cell differentiation in primates and mice (Ashar et al., 2012a; Ashar et al., 2012b). Additionally, P increased the levels of transcriptionally active Notch 1 intracellular domain, which can form a functional complex with PGR (Afshar et al., 2012a). The potential complexity of the regulatory networks involving PGR and Notch/Hh signaling suggests that no single mechanism may fully account for each KLF acting through these pathways.
A core KLF circuitry comprised of KLF2, KLF4 and KLF5 has been recently implicated in regulating the self-renewal of embryonic stem cells involving key pluripotency genes (Jiang et al., 2008). By regulating adult stem cell signaling pathways (e.g., Wnt, Notch), KLFs may similarly control the regenerative capacity of endometrial and myometrial stem/progenitor cells. In this regard, endometriosis (Sassoon & Taylor 2008) and leiomyoma (Ono et al., 2014) are increasingly considered to be a consequence of deregulated stem cell expansion. Indeed, endometrial epithelial stem/progenitor cells have been characterized from eutopic endometrium of women with endometriosis (Li et al., 2014), ovarian endometriotic cysts (Chan et al., 2011), endometrial carcinoma tissues (Hubbard et al., 2009) and uterine leiomyoma (Ono et al., 2012). KLF4 is a well-acknowledged regulator of stem cell biology and is the most highly implicated KLF in both cancer and normal stem cells (Tetrault et al., 2013). KLF4 also mediates PGR action in human endometrial epithelial cells (Shimizu et al., 2010) albeit unlike KLF9, KLF4 has not been shown to interact with PGR. However, KLF4 expression (Adammek et al., 2014), similar to that of KLF9 (Pabona et al., 2012), is reduced in eutopic endometria of women with endometriosis (relative to those of women without the disease) and in endometrial tumors relative to adjacent normal tissues (Simmons et al., 2011). Recent studies have shown that the loss of KLF9 expression promotes neurosphere formation (an in vitro measure of ‘stemness’) in neuroblastoma cells (Ying et al., 2011); this involved activation of Wnt signaling and KLF9 transcriptional repression of integrin-α6 expression (Ying et al., 2014). While the above studies provide causative support for loss of KLF9 expression in the aberrant promotion of ‘stemness’, direct evidence for KLF9 and KLF4 involvement in uterine diseases remains to be fully characterized.
Several KLFs have been directly linked to regulation of inflammatory signaling, defects of which may contribute to uterine pathology. In particular, uterine-specific Klf5 null mice are infertile due to aberrant expression of the prostaglandin synthesis gene Ptgs2, resulting in the enhanced expression of Cox2 (Sun et al., 2012). Similarly, KLF11, whose attenuated expression is linked to uterine leiomyoma, has been reported to inhibit prostaglandin E2 synthesis by transcriptionally silencing the gene promoter for phospholipase A2α, the key enzyme for prostaglandin biosynthesis (Buttar et al., 2010). Further, KLF4 was shown to stimulate monocyte differentiation in the human acute myeloid leukemia cell line HL60 (Alder et al., 2008) and to enhance macrophage activation in the macrophage cell line J774a (Feinberg et al., 2005), suggesting a role in immune modulation that is critical for uterine function. In women, prolonged pregnancy is associated with reduced expression of KLF9 and with aberrant down- and up-regulation of several pro-inflammatory and anti-inflammatory genes, respectively (Pabona et al., 2014). Given that a number of inflammation-associated genes are direct PGR targets (e.g., IL11, CXCL1) (Cordeaux et al., 2010; Kavandi et al., 2012), data suggest that the deregulated expression of numerous inflammatory mediators may be a direct outcome of aberrant PGR signaling involving KLFs. In a recent study, Rogatsky and colleagues (Chinenoy et al., 2014) described the functional cooperation between the glucocorticoid receptor and KLFs 2 and 9 in macrophages during inflammation. Since the glucocorticoid receptor can mediate progestin effects on uterine inflammatory response (Lei et al., 2012; Guo et al., 2012), KLF interaction with P-dependent transcriptional circuitry is a possible node by which KLFs may exert their control of inflammatory events in the uterus.
Additional pathways that have been linked to KLFs and which may underlie a number of uterine pathologies when these KLFs are aberrantly expressed include: KLF17 promotion of epithelial-mesenchymal transitions through induction of TWIST1 in endometrial cancer (Dong et al., 2014); KLF6-coactivation of NF-κB signaling via its induction of cytokines TNFα and IL-6 (Zhang et al., 2014) in the pathogenesis of endometriosis; KLF5-mediated activation of the JAK-STAT signaling pathway (Tetrault et al., 2012), the latter a key mediator of leukemia inhibitory factor control of embryo implantation and hence, successful pregnancy (Rosario et al., 2014); and KLF14- (de Assuncao et al., 2014) and KLF11-(Zheng et al., 2014) mediated activation of lipid and metabolic signaling, respectively, processes which when dysregulated can lead to abnormal metabolism and increased risk for endometrial cancer.
