Abstract
IMPORTANCE
Hearing sensitivity among adults has quality of life implications as individuals become older. There are limited data on hearing loss among aging HIV+ adults.
OBJECTIVES
1) To evaluate pure-tone hearing thresholds among HIV+ and HIV- adults with similar demographic characteristics. 2) To determine if HIV disease variables and antiretroviral therapy (ART) are associated with pure-tone threshold levels.
DESIGN, SETTING, AND PARTICIPANTS
262 men (117 HIV+) from the Baltimore/Washington, DC site of the Multicenter AIDS Cohort Study (MACS) and 134 women (105 HIV+) from the Washington, DC site of the Women's Interagency HIV Study (WIHS) participated. Pure-tone air-conduction thresholds were collected in a sound-treated room at octave frequencies from 250 through 8000 Hz, along with interoctave frequencies of 750, 3000, and 6000 Hz.
INTERVENTION(S)
None.
MAIN OUTCOME AND MEASURES
In each ear, a low-frequency pure-tone average (LPTA) was calculated using air-conduction thresholds at 250, 500, 1000, and 2000 Hz and a high-frequency PTA (HPTA) was calculated using air-conduction thresholds at 3000, 4000, 6000, and 8000 Hz. Linear mixed regression models tested the effect of HIV on hearing after adjusting for age, sex, race, and noise exposure history. Differential HIV effects for LPTA and HPTA and better/worse ear were also examined. CD4+ and CD8+ T-cell counts, log10 plasma HIV RNA concentrations, ever having had an AIDS-defining condition, and cumulative time on ART were included in the models for HIV+ participants only.
RESULTS
HPTA and LPTA were significantly higher (18% and 12%, respectively), for HIV+ participants compared to HIV- participants for the better ear. The direction of the effect was consistent across both the better and worse ear. There were no significant associations between HIV disease variables or treatment variables and LPTA or HPTA.
CONCLUSIONS AND RELEVANCE
HIV+ adults had significantly poorer lower and higher frequency hearing compared to HIV- adults. High frequency hearing loss is consistent with an accelerated aging (presbycusis); hearing loss in the low frequency range among middle-aged individuals is an unexpected finding. Since some vowels and consonants have predominantly low frequency acoustic energy, poorer hearing in lower frequencies may lead to increased communication difficulties in HIV+ individuals.
Keywords: Hearing loss, Human immunodeficiency virus (HIV), Antiretroviral therapy (ART), Multicenter AIDS Cohort Study (MACS), Women's Interagency HIV Study (WIHS)
The relationship between human immunodeficiency virus (HIV) infection and hearing loss in the era of highly active antiretroviral therapy (HAART) has not been extensively investigated. In one of the few studies to prospectively evaluate possible changes in hearing sensitivity in HIV+ individuals, Schouten et al1 evaluated pure-tone averages (PTAs) in adults who began receiving zidovudine (AZT) or didanosine (ddI), alone or in combination, from 1996-1999 with other ART not specified. Low-frequency PTAs (500, 1000, and 2000 Hz) and high-frequency PTAs (4000, 8000, and 12000 Hz) were calculated at baseline, 16 weeks, and 32 weeks. For the participants who had PTA data and returned at 32 weeks (n=19), there were no significant changes in either high or low frequency PTA, after accounting for age, noise exposure, CD4+ T-cell count, and viral load.
Recently, van der Westhuizen et al2 collected pure-tone threshold data among HIV+ and HIV- adults matched for age, gender, and race. HIV+ participants had a significantly higher prevalence of hearing loss (calculated using PTA of 500, 1000, and 2000 Hz >25 dB hearing level) compared to controls; furthermore, this was true across all of the individual frequencies measured (500, 1000, 2000, 3000, and 4000 Hz). Centers for Disease Control and Prevention (CDC) Classifications for HIV were also evaluated. CDC classifications are defined as Stage 1, 2, and 3, with CDC Stage 3 defined as the greatest HIV disease severity (< 200 CD4+ T-cell count/uL).3 There was a significantly higher prevalence of sensorineural hearing loss in those individuals with CDC Stage 3 disease status, who were the only patients in that study receiving HAART. In the disease status analysis, PTA was defined using the same frequencies as mentioned previously, but with a >15 dB HL cutpoint. This definition of hearing loss, however, is commonly used for children and one that is rarely used in adult populations.
There have been limited data obtained on the effects of HIV-related medications on hearing loss, and in the few published studies, it is difficult to attribute the increases in hearing loss specifically to HIV medications rather than age or cumulative noise exposure. In one of the earliest studies Bankaitis and Schountz4 reported drug-induced hearing loss in HIV+ individuals regardless of the stage of the disease. Some researchers have found ototoxic effects in HIV+ individuals treated with nucleoside analog reverse transcriptase inhibitors (NRTIs) such as AZT, combinations of stavudine (d4T) and lamivudine (3TC),5 and combinations of AZT and ddI6. Simdon et al5 suggested caution when interpreting the associations between NRTIs and hearing loss because of confounding variables such as age, previous hearing loss and noise exposure.
Therefore, the specific aims of this study were: 1) to evaluate the hearing sensitivity characteristics, using pure-tone threshold data, of immunologically and virologically controlled, due to effective use of HAART, HIV-seropositive (HIV+) and demographically similar HIV-seronegative (HIV-) men and women, after adjusting for age, sex, race, and noise exposure history, and 2) to determine if HIV disease status and ART are associated with hearing sensitivity. The primary hypothesis for this study was that being HIV+ was associated with greater loss of hearing sensitivity at both low and high frequencies compared to being HIV-.
