Abstract
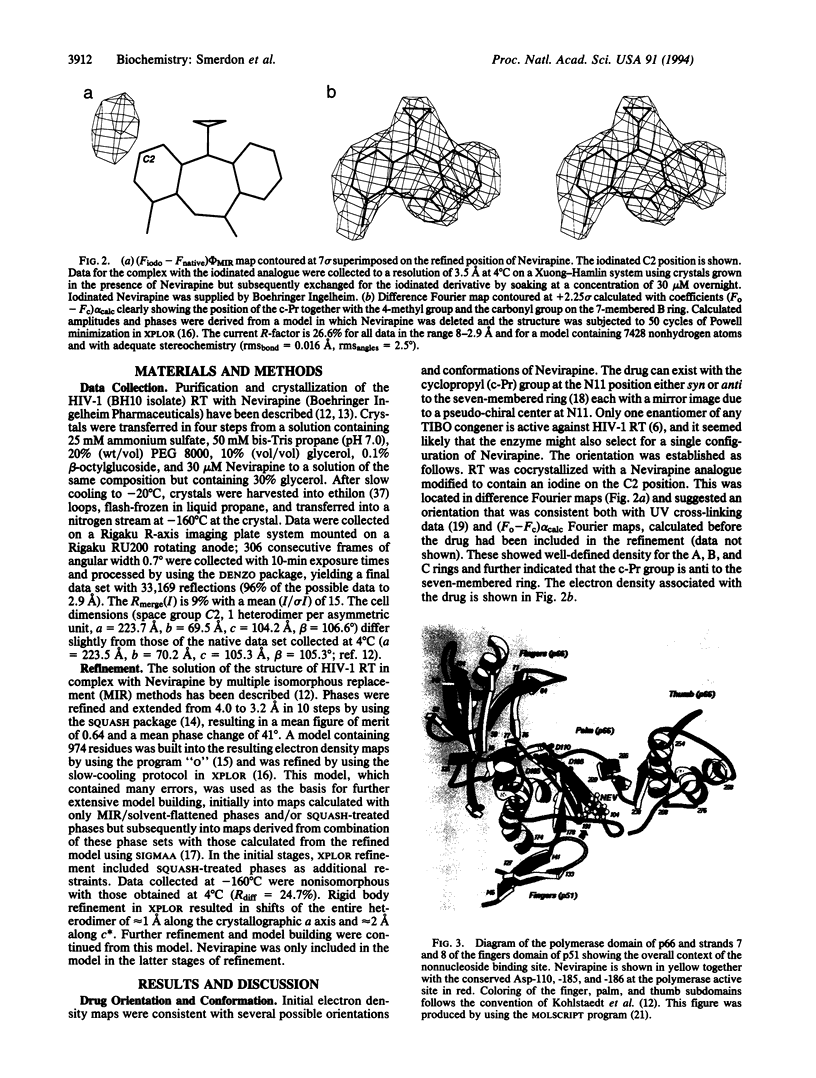
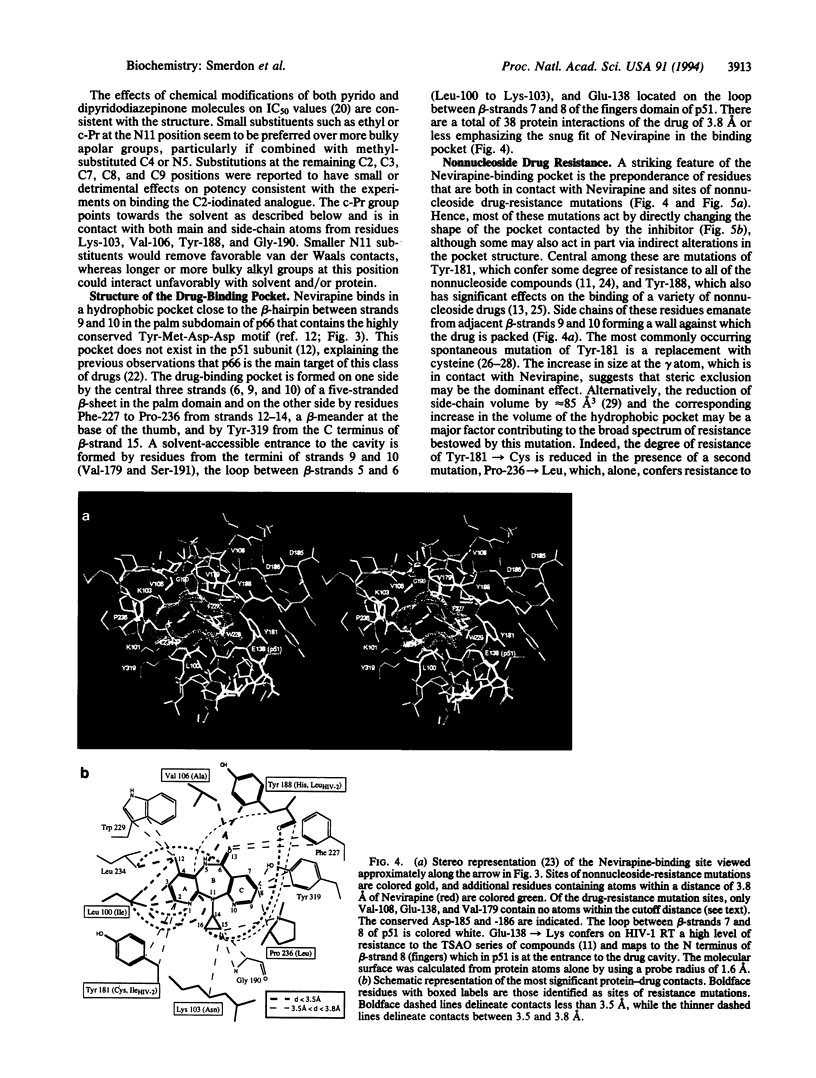
The dipyridodiazepinone Nevirapine is a potent and highly specific inhibitor of the reverse transcriptase (RT) from human immunodeficiency virus type 1 (HIV-1). It is a member of an important class of nonnucleoside drugs that appear to share part or all of the same binding site on the enzyme but are susceptible to a variety of spontaneous drug-resistance mutations. The co-crystal-structure of HIV-1 RT and Nevirapine has been solved previously at 3.5-A resolution and now is partially refined against data extending to 2.9-A spacing. The drug is bound in a hydrophobic pocket and in contact with some 38 protein atoms from the p66 palm and thumb subdomains. Most, but not all, nonnucleoside drug-resistance mutations map to residues in close contact with Nevirapine. The major effects of these