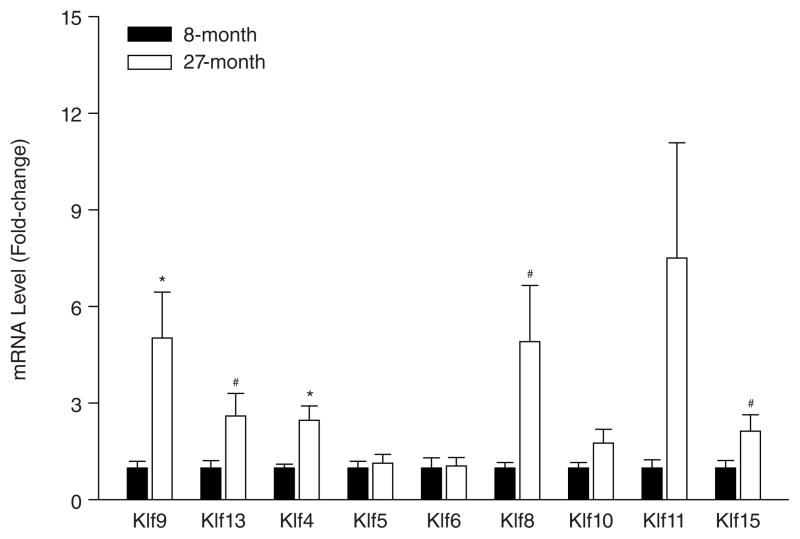
Reproductive aging is a natural biological process and does not fall into the category of a uterine pathology (Nelson et al., 2013); however, societal demands based on a woman’s choice to time her pregnancy have raised the need to further understand age-related co-morbidities in the uterus and ovary that can be modified for successful pregnancy outcome. To begin to evaluate a potential role for KLFs in this process, we measured the transcript levels of several KLF family members in uteri of young (8 months; n=7) and old (27 months; n=7) C57BL/6 mice, by quantitative RT-PCR. Of the nine KLFs analyzed, only the mRNAs for Klf9 and Klf4 displayed significant differences as a function of age; levels of Klf8, Klf13 and Klf15 transcripts only showed tendencies for differences (Fig. 3). Using a Stem Cell-Focused PCR Array, we also searched for potential differentially-regulated genes associated with the aging uterus. Transcripts for a number of cell-cycle regulators, cytokines and self-renewal markers were distinctly regulated during aging (Table 3). While it is premature to establish a correlation between aging and the KLFs based on this pilot study, the well-recognized reductions in the reservoir of uterine stem cells with aging together with the suggested roles of KLFs in stem cell self-renewal provide a reasonable basis for utilizing the aging uterus as a unique model to further evaluate a potential link between stem cell biology and KLFs.
Figure 3.
Expression of select KLFs in young and aging mouse uteri. Transcript levels of various KLFs were quantified in 8- and 27-month old C57BL/6 mouse uteri by quantitative RT-PCR. Data (mean ± SEM) are expressed as fold-change and were obtained from n=7 individual mice per age group. Transcript levels were normalized to corresponding levels of 18S, and then to control (8-month old uteri) and were calibrated to a standard curve using pooled cDNA stocks. *, significant at P<0.05, by t-test; #, tending to significance with 0.05<P<0.10
Table 3.
Differentially-Expressed Genes in the Aging Uterus
| Genesa | Fold-changeb |
|---|---|
| Cell Cycle | |
| Regulators/Wnt signaling | |
| APC | −2.10 |
| Axin 1 | −2.75 |
| Ccna2 | −8.40 |
| Ccnd1 | −7.36 |
| Ccne1 | −4.99 |
| Myc | −2.48 |
| Cytokines/Growth Factors | |
| Bmp1 | −6.06 |
| Cxcl12 | −4.56 |
| Fgf1 | 2.17 |
| Fgf2 | −2.64 |
| Igf1 | −3.81 |
| Self-Renewal Markers | |
| Cd44 | −9.19 |
| Msx1 | −7.26 |
| Jag1 | −1.64 |
| Hspa9 | −2.58 |
| Myst1 | −3.48 |
Identified using Stem Cell Signaling QPCR array
Aging vs. Young Uterus: (-) down-regulation
KLFs and Targeted Signaling Pathways in Ovarian Pathologies
It is notable that for those mice with global null-mutations of specific KLFs (e.g. KLF9, KLF11, KLF13) and surviving through adulthood, an ovarian phenotype characterized by dysfunctions in steroid hormone synthesis is not manifested throughout the reproductive years (Simmen et al., 2004; Zeng et al., 2007; Heard et al., 2012; Daftary et al., 2013). This finding is not congruent with the demonstrated regulation of several key steroidogenic genes transcript levels (LDLR, StAR and CYP11A) by KLF13 in ovarian granulosa cells (Natesampillai et al., 2008). Interestingly, the pathologic ovary (i.e., ovarian carcinoma) is characterized by reduced (KLF2, KLF4, KLF6) and enhanced (KLF5, KLF8) expression of several KLFs; contradicting results have been reported for KLF9 (Fig. 2B). Analyses of currently identified target genes associated with dysregulation of distinct KLF expression in ovarian cancer cells revealed perturbations in those related to proliferation and differentiation (cyclin D1); apoptosis (Bcl2, Bax, survivin); epithelial-mesenchymal interactions (E-cadherin, vimentin, Extracellular matrix receptor); stem cell differentiation (USP44, ErbB), and angiogenesis (VEGF). These findings raise important questions on how KLFs alone or together may integrate the physiological processes in the ovary and whether pathways defined for uterine pathologies in which multiple KLFs (e.g., KLF4, KLF5) are similarly dysregulated, may be relevant to ovarian diseases.
KLF Networks: A Case For and Against Functional Redundancy
Since KLF expression is ubiquitous yet known reproductive system pathologies appear to involve select subsets of KLFs (Table 1), functional redundancies and compensatory regulation among KLFs must exist to ensure robust physiological responses to cellular perturbations for maintaining homeostasis. Recent elegant studies have demonstrated this concept for KLF3 and KLF8 in a non-reproductive (i.e., erythroid) system (Eaton et al., 2008; Funnell et al., 2013). The lack of distinct uterine phenotypes in mouse knockout models for several KLF genes support this concept in the reproductive tract. A prime example involves the highly-related members KLF9 and KLF13. Albeit a definitive conclusion is limited by the lack of functional studies in mice deficient in both KLFs, support for a KLF9/KLF13 genetic interaction comes from findings that Klf9-null mouse uteri at peri-implantation displayed increased Klf13 expression, which was confirmed in siKLF9-targeted human endometrial stromal cells (Pabona et al., 2010). Moreover, Klf13-null mice are reproductively normal, perhaps due to the accompanying increase in nuclear KLF9 protein levels shown for Klf13-null endometrial cells (Heard et al., 2013). Thus, the absence of an association between KLF13 and any reproductive dysfunctions reported to date (Table 1) may be a consequence of the placement of KLF9 at a higher functional hierarchy relative to KLF13. In this scenario, potential transcriptional dysregulation that may occur with KLF13 loss-of-expression is abrogated by the compensatory actions of KLF9.