Methods
The Institutional Review Boards for San Diego State University, Johns Hopkins Bloomberg School of Public Health, Georgetown University, and Whitman-Walker Health approved this study. All participants provided signed informed consent.
Participants in the Multicenter AIDS Cohort Study (MACS) and Women's Interagency HIV Study (WIHS)
The MACS is an ongoing, prospective study of the natural and treated history of HIV infection among men who have sex with men in the United States. Approximately 7,000 men were recruited beginning in 1984-1985 at four centers located in Baltimore, MD/Washington, DC, Chicago, IL, Los Angeles, CA, and Pittsburgh, PA. HIV+ and HIV- men were recruited from a combination of sources including gay-focused public media, personal referrals, promotional events, and through medical practices and other research studies that targeted gay men. Other details about the recruitment and study design have been described elsewhere.7,8 Participants return every 6 months for a detailed interview, a physical examination, and collection of blood for laboratory testing and storage. For the present study men were recruited only from the Baltimore/Washington, DC MACS site.
The WIHS is a multicenter prospective cohort study that was established in 1994 to study women with or at risk for HIV infection. A total of 3,766 HIV+ and at risk HIV- women were enrolled beginning in 1994-1995 at six centers located in New York City (Bronx and Brooklyn), NY, Chicago, IL, Los Angeles, CA, San Francisco, CA, and Washington, DC. The HIV+ and HIV- women were recruited from primary care and hospital-based clinics, research studies, community centers, women's support groups, HIV testing sites and referrals from enrolled participants. Participants return every 6 months for a detailed interview, a physical examination, and collection of blood for laboratory testing and storage. Further details of WIHS study methodology have been previously reported.9,10 For this study women were recruited from the Washington, DC site of the WIHS.
Procedures
The hearing research protocol was added to the existing MACS/WIHS protocol. A hearing-related questionnaire was interviewer-administered and assessed the participant's self-reported hearing loss due to various factors, including perinatal exposure to rubella or cytomegalovirus, factors present at birth other than genetic or infectious disease, measles or meningitis, otitis media, ear trauma, or Meniere's disease or otosclerosis. Questions regarding tinnitus and noise exposure at work or during leisure activities were also included. All questions were from the National Institute on Deafness and Other Communication Disorders (NIDCD) funded adult Hearing Supplement to the 2007 National Health Interview Survey (NHIS) (ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS /2007/English/qadult.pdf).
The hearing examination consisted of an otoscopic examination, tympanometry, and pure-tone air- and bone-conduction testing. Otoscopy and tympanometry were used to examine for possible outer and middle ear pathologies. Equivalent ear canal volume (Vec), peak acoustic admittance (Ytm), and tympanogram peak pressure (TPP) were determined from the tympanogram using a GSI Tympstar (Grason Stadler Inc.). Pure-tone thresholds, in dB HL, were obtained according to American Speech-Language-Hearing Association (ASHA) guidelines11 in a sound-treated booth (Industrial Acoustics Company, New York, New York) using a clinical audiometer (GSI 61; Grason Stadler Inc.) with supra-aural earphones (TDH-50P). Pure-tone air-conduction (AC) thresholds were completed in each ear at 250, 500, 750, 1000, 2000, 3000, 4000, 6000, and 8000 Hz. Bone-conduction (BC) thresholds were completed at 500, 1000, 2000, and 4000 Hz. Pure-tone averages (PTAs) were calculated as the mean of air-conduction thresholds at 250, 500, 1000, and 2000 Hz for the low-frequency PTA (LPTA), and at 3000, 4000, 6000, and 8000 Hz for the high-frequency PTA (HPTA). Left and right ear PTA measurements at lower frequencies and higher frequencies were differentiated as 1) LPTA or HPTA and 2) by ear with the better or worse PTA measurement. Better ear was defined as the ear with the lower PTA. When the ears were equal, the ear with a lower threshold at a subsequent frequency, not used in the calculation of the PTA, established the better ear. For the purpose of the present study, an air-bone gap (ABG) was defined as a difference between AC thresholds and BC thresholds that was ≥15 dB at any two of the four frequencies tested in either ear.
In both MACS and WIHS, ART use was assessed at the study visit, and, beginning in October 1998, ART adherence was also captured at each visit. The ART medications were classified as NRTI, protease inhibitors (PI), and non-NRTI (NNRTI). Cumulative time (years) of use of each class of ART was calculated based on the number of ART medications reported in each classification and weighted for self-reported adherence. Weights were calculated by multiplying the number of ART medications at each visit by the adherence level, and the weighted values then cumulated. The weights were 1, 0.975, 0.85, 0.375, 0 for adherence levels of 100%, 95-99%, 75-94%, <75%, 0%, respectively. ART use prior to October 1998 was considered 100% adherent. AIDS-defining illnesses including a history of pulmonary tuberculosis (TB) were self-reported according to the 1993 CDC definition of AIDS.12 Data on prevalent diabetes13,14 and ever use of hormone replacement therapy or thyroid medication were collected from the medical history questionnaire and/or laboratory results from the enrollment MACS or WIHS study visit through the study visit when the hearing test was performed (August 2008-October 2012).
In the MACS, plasma HIV RNA concentration were measured using the COBAS Ultrasensitive Amplicor HIV-1 monitor assay (Roche Molecular Systems, Branchburg, NJ), sensitive to 50 copies HIV RNA/mL or Taqman HIV-1 Test (Roche molecular Systems, Branchburg, NJ), sensitive to 20 copies HIV RNA/mL. In the WIHS, plasma HIV RNA was measured using COBAS AmpliPrep/COBAS TaqMan HIV-1 Test (Roche Molecular Systems, Branchburg, NJ), sensitive to 20 or 48 copies HIV RNA/mL. Values were log10 transformed for statistical analysis. CD4+ and CD8+ T-cell counts were measured for HIV+ men and women at each study visit using standardized flow cytometry and a complete blood count.15 Lab results (CD4+, CD8+, HIV RNA) collected within one year prior to the hearing testing date were used for this analysis.
Statistical Analysis
These cross-sectional data were analyzed using two multivariable linear mixed (containing both fixed and random effects) models constructed using PROC MIXED (SAS, Version 9.3, Cary, NC). The first mixed model was designed to examine the relationship of the primary predictor, HIV status (HIV- as the reference), with the PTA outcome defined as the HPTA and LPTA for each ear, a total of four continuous PTA outcomes for each participant. A random subject effect was included in each model to account for within-person correlation of the four repeated measurements (i.e., PTA outcomes). The second mixed model was similar to the first model but for HIV+ participants only. Covariables in the multivariable models included frequency (indicator variables LPTA/HPTA, with HPTA as the reference), ear (better/worse, with worse as the reference), sex (female as the reference), age (in decades), race (black/non-black, with non-black as the reference), and history of noise exposure (occupational/none and non-occupational/none, with none as the reference for both variables).
To examine effect modification by frequency and ear, we included the three-way interaction between HIV status, LPTA/HPTA and better/worse ear in the mixed model. Since we were primarily interested in the effect of HIV status, we chose to estimate separate HIV+ and HIV- effects for each combination of the four groups (HPTA/worse-ear, HPTA/better-ear, LPTA/worse-ear, LPTA/better-ear), a total of 8 effects for the 8 categories of the combination of three binary variables. In addition, similar three-way interactions were also included for sex × LPTA/HPTA × better/worse-ear (each of four regression coefficients compares a specific combination of the three variables involving males to the other four combinations involving females), race × LPTA/HPTA × better/worse-ear (each regression coefficient compares a specific combination involving blacks to the other four combinations involving non-blacks) and age × LPTA/HPTA × better/worse-ear (with age as a continuous variable, separate linear regression slopes for each of the four categories of LPTA/HPTA and better/worse ear were generated). Each analysis included an examination of residuals as a check on the required assumptions of normally distributed errors with constant variance. Standard residual plots indicated that the error distribution was skewed to the right, and a logarithmic transformation of PTA was employed to stabilize the variance. Estimates (regression coefficients) from the linear mixed model are presented on the transformed scale. The proportionate difference, expressed as a ratio, between HIV+ and HIV- in each of the 4 LTPA/HTPA × better/worse-ear categories was estimated by the exponentiated difference between coefficients (HIV+/HIV-). Separate preliminary analyses also revealed that the error variance was smaller for the LPTA outcome than for HPTA, and we therefore allowed the two error variances to be different in the mixed model.
For models restricted to the HIV+ participants, which did not include any three-way interactions, additional covariables included CD4+ and CD8+ T-cell counts, log10 plasma HIV RNA at the study visit closest to the date of the test, ever having had an AIDS-defining condition,12 and cumulative time on PI/NNRTI/NRTI, adjusted for adherence.
Results
Three hundred ninety-six adults completed pure-tone audiometry testing (90% enrollment of targeted sample); there were 262 men (mean age=57.1 years, SD=8.8 years) with 117 (44.7%) being HIV+ and 134 women (mean age=47.7 years, SD=8.3 years) with 105 (n=78.4%) being HIV+. The proportions of HIV+ in this sample are consistent with those of the entire MACS and WIHS, respectively. The demographic characteristics of the study participants, stratified by HIV status and sex, are presented in Table 1. HIV+ participants were younger and more likely to be female and of black race compared to the HIV- participants. Self-reported occupational noise exposure was similar between HIV+ and HIV- participants, but men had a higher proportion with occupational noise exposure. HIV- participants had a slightly higher proportion with non-occupational noise exposure, but men and women had similar rates for this exposure. Among HIV+ participants, men had longer total time on NRTIs and NNRTIs compared to women, but women had longer time on PIs. HIV+ men and women had similar CD4+ cell counts, but men had a higher proportion with full virologic suppression and higher CD8+ cell counts. Lastly, more HIV+ women had at least one AIDS-defining illness compared to men.
Table 1.