The co-reduction of KLF9 and KLF4 expression noted in endometrial cancer and in endometriosis and those of KLF9 and KLF11 in endometriosis and in leiomyoma (Table 1) on the other hand, support the concept of distinct programs of gene expression being controlled by these KLFs. Alternatively, this may indicate that there is an obligatory pathway that is mediated by both KLFs occurring through a linear mechanism. There is evidence for the latter possibility, at least for KLF9 and KLF4. KLF9 siRNA knockdown in the human endometrial carcinoma Ishikawa cell line reduced KLF4 transcript levels (Simmons et al., 2011) and conversely, KLF9 over-expression in HEC-1A cells induced KLF4 gene expression (Simmen et al., 2008); these observations are in accord (albeit yet to be proven) that KLF4 serves as a downstream target of KLF9 either directly or indirectly. Parallel transcriptome and ChiP-Seq analyses of uterine cells subjected to siKLF9 and siKLF4 targeting, alone and in combination, will be required to identify unique and shared networks regulated by both KLFs and could provide insight into whether KLF4 is an early target of KLF9. Importantly, such studies may allow the identification of an obligate response (gene target, signaling pathway) mediated by both. In regards to KLF9 and KLF11, there are limited data to support or refute redundant functions; however, they are likely to differentially mediate PGR-driven transcriptional events in uterine cells based on their distinct reproductive phenotypes upon targeted gene inactivation (Klf11-null mice breed normally and are fertile in contrast to Klf9-null mice which are subfertile) (Song et al., 2010; Simmen et al., 2004) and the distinct mechanisms by which they mediate PGR transactivity (Zhang et al., 2003; Yin et al., 2010).
The opposing actions of KLF4 and KLF15 in uterine epithelial cells constitute additional support for non-redundant functions of KLF family members. In these cells, KLFs 4 and 15 are inversely expressed, and are found to discretely regulate initiation of DNA synthesis by virtue of their distinct responses to E- and P-treatments (Ray & Pollard, 2012). By inhibiting E-enhanced transcription of the DNA synthesis initiator protein minichromosome maintenance-2, KLF15 functions as a downstream mediator of P-inhibition of the cell cycle. What factors direct the inverse expression of KLFs 4 and 15 and their opposing responses to steroid hormones in the uterine epithelium have yet to be determined. Clearly, the biology underlying optimal uterine function involving KLF regulatory networks is wide-open for further investigations.
Regulation of KLF Expression
Factors that contribute to the aberrant expression and activity of KLFs in the reproductive tract leading to pathology have not been well-characterized, in contrast to other systems. In embryonic stem cells, induction of KLF2 by Oct4 and of KLF4 by LIF has been demonstrated, reinforcing these KLFs’ function in stem cell renewal (Hall et al., 2009). KLF4 expression was suppressed by transcription factor FOXO in B-lymphocytes (Yusuf et al., 2008) and by an inhibitor of notch signaling in the mouse gastrointestinal tract (Zheng et al., 2009), and conversely, was induced by Notch 1 intracellular domain in ocular surface epithelia (Zhang et al., 2013). KLF6 expression was stimulated by IGF-1 in human colon cancer cell lines (Bentov et al., 2008) and the binding of carbohydrate response element-binding protein (ChREBP), a glucose-activated transcription factor, induced KLF10 promoter activity and expression in rat hepatocytes (Iizuka et al., 2011). The identity of factors that regulate KLF expression in uterine cells is currently limited to that for KLF9 in human endometrial stromal cells; in these cells, BMP2 inhibited KLF9 expression indirectly through KLF13 (Pabona et al., 2010) while E and P had no influence on its expression (Pabona et al., 2012). In ovarian granulosa cells, IGF1 and LH were reported to increase KLF13 expression (Natesampillai et al., 2008). The comprehensive analyses of cellular components responsible for maintaining KLF expression will be required to understand and ultimately manipulate KLF regulatory circuits for optimal reproductive function.
The Next Steps: A Perspective
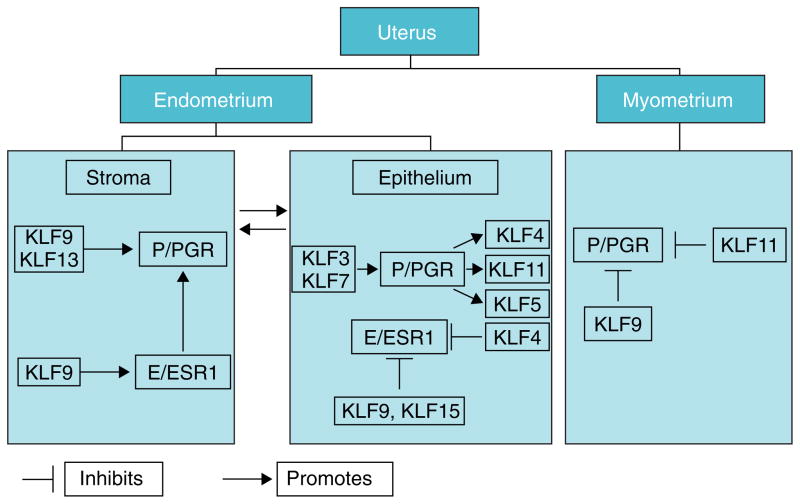
In the last decade, multiple molecular pathways mediated by KLFs have been elucidated in uterine and ovarian cells and tissues. Nevertheless, direct evidence linking described KLF effects to health outcomes and disease states remain elusive. How may we address this gap in knowledge? In most cases, the difficulty lies in the absence of mouse models that recapitulate the human disease and in the possible biological redundancies among subsets of KLFs that may prevent abnormal responses to be gleaned when one KLF is absent. Thus, it is imperative to establish which subsets of KLFs compensate for each other, using relevant cell lines in vitro by siRNA targeting and by characterizing uterine (or ovarian)-targeted KLF-combination knockouts in vivo. Many of the mouse mutants for KLFs have modest or no reproductive phenotypes when they survive through adulthood (e.g. Klf9, Klf11, and Klf13 null mice). For other KLFs, homozygous disruptions result in early embryo (for Klf4, Klf5 and Klf6), in utero (for Klf2) and neonatal (for Klf7) lethality (Wani et al., 1999; Matsumoto et al., 2006; Laub et al., 2006; Ema et al., 2008). For these KLFs, therefore, conditional mutations using uterine epithelial, stromal and myometrial-specific promoters driving the Cre-recombinase may serve as a powerful strategy for studying gene function in each cell type. Such studies are anticipated to be labor-intensive and complex, given that the uterus has multiple cellular compartments and several KLFs exhibit preferential cellular expression (e.g., KLF9 in endometrial stroma and myometrium) (Simmen et al., 2004). Indeed, the complexity of ‘teasing out’ the details of KLF signaling in each compartment is best illustrated when one considers that for the P/PGR signaling pathway alone, distinct KLFs are involved either as regulators or integrators of P/PGR transactivity, albeit not necessarily under the same physiological contexts (Fig. 4). To date, the proliferative, survival, and pro-/anti-inflammatory molecular signatures elicited by each KLF family member when null-mutated in specific uterine compartments have not been defined. The power of increasingly sophisticated approaches such as ChiP-Seq, various ‘omics’ technologies and precise genome editing methodologies using engineered nucleases offered by the clustered regularly interspaced short palindromic repeats (CRISPR) with CRISPR-associated (Cas) proteins should be harnessed to address this question.