Characteristics of Participants, Stratified by HIV Status and Sex
| HIV+ | HIV− | ||||||
|---|---|---|---|---|---|---|---|
| Men (n=117) | Women (n=105) | All HIV+ (n=222) | Men (n=145) | Women (n=29) | All HIV− (n=174) | P Valuea | |
| Age, mean (SD), yrs. | 52.3 (7.9) | 46.3 (8.0) | 49.5 (8.5) | 57.8 (9.0) | 41.7 (10.5) | 55.1 (11.0) | <0.001 |
| Race, n (%) | <0.001 | ||||||
| Non-black | 56 (47.9) | 18 (17.1) | 74 (33.3) | 117 (80.7) | 9 (31.0) | 126 (72.4) | |
| Black | 61 (52.1) | 87 (82.9) | 148 (66.7) | 28 (19.3) | 20 (69.0) | 48 (27.6) | |
| Occupational noise exposure, n (%) | 31 (26.5) | 14 (13.3) | 45 (20.3) | 35 (24.1) | 3 (10.3) | 38 (21.8) | 0.70 |
| Non-occupational noise exposure, n (%) | 63 (53.8) | 55 (52.4) | 118 (53.2) | 91 (62.8) | 19 (65.5) | 110 (63.2) | 0.06 |
| Ever diabetes, n (%) | 16 (13.9) | 21 (20.0) | 37 (16.7) | 15 (10.3) | 7 (24.1) | 22 (12.6) | 0.26 |
| Ever HRT, n (%) | 28 (26.7) | 5 (17.2) | <0.001 | ||||
| Ever AIDS, n (%) | 19 (16.2) | 40 (38.1) | 59 (26.6) | ||||
| Total years spent on NRTI, median (IQR) | 20.5 (10.3, 29.7) | 15.3 (6.2, 23.5) | 17.4 (8.0, 26.4) | ||||
| Total years spent on NNRTI, median (IQR) | 4.4 (0.4, 8.1) | 1.4 (0, 2.9) | 2.1 (0, 6.4) | ||||
| Total years spent on PI, median (IQR) | 3.1 (0, 10.0) | 5.0 (0, 9.4) | 3.8 (0, 9.6) | ||||
| Current CD4+ cell count, mean (SD), cells/μl | 603 (287) | 549 (305) | 577 (296) | ||||
| Current CD8+ cell count, mean (SD), cells/μL | 940 (417) | 782 (356) | 865 (397) | ||||
| Log10 HIV RNA, (median IQR), copies/ml | 1.6 (1.6, 1.6)b | 1.9 (1.7, 3.1) | 1.7 (1.6, 2.5) | ||||
Abbreviations: HRT, Hormone replacement therapy; Ever AIDS, ever diagnosed with AIDS; NRTI, Nucleoside analog reverse transcriptase inhibitor; NNRTI, Non-NRTI; PI, Protease inhibitor; IQR, Interquartile range; SD, standard deviation.
Comparisons between all HIV+ and all HIV− participants.
1.6 denotes a plasma HIV RNA value of <50 copies/ml, or undetectable by the assay used.
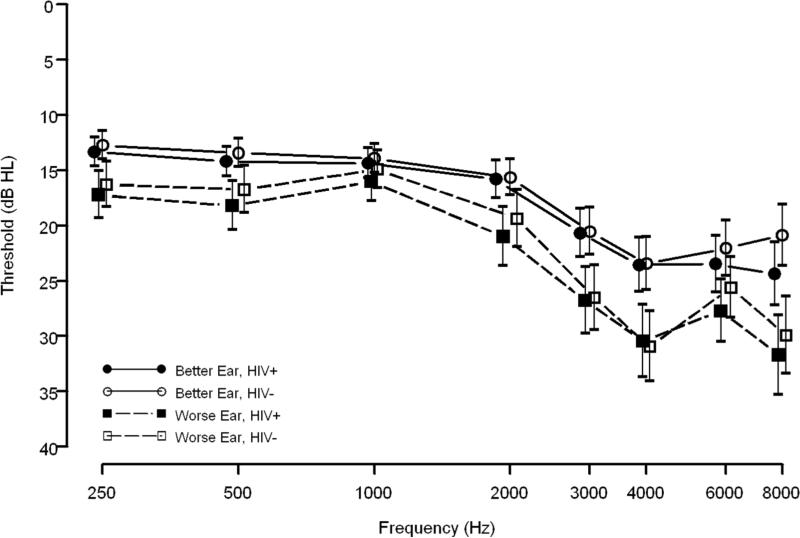
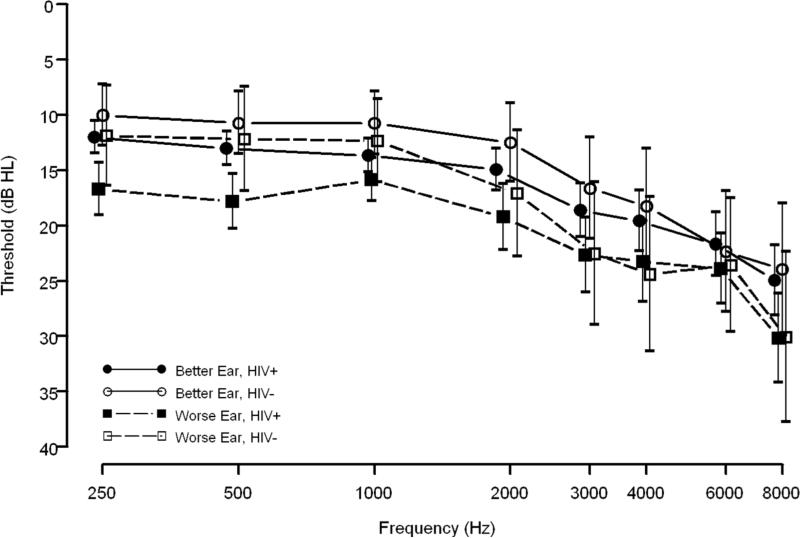
Overall, both HIV+ and HIV- men and women demonstrated a high-frequency sloping configuration for better and worse ear data (Figures 1 and 2). Additionally, mean threshold data, in dB HL, show a notched configuration for both HIV+ and HIV- men at 4000 Hz (Figure 1). Also in Figure 1, HIV+ and HIV- men had similar better ear threshold data although HIV+ men had slightly poorer worse ear threshold than HIV- men. Conversely, HIV+ women had poorer mean thresholds for better and worse ear data across most frequencies tested than HIV- women. The difference between HIV+ and HIV- women, however, was larger for the worse ear than for the better ear, at least up to 2000 Hz (Figure 2). Lastly, only 3% (n=12, 10 HIV+ and 2 HIV-) of participants had an air-bone gap, implying that the great majority of hearing loss was of the sensorineural type, not the conductive or mixed types of hearing loss.