Figure 4.
Complex and redundant control by KLFs of PGR and ESR1 signaling in distinct uterine compartments. Promotion or inhibition of PGR and ESR1 activity may occur by direct or indirect mechanisms. Arrows originating from P/PGR (in epithelium) depict KLFs as integrators of P/PGR signaling. Bi-directional arrows between stroma and epithelium signify the dynamic communication between the two endometrial compartments.
So why study KLFs in the face of their seeming complexity? The data presented in this review documenting: 1) their association with many reproductive disorders, whose etiologies remain not well-understood; 2) their control of a plethora of signaling pathways; and 3) the considerable diversity of their target genes due to their ability to act as transcriptional activators or repressors, collectively suggest their prominent roles as integrators of uterine (and ovarian) biology. Perhaps an exciting direction for KLF research is one that focuses on their transcriptional roles in uterine and ovarian stem cell biology. It is well-known that the endometrium displays dramatic regenerative properties, estimated to occur ~400-times during a woman’s reproductive years; these have been linked to the presence of adult stem cells displaying key properties of mesenchymal stem cells (Figueira et al., 2011; Spitzer et al., 2012). In a recent study, Taylor and colleagues (Sakr et al., 2014) demonstrated that mesenchymal stem cells are recruited to endometriosis lesions and that reduction of this recruitment can diminish lesion incidence. Similarly, a small population of cells (~1% of tumor cells) showing stem-progenitor properties was found to be essential for E+P-dependent growth of uterine leiomyomas (Ono et al., 2012). Interestingly, the growth of this cell population involves ESR/PGR and Wnt signaling pathway cross-talk via E+P-induced β-catenin translocation, leading to Axin2 promoter activation (Ono et al., 2013). Since loss of KLF11 expression is associated with increased PGR signaling and proliferation of leiomyoma cells (Yin et al., 2010), it is tempting to consider that inhibition of the aberrant expansion of myometrial smooth muscle stem cells by KLF11 may avert tumor initiation and leiomyoma.
How will understanding the biology of KLFs lead to novel and more effective therapies for female reproductive disorders? To date, treatment options for most uterine disorders involve aromatase inhibitors and progestins; however, prolonged treatments with these agents can result in drug resistance, with disease recurring often times after cessation of treatment. If current data indicating that KLFs integrate P/PGR and E/ESR cross-talk with Notch and Wnt pathways to control aberrant stem/progenitor cell proliferation, are verified, it may be possible to develop non-steroidal treatments that target specific ‘stemness’ factors such as the Notch ligand Jagged1, that promote the survival of this subpopulation and hence, progression/recurrence of uterine pathologies. Thus, targeting Notch signaling with γ-secretase inhibitors that inhibit the intracellular localization of transcriptional mediator Notch intracellular domain may offer a viable therapeutic strategy. A proof-of-concept for the latter has been recently demonstrated for uterine serous carcinoma in a human xenograft model in mice (Groeneweg et al., 2014). In a recent report, small molecule inhibitors of the expression of the colorectal cancer oncogene KLF5 were identified by high-throughput screening of compound libraries (Bialkowska et al., 2011). The isolated compounds, screened using a rat intestinal cell line stably expressing a luciferase reporter driven by the human KLF5 promoter, reduced endogenous KLF5 protein levels and decreased the viability of a number of colorectal cancer cell lines. A similar strategy may also be employed to elude reproductive pathologies, although compounds promoting, rather than inhibiting, KLF expression will need to be identified since uterine pathologies are mostly associated with reduced KLF expression (Table 1). Such approaches could yield novel research outcomes valuable for translation into the clinic.
Finally, it is worth noting that the major male reproductive disease namely prostate cancer is also highly associated with dysfunctions in numerous KLFs including KLF4 (Wang et al., 2010), KLF5 (Frigo et al., 2009), KLF6 (Narla et al., 2001), KLF8 (He et al., 2013) and KLF9 (Shen et al., 2014). Importantly, a number of signaling pathways reported for KLF (dys) regulation of prostate epithelial cell proliferation, differentiation and survival overlap with those elucidated for KLF-mediated uterine function. In particular, KLFs have been reported to participate in androgen receptor-dependent signaling (Liu et al., 2012; He et al., 2013), the male counterpart of PGR/ESR signaling in females; in regulating Hedgehog pathway components (Leow et al., 2009); and in stem cell signaling involving the Notch pathway (Oklem et al., 2014). However, no KLFs have been demonstrated so far to be indispensable for spermatogenesis.
Conclusion
The growing evidence for the functional and correlative association of KLFs in various female (and male) reproductive pathologies underscores the importance of extending and expanding current knowledge of this multi-faceted transcription factor family in reproductive health. New possibilities for targeting KLFs may soon be available from reproductive systemwide analyses of KLF signaling. Other reproductive pathologies including preeclampsia, fallopian tube cancers, and recurrent pregnancy loss as well as male infertility may similarly benefit from an understanding of KLF biology.
Acknowledgments
Funding
Work from our laboratory described in this review was supported in part by the United States National Institutes of Health grants HD21961 and CA136493.
We thank Dr. Frank A Simmen for his critical review of this manuscript.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this report.