Figure 1. Age-adjusted Means and Standard Errors of Pure-tone Threshold Data for Men (n=262).
Data are shown for 117 HIV+ (filled symbols) and 145 HIV- (open symbols) men. Solid lines and circles represent the better ear; dashed lines and squares represent the worse ear.
Figure 2. Age-adjusted Means and Standard Errors of Pure-tone Threshold Data for Women (n=134).
Data are shown for 105 HIV+ (filled symbols) and 29 HIV- (open symbols) women. Solid lines and circles represent the better ear; dashed lines and squares represent the worse ear.
The results of the multivariable analysis are shown in Table 2. The regression coefficients of the HIV+/HIV- × High/Low Frequency × Better/Worse ear combinations represent the difference between each combination and the reference group. The log PTAs for the other seven groups were all significantly higher than the reference (High frequency × better ear × HIV-). The regression coefficients of the combinations involving gender and race represent effects compared to females and non-blacks, respectively. For high frequencies only, being black was negatively associated with hearing loss compared to being non-black. In other words, black participants were less likely to have poorer high frequency hearing. Age was significantly associated with higher log PTAs in both ears. Having a reported history of noise exposure, both occupational and non-occupational, was not significantly associated with poorer hearing sensitivity.
Table 2.
Estimated Regression Coefficients,a Standard Errors, and Confidence Intervals from the Multivariable Linear Mixed Model
| Estimate (SE)a | 95% CI | P Value | |
|---|---|---|---|
| Intercept | 0.95 (0.21) | (0.54, 1.36) | <0.001 |
| High frequency × better ear × HIV−b | 0.00 (reference) | -- | -- |
| High frequency × better ear × HIV+ | 0.16 (0.07) | (0.02, 0.29) | 0.03 |
| High frequency × worse ear × HIV− | 0.67 (0.20) | (0.28, 1.05) | 0.001 |
| High frequency × worse ear × HIV+ | 0.78 (0.20) | (0.38, 1.17) | <0.001 |
| Low frequency × better ear × HIV− | 0.27 (0.16) | (−0.05, 0.58) | 0.10 |
| Low frequency × better ear × HIV+ | 0.38 (0.17) | (0.05, 0.71) | 0.02 |
| Low frequency × worse ear × HIV− | 0.75 (0.16) | (0.43, 1.07) | <0.001 |
| Low frequency × worse ear × HIV+ | 0.85(0.17) | (0.52, 1.18) | <0.001 |
| Male × high frequency × worse ear | 0.15 (0.08) | (−0.01, 0.31) | 0.06 |
| Male × high frequency × better ear | 0.07 (0.08) | (−0.09, 0.23) | 0.39 |
| Male × low frequency × worse ear | 0.12 (0.07) | (−0.01, 0.25) | 0.08 |
| Male × low frequency × better ear | 0.17 (0.07) | (0.04, 0.30) | 0.01 |
| Black × high frequency × worse ear | −0.23 (0.07) | (−0.37, −0.08) | 0.003 |
| Black × high frequency × better ear | −0.26 (0.07) | (−0.40, −0.11) | <0.001 |
| Black × low frequency × worse ear | −0.02 (0.06) | (−0.14, 0.10) | 0.74 |
| Black × low frequency × better ear | −0.05 (0.06) | (−0.17, 0.08) | 0.45 |
| Age/10c × high frequency × worse ear | 0.28 (0.04) | (0.21, 0.36) | <0.001 |
| Age/10 × high frequency × better ear | 0.37 (0.04) | (0.29, 0.44) | <0.001 |
| Age/10 × low frequency × worse ear | 0.17 (0.03) | (0.11, 0.23) | <0.001 |
| Age/10 × low frequency × better ear | 0.21 (0.03) | (0.15, 0.27) | <0.001 |
| Occupational noise exposure | 0.08 (0.06) | (−0.04, 0.20) | 0.18 |
| Non-occupational noise exposure | −0.03 (0.05) | (−0.13, 0.07) | 0.52 |
Abbreviations: SE, Standard error; CI, Confidence interval.
in natural log dB
see Methods section for definitions of interaction terms
age expressed in decades
The estimates from Table 2 were used to calculate the ratios of PTAs for HIV+ and HIV- participants (Table 3). After adjusting for age, sex, race, and noise exposure, for the better ear, HPTA was 18% higher for HIV+ participants than for HIV- participants, and LPTA was 12% higher; both differences were significant. For worse ear data, HIV+ participants again had higher LPTA and HPTA data compared to HIV- participants, and these ratios were marginally significant. The HIV-related variables studied (i.e., CD4+ and CD8+ T-cell counts, plasma HIV RNA, history of AIDS, and total years of receipt of any class of ART medications) were not significantly associated with hearing sensitivity (Table 4) after adjusting for age, sex, race, and noise exposure.