References
- Adammek M, Greve B, Kässens N, Schneider C, Brüggemann K, Schüring AN, Starzinski-Powitz A, Kiesel L, Götte M. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertility & Sterility. 2013;99:1346–1355. doi: 10.1016/j.fertnstert.2012.11.055. [DOI] [PubMed] [Google Scholar]
- Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012a;153:2884–2896. doi: 10.1210/en.2011-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, Radnor R, Miele L, Fazleabas A. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB Journal. 2012b;26:282–294. doi: 10.1096/fj.11-184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Georgantas RW, III, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. Journal of Immunology. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov I, Narla G, Schayek H, Akita K, Plymate SR, LeRoith D, Friedman SL, Werner H. Insulin-like growth factor-i regulates Kruppel-like factor-6 gene expression in a p53-dependent manner. Endocrinology. 2008;149:1890–1897. doi: 10.1210/en.2007-0844. [DOI] [PubMed] [Google Scholar]
- Bialkowska AB, Crisp M, Bannister T, He Y, Chowdhury S, Schürer S, Chase P, Spicer T, Madoux F, Tian C, et al. Identification of small-molecule inhibitors of the colorectal cancer oncogene Krüppel-like factor 5 expression by ultrahigh-throughput screening. Molecular Cancer Therapeutics. 2011;10:2043–2051. doi: 10.1158/1535-7163.MCT-11-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttar NS, DeMars CJ, Lomberk G, Rizvi S, Bonilla-Velez J, Achra S, Rashtak S, Wang KK, Fernandez-Zapico ME, Urrutia R. Distinct role of Kruppel-like factor 11 in the regulation of prostaglandin E2 biosynthesis. Journal of Biological Chemistry. 2010;285:11433–11444. doi: 10.1074/jbc.M109.077065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Vanegas O, Till J, Miranda-Lorenzo I, Ozturk B, Camacho SC, Martignetti JA. Shaking the family tree: identification of novel and biologically active alternatively spliced isoforms across the KLF family of transcription factors. FASEB J. 2013;27:432–436. doi: 10.1096/fj.12-220319. [DOI] [PubMed] [Google Scholar]
- Chan RW, Ng EH, Yeung WS. Identification of cells with colony-forming activity, self-renewal capacity, and multipotency in ovarian endometriosis. American Journal of Pathology. 2011;178:2832–2844. doi: 10.1016/j.ajpath.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Y, Liu W, Zhao G, Lee S, Balogh A, Zou Y, Guo Y, Zhang Z, Gu W, et al. Doxycycline inducible Krüppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS One. 2014;9:e105331. doi: 10.1371/journal.pone.0105331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Coppo M, Gupte R, Sacta MA, Rogatsky I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genomics. 2014;15:656. doi: 10.1186/1471-2164-15-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeaux Y, Tattersall M, Charnock-Jones DS, Smith GC. Effects of medroxyprogesterone acetate on gene expression in myometrial explants from pregnant women. Journal of Clinical Endocrinology & Metabolism. 2010;95:437–447. doi: 10.1210/jc.2010-1541. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Zheng Y, Tabbaa ZM, Schoolmeester JK, Gada RP, Grzenda AL, Mathison AJ, Keeney GL, Lomberk GA, Urrutia R. A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLoS One. 2013;8:e60165. doi: 10.1371/journal.pone.0060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, O’Malley BW. Transcriptional coregulators: emerging roles of SRC family of coactivators in disease pathology. Journal of Molecular Endocrinology. 2014;53:R47–59. doi: 10.1530/JME-14-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assuncao TM, Lomberk G, Cao S, Yaqoob U, Mathison A, Simonetto DA, Huebert RC, Urrutia RA, Shah VH. New role for Kruppel-like factor 14 as a transcriptional activator involved in the generation of signaling lipids. Journal of Biological Chemistry. 2014;289:15798–15809. doi: 10.1074/jbc.M113.544346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFeo A, Narla G, Camacho-Vanegas O, Nishio H, Rose SL, Buller RE, Friedman SL, Walsh MJ, Martignetti JA. E-cadherin is a novel transcriptional target of the KLF6 tumor suppressor. Oncogene. 2006;25:6026–6031. doi: 10.1038/sj.onc.1209611. [DOI] [PubMed] [Google Scholar]
- DiFeo A, Narla G, Hirshfeld J, Camacho-Vanegas O, Narla J, Rose SL, Kalir T, Yao S, Levine A, Birrer MJ, et al. Roles of KLF6 and KLF6-SV1 in ovarian cancer progression and intraperitoneal dissemination. Clinical Cancer Research. 2006;12:3730–3739. doi: 10.1158/1078-0432.CCR-06-0054. [DOI] [PubMed] [Google Scholar]
- Dong P, Kaneuchi M, Xiong Y, Cao L, Cai M, Liu X, Guo SW, Ju J, Jia N, Konno Y, et al. Identification of KLF17 as a novel epithelial to mesenchymal transition inducer via direct activation of TWIST1 in endometrioid endometrial cancer. Carcinogenesis. 2014;35:760–768. doi: 10.1093/carcin/bgt369. [DOI] [PubMed] [Google Scholar]
- Dong Z, Yang L, Lai D. KLF5 strengthens drug resistance of ovarian cancer stem-like cells by regulating survivin expression. Cell Proliferation. 2013;46:425–435. doi: 10.1111/cpr.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SA, Funnell AP, Sue N, Nicholas H, Pearson RC, Crossley M. A network of Krüppel-like Factors (Klfs). Klf8 is repressed by Klf3 and activated by Klf1 in vivo. Journal of Biological Chemistry. 2008;283:26937–26947. doi: 10.1074/jbc.M804831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Mori D, Niwa H, Hasegawa Y, Yamanaka Y, Hitoshi S, Mimura J, Kawabe Y, Hosoya T, Morita M, et al. Krüppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3:555–567. doi: 10.1016/j.stem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. Journal of Biological Chemistry. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- Figueira PG, Abrão MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Annual New York Academy of Science. 2011;1221:10–17. doi: 10.1111/j.1749-6632.2011.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo DE, Sherk AB, Wittmann BM, Norris JD, Wang Q, Joseph JD, Toner AP, Brown M, McDonnell DP. Induction of Krüppel-like factor 5 expression by androgens results in increased CXCR4-dependent migration of prostate cancer cells in vitro. Molecular Endocrinology. 2009;23:1385–1396. doi: 10.1210/me.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell AP, Mak KS, Twine NA, Pelka GJ, Norton LJ, Radziewic T, Power M, Wilkins MR, Bell-Anderson KS, Fraser ST, et al. Generation of mice deficient in both KLF3/BKLF and KLF8 reveals a genetic interaction and a role for these factors in embryonic globin gene silencing. Molecular & Cell Biology. 2013;33:2976–2987. doi: 10.1128/MCB.00074-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg JW, Hall TR, Zhang L, Kim M, Byron VF, Tambouret R, Sathayanrayanan S, Foster R, Rueda BR, Growdon WB. Inhibition of gamma-secretase activity impedes uterine serous carcinoma growth in a human xenograft model. Gynecology & Oncology. 2014;133:607–615. doi: 10.1016/j.ygyno.2014.03.560. [DOI] [PubMed] [Google Scholar]