Table 3.
Estimated Ratios of PTAs for HIV+ People to Those for HIV− People, for High and Low Frequencies and Better and Worse Ear
| Condition | Ratio of PTA (HIV+ vs. HIV−) (95% CI)a | P Valueb |
|---|---|---|
| High frequency and worse ear | 1.12 (0.97, 1.29) | 0.12 |
| High frequency and better ear | 1.18 (1.02, 1.36) | 0.02 |
| Low frequency and worse ear | 1.11 (0.98, 1.25) | 0.09 |
| Low frequency and better ear | 1.12 (1.00, 1.26) | 0.05 |
Abbreviation: PTA, Pure-tone average.
Adjusted for age, sex, race, and occupational/non-occupational noise exposure.
P values for comparing the estimated ratios to the null value of one.
Table 4.
Estimated Regression Coefficients,a Standard Errors, and Confidence Intervals from the Multivariable Linear Mixed Model among the HIV+ Participants Only
| Estimate (SE)a | 95% CI | P Value | |
|---|---|---|---|
| Intercept | 1.72 (0.27) | (1.19, 2.26) | <0.001 |
| High frequency | 0.36 (0.02) | (0.31, 0.40) | <0.001 |
| Male | 0.21 (0.09) | (0.04, 0.38) | 0.02 |
| Black | −0.07 (0.08) | (−0.24, 0.09) | 0.38 |
| Better ear | −0.26 (0.02) | (−0.31, −0.21) | <0.001 |
| Age, each 10-yr increase | 0.20 (0.05) | (0.11, 0.29) | <0.001 |
| Occupational noise exposure | 0.02 (0.09) | (−0.15, 0.20) | 0.79 |
| Non-occupational noise exposure | −0.02 (0.07) | (−0.17, 0.12) | 0.74 |
| CD4+ cell count, 100-count increase | −0.00 (0.01) | (−0.03, 0.02) | 0.84 |
| CD8+ cell count, 100-count increase | −0.00 (0.01) | (−0.02, 0.02) | 0.66 |
| Total years spent on NRTI | −0.00 (0.00) | (−0.01, 0.01) | 0.50 |
| Total years spent on NNRTI | −0.01 (0.01) | (−0.03, 0.02) | 0.61 |
| Total years spent on PI | 0.01 (0.01) | (−0.01, 0.02) | 0.38 |
| Log10 (HIV RNA, copies/ml) | −0.01 (0.04) | (−0.08, 0.07) | 0.90 |
| Ever AIDS | 0.07 (0.09) | (−0.10, 0.25) | 0.42 |
Abbreviations: SE, Standard error; CI, Confidence interval; NRTI, Nucleoside analog reverse transcriptase inhibitor; NNRTI, Non-NRTI; PI, Protease inhibitor; Ever AIDS, ever diagnosed with AIDS.
in natural log dB
Discussion
In this study, independent of long-term exposure to anti-retroviral medications, current CD4+ cell count and HIV viral load, HIV+ participants had significantly higher (i.e., poorer hearing sensitivity) better ear HPTA (18%) and LPTA (12%) values than HIV- participants, and both results are important. The participants were middle-aged (mean age approximately 50 years), so an HIV effect on LPTA was not expected, given the speculation that long-term ART exposure or HIV itself contributes to premature aging.16
Reports of the prevalence of hearing loss in HIV+ adults have ranged from as low as 14%2 or 23%17 to as high as 49%18. Hearing loss, in some studies, has been defined as any threshold measured >25 dB HL,17,18 but this definition will overestimate the prevalence of hearing loss and is rarely used in clinical settings or by the World Health Organization (WHO)19. van der Westhuizen et al2 utilized an HIV- control group that was age-, gender-, race-, and work environment-matched to HIV+ adults and defined hearing loss as a PTA of 500, 1000, and 2000 Hz >25 dB HL, and found significantly poorer thresholds at 500, 1000, 2000, 3000, and 4000 Hz among HIV+ adults compared to controls. That study was limited, however, by the range of frequencies tested (i.e., only 500 through 4000 Hz) so an HIV effect above 4000 Hz was potentially missed by van der Westhuizen et al2. In the current study pure-tone thresholds were extensively evaluated over a range of frequencies that are commonly tested clinically. Additionally, the analyses included age, sex, race, and occupational and non-occupational noise exposure using a clear definition of LPTA and HPTA.
An effect on LPTA, although unexpected, has been reported previously. Bainbridge et al20 showed that adults with diabetes were significantly more likely to have hearing loss at both low (LPTA) and high frequencies (HPTA). In fact, the definitions of HPTA for the current study were the same as those used by Bainbridge et al20; the only difference was that for the LPTA, 250 Hz was included in the definition for the current study and not by Bainbridge et al20. It is possible that both HIV infection and diabetes, being systemic diseases, could affect the neural function of the cochlea.
Our findings that HIV disease- and treatment-related variables were not significantly associated with a higher LPTA or HPTA contrasts with previous research. Ongulo and Oburra21 reported that among HIV+ adults not receiving ART, those with lower CD4+ T-cell counts and advanced disease were more likely to have hearing loss. Van der Westhuizen et al2 also found greater hearing loss in those with more advanced HIV infection (stage 3), despite HAART. These discrepancies are probably due to differences in study populations, because most of the people in the present study were virologically suppressed on ART.