- Guo W, Li P, Zhao G, Fan H, Hu Y, Hou Y. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. American Journal of Reproductive Immunology. 2012;67:463–473. doi: 10.1111/j.1600-0897.2012.01114.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, et al. Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reproductive Biology. 2014;14:3–8. doi: 10.1016/j.repbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HJ, Gu XF, Xu WH, Yang DJ, Wang XM, Su Y. Krüppel-like factor 8 is a novel androgen receptor co-activator in human prostate cancer. Acta Pharmacologica Sinica. 2013;34:282–288. doi: 10.1038/aps.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard ME, Pabona JM, Clayberger C, Krensky AM, Simmen FA, Simmen RC. The reproductive phenotype of mice null for transcription factor Krüppel-like factor 13 suggests compensatory function of family member Krüppel-like factor 9 in the peri-implantation uterus. Biology of Reproduction. 2012;87:115. doi: 10.1095/biolreprod.112.102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard ME, Simmons CD, Simmen FA, Simmen RC. Krüppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology. 2014;155:1532–1546. doi: 10.1210/en.2013-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Ju B, Tian J, Liu F, Yu H, Xiao H, Liu X, Liu W, Yao Z, Hao Q. Ovarian cancer stem cell-specific gene expression profiling and targeted drug prescreening. Oncology Reports. 2014;31:1235–1248. doi: 10.3892/or.2014.2976. [DOI] [PubMed] [Google Scholar]
- Hubbard SA, Friel AM, Kumar B, Zhang L, Rueda BR, Gargett CE. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Research. 2009;69:8241–8248. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Takeda J, Horikawa Y. Krüppel-like factor-10 is directly regulated by carbohydrate response element-binding protein in rat primary hepatocytes. Biochemical Biophysical Research Communications. 2011;412:638–643. doi: 10.1016/j.bbrc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nature Cell Biology. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. Sp1- and Krüppel-like transcription factors. Genome Biology. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavandi L, Collier MA, Nguyen H, Syed V. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. Journal of Cell Biochemistry. 2012;113:3143–3152. doi: 10.1002/jcb.24191. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine Reviews. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korani M, Fallah S, Tehranian A, Nourbakhsh M, Samadikuchaksaraei A, Pour MS, Maleki J. The evaluation of the FOXO1, KLF9 and YT521 genes expression in human endometrial cancer. Clinical Laboratory. 2013;59:483–489. doi: 10.7754/clin.lab.2012.120626. [DOI] [PubMed] [Google Scholar]
- Knoedler JR, Denver RJ. Krüppel-like factors are effectors of nuclear receptor signaling. General and Comparative Endocrinology. 2014;203:49–59. doi: 10.1016/j.ygcen.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- Laub F, Dragomir C, Ramirez F. Mice without transcription factor KLF7 provide new insight into olfactory bulb development. Brain Research. 2006;1103:108–113. doi: 10.1016/j.brainres.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biology of Reproduction. 2008;80:79–85. doi: 10.1095/biolreprod.108.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Chen L, Georgiou EX, Sooranna SR, Khanjani S, Brosens JJ, Bennett PR, Johnson MR. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells. PLoS One. 2012;7:e50167. doi: 10.1371/journal.pone.0050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow CC, Wang BE, Ross J, Chan SM, Zha J, Carano RA, Frantz G, Shen MM, de Sauvage FJ, Gao WQ. Prostate-specific Klf6 inactivation impairs anterior prostate branching morphogenesis through increased activation of the Shh pathway. Journal of Biological Chemistry. 2009;284:21057–21065. doi: 10.1074/jbc.M109.001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, He H, Liu R, Wang SX, Pu DM. Isolation and identification of epithelial and stromal stem cells from eutopic endometrium of women with endometriosis. European Journal of Obstetrics, Gynecology and Reproductive Biology. 2014;178:89–94. doi: 10.1016/j.ejogrb.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Limame R, Op de Beeck K, Lardon F, De Wever O, Pauwels P. Krüppel-like factors in cancer progression: three fingers on the steering wheel. Oncotarget. 2014;5:29–48. doi: 10.18632/oncotarget.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gomez-Pinillos A, Loder C, Carrillo-de Santa Pau E, Qiao R, Unger PD, Kurek R, Oddoux C, Melamed J, Gallagher RE, et al. KLF6 loss of function in human prostate cancer progression is implicated in resistance to androgen deprivation. American Journal of Pathology. 2012;181:1007–1016. doi: 10.1016/j.ajpath.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Wang X, Urvalek AM, Li T, Xie H, Yu L, Zhao J. Transformation of human ovarian surface epithelial cells by Krüppel-like factor 8. Oncogene. 2012;33:10–18. doi: 10.1038/onc.2012.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero-Rodríguez D, Taniguchi-Ponciano K, Jimenez-Vega F, Romero-Morelos P, Mendoza-Rodríguez M, Mantilla A, Rodriguez-Esquivel M, Hernandez D, Hernandez A, Gomez-Gutierrez G, et al. Krüppel-like factor 5 as potential molecular marker in cervical cancer and the KLF family profile expression. Tumour Biology. 2014;35:11399–11407. doi: 10.1007/s13277-014-2380-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Kubo A, Liu H, Akita K, Laub F, Ramirez F, Keller G, Friedman SL. Developmental regulation of yolk sac hematopoiesis by Krüppel-like factor 6. Blood. 2006;107:1357–1365. doi: 10.1182/blood-2005-05-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Liang S, Tseng L. Expression of Delta-like protein 4 in the human endometrium. Endocrinology. 2008;149:15–19. doi: 10.1210/en.2007-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla G, Heath KE, Reeves HL, Li D, Giono LE, Kimmelman AC, Glucksman MJ, Narla J, Eng FJ, Chan AM, et al. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science. 2001;294:2563–2566. doi: 10.1126/science.1066326. [DOI] [PubMed] [Google Scholar]