In other research, the prevalence of sensorineural hearing loss increased significantly with disease progression2,17,22 and pure-tone thresholds were significantly higher in individuals with higher CDC classifications23. Comparisons between these studies and the present study are difficult as hearing loss was not defined21-23 and in one study21 HIV+ participants were not receiving ART. Additionally, HIV-specific variables (e.g., CD4+ and CD8+ T-cell counts, and plasma HIV RNA) in the present study were clearly defined whereas in previous research, only CDC classification and CD4+ T-cell count17 were examined. Thus, the present study has evaluated the association between HIV disease characteristics, HIV treatment, and hearing threshold levels more thoroughly than previous studies.
Total years taking any ART were not associated with higher PTAs in our study, which is consistent with other reports. Schouten et al1 reported no effect of initiating AZT or ddl on hearing sensitivity over a few months. The LPTA and HPTA definitions between the current study and Schouten et al1 are slightly different. Marra et al24 found a significant association between ART and hearing loss among older adults, after adjusting for confounding variables. Hearing loss in that study, however, was defined as either a unilateral or bilateral threshold >25 dB HL at 4000 Hz only, an approach rarely used to define hearing loss clinically. Furthermore, Marra et al24 did not present any characteristics of the HIV+ participants studied. The lack of a significant association between ART and hearing loss in the present study does not eliminate the possibility that ART exposure may be a risk factor for hearing loss. NRTIs have been associated with possible mitochondrial mutation both in perinatally HIV-infected children25 and HIV+ adults26 including nonsyndromic sensorineural hearing loss27.
The current study has expanded our knowledge of the relationship between HIV infection and hearing loss because aspects of HIV, disease-specific and treatment-specific, and definitions of hearing loss had not been examined previously. It is well known that higher pure-tone thresholds are the best predictor of poorer word recognition abilities28,29, it is not known, however, how HIV and poorer hearing, specifically higher LPTA, affect communication or word recognition. Most of acoustic energy for English vowels, specifically the first and second formants, occurs below 2000 Hz and these formants are the most important for vowel identification.30 Additional, stop consonants (/p, b/, /t, d/, /k, g/), for example, have lower frequency energy and formant transitions31 although other consonants, such as fricatives (/s, z/, /f, v/) have higher frequency energy32. Hence, elevated low frequency hearing threshold levels can affect the perception of vowels and certain consonants.
Conclusions
This is the first study to demonstrate that HIV+ individuals have poorer hearing across the frequency range after controlling for many other factors known to affect hearing. While the early literature on possible hearing loss associations with diabetes33,34 tended to focus on specific frequency ranges (namely, higher frequencies), additional data from the follow-up studies have demonstrated hearing loss in both the low/middle and high frequency range.35 The association reported by Bainbridge et al 20between diabetes and higher audiometric thresholds across the frequency range based on the 1999-2004 U.S. National Health and Nutrition Examination Survey (NHANES) was also investigated by Agrawal et al36 who supported the association of diabetes, hypertension, and smoking on hearing loss at both high and low PTA frequency ranges. While we do not understand the mechanism of hearing loss found in our study, our results suggest that HIV+ individuals may have physiologic changes that mimic other chronic conditions that affect hearing levels.
Acknowledgments
Funding/Support: This research was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH) via interagency agreement with the National Institute of Allergy and Infectious Diseases (NIAID), NIH for Cooperative Agreements U01 AI-035042-18 (MACS) and U01 AI-034994-17 (WIHS). Support of the Baltimore-Washington, DC MACS site was provided by the NIAID, with additional supplemental funding from the National Cancer Institute (U01-AI-35042) and ICTR (UL1-RR025005). Support of the Metropolitan Washington, DC WIHS site was provided by the NIAID (U01-AI-34994) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH (U01-HD-32632).
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest Disclosures: The authors have no conflicts of interest to declare.
Authors’ contributions: Torre, Springer, Cox, and Plankey had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Torre, Hoffman, Plankey.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Torre.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Cox, Hoffman, Springer.
Obtaining funding: Margolick, Young, Plankey.
Administrative, technical, or material support: Margolick, Young, Plankey.
Study supervision: Torre, Plankey.
Additional Contributions: We thank Dr. Chuan-Ming Li for providing ancillary statistical analyses on a portion of the dataset and Dr. Ying Li, Georgetown University Medical Center, for technical assistance in the preparation of this manuscript.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of their respective academic or government agencies.
REFERENCES
- 1.Schouten JT, Lockhart DW, Rees TS, Collier AC, Marra CM. A prospective study of hearing changes after beginning zidovudine or didanosine in HIV-1 treatment-naïve people. BMC Infect Dis. 2006;6:28. doi: 10.1186/1471-2334-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Westhuizen Y, Swanepoel de W, Heinze B, Hofmeyr LM. Auditory and otological manifestations in adults with HIV/AIDS. Int J Audiol. 2013;52(1):37–43. doi: 10.3109/14992027.2012.721935. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention Revised surveillance case definition for HIV infection--United States, 2014. MMWR Recomm Rep. 2014;63(RR-03):1–10. [PubMed] [Google Scholar]
- 4.Bankaitis A, Schountz T. HIV-related ototoxicity. Semi Hear. 1998;19(2):155–163. [Google Scholar]
- 5.Simdon J, Watters D, Bartlett S, Connick E. Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: a report of 3 possible cases and review of the literature. Clin Infect Dis. 2001;32(11):1623–1627. doi: 10.1086/320522. [DOI] [PubMed] [Google Scholar]
- 6.Christensen LA, Morehouse CR, Powell TW, Alchediak T, Silio M. Antiviral therapy in a child with pediatric human immunodeficiency virus (HIV): case study of audiologic findings. J Am Acad Audiol. 1998;9(4):292–298. [PubMed] [Google Scholar]
- 7.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142(3):323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 8.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 9.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 10.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Speech-Language-Hearing Association [August 16, 2013];Guidelines for manual pure-tone threshold audiometry [Guidelines] 2005 www.asha.org/policy.