- Natesampillai S, Kerkvliet J, Leung PC, Veldhuis JD. Regulation of Krüppel-like factor 4, 9, and 13 genes and the steroidogenic genes LDLR, StAR, and CYP11A in ovarian granulosa cells. American Journal of Physiology: Endocrinology & Metabolism. 2008;294:E385–91. doi: 10.1152/ajpendo.00480.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Human Reproduction Update. 2013;19:67–83. doi: 10.1093/humupd/dms043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem G, Bilir A, Uslu R, Inan SV, Demiray SB, Atmaca H, Ayla S, Sercan O, Uysal A. Expression profiling of stem cell signaling alters with spheroid formation in CD133high/CD44high prostate cancer stem cells. Oncology Letters. 2014;7:2103–2109. doi: 10.3892/ol.2014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Bulun SE, Maruyama T. Tissue-specific stem cells in the myometrium and tumor-initiating cells in leiomyoma. Biology of Reproduction. 2014;91:149. doi: 10.1095/biolreprod.114.123794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Qiang W, Serna VA, Yin P, Coon JS, Navarro A, Monsivais D, Kakinuma T, Dyson M, Druschitz S, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7:e36935. doi: 10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Yin P, Navarro A, Moravek MB, Coon JS, Druschitz SA, Serna VA, Qiang W, Brooks DC, Malpani SS, et al. Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proceedings of the National Academy of Science U S A. 2013;110:17053–17058. doi: 10.1073/pnas.1313650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabona JMP, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenco Z, Giudice LC, Simmen RC. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. Journal of Clinical Endocrinology & Metabolism. 2012;97:376–392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabona JM, Zeng Z, Simmen FA, Simmen RC. Functional differentiation of uterine stromal cells involves cross-regulation between bone morphogenetic protein 2 and Krüppel-like factor (KLF) female family members KLF9 and KLF13. Endocrinology. 2010;151:3396–3406. doi: 10.1210/en.2009-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabona JM, Zhang D, Ginsburg DS, Simmen FA, Simmen RC. Prolonged Pregnancy in Women is Associated with Attenuated Myometrial Expression of Progesterone Receptor Co-Regulator Krüppel-like Factor 9. Journal of Clinical Endocrinology & Metabolism. 2015;100:166–174. doi: 10.1210/jc.2014-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Grishin NV. A new family of predicted Krüppel-like factor genes and pseudogenes in placental mammals. PLoS One. 2013;8:e81109. doi: 10.1371/journal.pone.0081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Pollard JW. KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing. Proceedings National Academy of Science U S A. 2012;109:1334–1343. doi: 10.1073/pnas.1118515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertility & Sterility. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario GX, Hondo E, Jeong JW, Mutalif R, Ye X, Yee LX, Stewart CL. The LIF-mediated molecular signature regulating murine embryo implantation. Biology of Reproduction. 2014;91:66. doi: 10.1095/biolreprod.114.118513. [DOI] [PubMed] [Google Scholar]
- Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155:1489–1497. doi: 10.1210/en.2013-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Annals New York Academy of Science. 2008;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Sun J, Xu G, Zhang L, Yang Z, Xia S, Wang Y, Liu Y, Shi G. KLF9, a transcription factor induced in flutamide-caused cell apoptosis, inhibits AKT activation and suppresses tumor growth of prostate cancer cells. Prostate. 2014;74:946–958. doi: 10.1002/pros.22812. [DOI] [PubMed] [Google Scholar]
- Shen X, Hu Y, Jiang Y, Liu H, Zhu L, Jin X, Shan H, Zhen X, Sun L, Yan G, et al. Krüppel-like factor 12 negatively regulates human endometrial stromal cell decidualization. Biochemical Biophysical Research Communications. 2013;433:11–17. doi: 10.1016/j.bbrc.2013.02.078. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Takeuchi T, Mita S, Notsu T, Mizuguchi K, Kyo S. Krüppel-like factor 4 mediates anti-proliferative effects of progesterone with G0/G1 arrest in human endometrial epithelial cells. Journal of Endocrinological Investigation. 2010;33:745–750. doi: 10.1007/BF03346681. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Krüppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. Journal of Biological Chemistry. 2004;279:29286–29294. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- Simmen FA, Su Y, Xiao R, Zeng Z, Simmen RC. The Krüppel-like factor 9 (KLF9) network in HEC-1-A endometrial carcinoma cells suggests the carcinogenic potential of dys-regulated KLF9 expression. Reproductive Biology & Endocrinology. 2008;10(6):41. doi: 10.1186/1477-7827-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C, Pabona JM, Heard ME, Friedman TM, Spataro MT, Godley AL, Simmen FA, Burnett AF, Simmen RC. Krüppel-like factor 9 loss-of-expression in human endometrial carcinoma links altered expression of growth-regulatory genes and aberrant proliferative response to estrogen. Biology of Reproduction. 2011;85:378–385. doi: 10.1095/biolreprod.110.090654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Spiewak KA, Ekman GC, Kim J, Lydon JP, Bagchi MK, Bagchi IC, DeMayo FJ, Cooke PS. Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology. 2009;150:3871–3876. doi: 10.1210/en.2008-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CZ, Gavriilidis G, Asano H, Stamatoyannopoulos G. Functional study of transcription factor KLF11 by targeted gene inactivation. Blood Cells Molecular Disease. 2005;34:53–59. doi: 10.1016/j.bcmd.2004.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biology of Reproduction. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhang L, Xie H, Wan H, Magella B, Whitsett JA, Dey SK. Krüppel-like factor 5 (KLF5) is critical for conferring uterine receptivity to implantation. Proceedings National Academy of Science USA. 2012;109:1145–1150. doi: 10.1073/pnas.1118411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Tamaresis JS, Irwin JC, Goldfien GA, Rabban JT, Burney RO, Nezhat C, DePaolo LV, Giudice LC. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–4999. doi: 10.1210/en.2014-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault MP, Alrabaa R, McGeehan M, Katz JP. Krüppel-like factor 5 protects against murine colitis and activates JAK-STAT signaling in vivo. PLoS One. 2012;7:e38338. doi: 10.1371/journal.pone.0038338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault MP, Yang Y, Katz JP. Krüppel-like factors in cancer. Nature Reviews Cancer. 2013;13:701–713. doi: 10.1038/nrc3582. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC. Null mutation of Krüppel-like factor 9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biology of Reproduction. 2005;73:472–481. doi: 10.1095/biolreprod.105.041855. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RC. Krüppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor alpha signaling in Ishikawa endometrial adenocarcinoma cells. Molecular Endocrinology. 2007;21:2988–3001. doi: 10.1210/me.2007-0242. [DOI] [PubMed] [Google Scholar]
- Wani MA, Wert SE, Lingrel JB. Lung Krüppel-like factor, a zinc finger transcription factor, is essential for normal lung development. Journal of Biological Chemistry. 1999;274:21180–21185. doi: 10.1074/jbc.274.30.21180. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu Y, Huang Y, McAvoy S, Johnson WB, Cheung TH, Chung TK, Lo KW, Yim SF, Yu MM, et al. Transcriptional repression of WEE1 by Krüppel-like factor 2 is involved in DNA damage-induced apoptosis. Oncogene. 2005;24:3875–3885. doi: 10.1038/sj.onc.1208546. [DOI] [PubMed] [Google Scholar]
- Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE, Huang J, Li LC. Prognostic value and function of KLF4 in prostate cancer: RNAi and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Research. 2010;70:10182–10191. doi: 10.1158/0008-5472.CAN-10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Michel FJ, Wing A, Simmen FA, Simmen RC. Cell-type expression, immunolocalization, and deoxyribonucleic acid-binding activity of basic transcription element binding transcription factor, an Sp-related family member, in porcine endometrium of pregnancy. Biology of Reproduction. 1997;57:707–714. doi: 10.1095/biolreprod57.4.707. [DOI] [PubMed] [Google Scholar]
- Yang WT, Zheng PS. Promoter hypermethylation of KLF4 inactivates its tumor suppressor function in cervical carcinogenesis. PLoS One. 2014;9:e88827. doi: 10.1371/journal.pone.0088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Lin Z, Reierstad S, Wu J, Ishikawa H, Marsh EE, Innes J, Cheng Y, Pearson K, et al. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Research. 2010;70:1722–30. doi: 10.1158/0008-5472.CAN-09-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying M, Sang Y, Li Y, Guerrero-Cazares H, Quinones-Hinojosa A, Vescovi AL, Eberhart CG, Xia S, Laterra J. Krüppel-like family of transcription factor 9, a differentiation-associated transcription factor, suppresses Notch1 signaling and inhibits glioblastoma-initiating stem cells. Stem Cells. 2011;29:20–31. doi: 10.1002/stem.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying M, Tilghman J, Wei Y, Guerrero-Cazares H, Quinones-Hinojosa A, Ji H, Laterra J. KLF9 inhibits glioblastoma stemness through global transcription repression and integrin-α6 inhibition. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.588988. jbc.M114.588988. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon O, Roh J. Downregulation of KLF4 and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer. Oncology Letters. 2012;4:1033–1036. doi: 10.3892/ol.2012.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf I, Kharas MG, Chen J, Peralta RQ, Maruniak A, Sareen P, Yang VW, Kaestner KH, Fruman DA. KLF4 is a FOXO target gene that suppresses B cell proliferation. International Immunology. 2008;20:671–681. doi: 10.1093/intimm/dxn024. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Velarde MC, Simmen FA, Simmen RCM. Delayed parturition and altered myometrial progesterone receptor isoform A expression in mice null for Krüppel-like factor 9. Biology of Reproduction. 2008;78:1039–1037. doi: 10.1095/biolreprod.107.065821. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. Direct interaction of the Krüppel-like family (KLF) member, BTEB1 and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143:62–73. doi: 10.1210/endo.143.1.8590. [DOI] [PubMed] [Google Scholar]
- Zhang QH, Dou HT, Tang YJ, Su S, Liu PS. Lentivirus-mediated knockdown of Krüppel-like factor 9 inhibits the growth of ovarian cancer. Archives of Gynecology & Obstetrics. 2014;291:377–382. doi: 10.1007/s00404-014-3405-3. [DOI] [PubMed] [Google Scholar]
- Zhang XLD, Michel FJ, Blum JL, Simmen FA, Simmen RC. Selective interactions of Krüppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. Journal of Biological Chemistry. 2003;278:21474–21482. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lam O, Nguyen MT, Ng G, Pear WS, Ai W, Wang IJ, Kao WW, Liu CY. Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development. 2013;140:594–605. doi: 10.1242/dev.082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lei CQ, Hu YH, Xia T, Li M, Zhong B, Shu HB. Krüppel-like factor 6 is a co-activator of NF-κB that mediates p65-dependent transcription of selected downstream genes. Journal of Biological Chemistry. 2014;289:12876–12885. doi: 10.1074/jbc.M113.535831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Pritchard DM, Yang X, Bennett E, Liu G, Liu C, Ai W. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. American Journal of Physiology: Gastrointestinal & Liver Physiology. 2009;296:G490–498. doi: 10.1152/ajpgi.90393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Tabbaa ZM, Khan Z, Schoolmeester JK, El-Nashar S, Famuyide A, Keeney GL, Daftary GS. Epigenetic regulation of uterine biology by transcription factor KLF11 via posttranslational histone deacetylation of cytochrome p450 metabolic enzymes. Endocrinology. 2014;155:4507–4520. doi: 10.1210/en.2014-1139. [DOI] [PubMed] [Google Scholar]