- 12.Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 13.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 14.Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61(3):334–340. doi: 10.1097/QAI.0b013e31826bfc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hultin LE, Menendez FA, Hultin PM, et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom. 2007;72(4):249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoza K, Ross E. Auditory function in a group of adults infected with HIV/AIDS in Gauteng, South Africa. S Afr J Commun Disord. 2002;49:17–27. [PubMed] [Google Scholar]
- 18.Sooy CD, Gerberding JL, Kaplan MJ. The risk for otolaryngologists who treat patients with AIDS and AIDS virus infection: report of an in-process study. Laryngoscope. 1987;97(4):430–434. doi: 10.1288/00005537-198704000-00005. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO), Prevention of Blindness and Deafness (PBD) Programme [May 1, 2014];Prevention of Deafness and Hearing Impairment. Grades of hearing impairment. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/.
- 20.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med. 2008;149(1):1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ongulo B, Oburra H. Hearing Disorders in HIV Positive Adult Patients East Cen Afr J Surg. 2010;15(1):96–101. [PubMed] [Google Scholar]
- 22.Teggi R, Ceserani N, Luce FL, Lazzarin A, Bussi M. Otoneurological findings in human immunodeficiency virus positive patients. J Laryngol Otol. 2008;122(12):1289–1294. doi: 10.1017/S0022215107001624. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekhar SS, Connelly PE, Brahmbhatt SS, Shah CS, Kloser PC, Baredes S. Otologic and audiologic evaluation of human immunodeficiency virus-infected patients. Am J Otolaryngol. 2000;21(1):1–9. doi: 10.1016/s0196-0709(00)80117-9. [DOI] [PubMed] [Google Scholar]
- 24.Marra CM, Wechkin HA, Longstreth WT, Jr., Rees TS, Syapin CL, Gates GA. Hearing loss and antiretroviral therapy in patients infected with HIV-1. Arch Neurol. 1997;54(4):407–410. doi: 10.1001/archneur.1997.00550160049015. [DOI] [PubMed] [Google Scholar]
- 25.Crain MJ, Chernoff MC, Oleske JM, et al. Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J Infect Dis. 2010;202(2):291–301. doi: 10.1086/653497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherry CL, Nolan D, James IR, et al. Tissue-specific associations between mitochondrial DNA levels and current treatment status in HIV-infected individuals. J Acquir Immune Defic Syndr. 2006;42(4):435–440. doi: 10.1097/01.qai.0000224974.67962.ce. [DOI] [PubMed] [Google Scholar]
- 27.Fischel-Ghodsian N. Mitochondrial deafness mutations reviewed. Hum Mutat. 1999;13(4):261–270. doi: 10.1002/(SICI)1098-1004(1999)13:4<261::AID-HUMU1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Jerger J, Jerger S, Pirozzolo F. Correlational analysis of speech audiometric scores, hearing loss, age, and cognitive abilities in the elderly. Ear Hear. 1991;12(2):103–109. doi: 10.1097/00003446-199104000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Garstecki DC, Erler SF. Hearing loss, control, and demographic factors influencing hearing aid use among older adults. J Speech Lang Hear Res. 1998;41(3):527–537. doi: 10.1044/jslhr.4103.527. [DOI] [PubMed] [Google Scholar]
- 30.Hillenbrand J, Getty LA, Clark MJ, Wheeler K. Acoustic characteristics of American English vowels. J Acoust Soc Am. 1995;97(5 Pt 1):3099–3111. doi: 10.1121/1.411872. [DOI] [PubMed] [Google Scholar]
- 31.Kewley-Port D. Measurement of formant transitions in naturally produced stop consonant-vowel syllables. J Acoust Soc Am. 1982;72(2):379–389. doi: 10.1121/1.388081. [DOI] [PubMed] [Google Scholar]
- 32.Jongman A, Wayland R, Wong S. Acoustic characteristics of English fricatives. J Acoust Soc Am. 2000;108(3 Pt 1):1252–1263. doi: 10.1121/1.1288413. [DOI] [PubMed] [Google Scholar]
- 33.Axelsson A, Sigroth K, Vertes D. Hearing in diabetics. Acta Otolaryngol Suppl. 1978;356:1–23. [PubMed] [Google Scholar]
- 34.Vaughan N, James K, McDermott D, Griest S, Fausti S. A 5-year prospective study of diabetes and hearing loss in a veteran population. Otol Neurotol. 2006;27(1):37–43. doi: 10.1097/01.mao.0000194812.69556.74. [DOI] [PubMed] [Google Scholar]
- 35.Austin DF, Konrad-Martin D, Griest S, McMillan GP, McDermott D, Fausti S. Diabetes-related changes in hearing. Laryngoscope. 2009;119(9):1788–1796. doi: 10.1002/lary.20570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med. 2008;168(14):1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]