mutations are to introduce steric clashes with the drug molecule or to remove favorable protein-drug contacts. Additionally, four residues (Phe-227, Trp-229, Leu-234, and Tyr-319) in contact with Nevirapine have not been selected as sites of drug-resistance mutations, implying that there may be limitations on the number and types of resistance mutations that yield viable virus. Strategies of inhibitor design that target interactions with these conserved residues may yield drugs that are less vulnerable to escape mutations.
Full text
PDF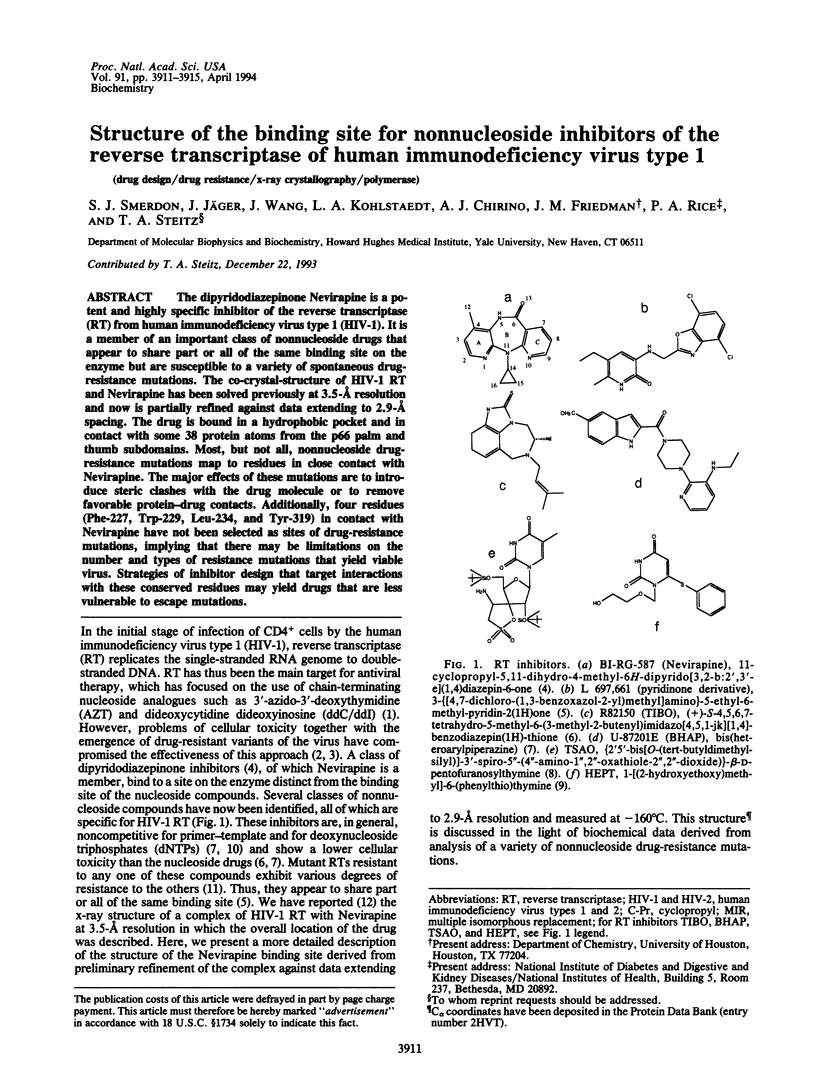


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacolla A., Shih C. K., Rose J. M., Piras G., Warren T. C., Grygon C. A., Ingraham R. H., Cousins R. C., Greenwood D. J., Richman D. Amino acid substitutions in HIV-1 reverse transcriptase with corresponding residues from HIV-2. Effect on kinetic constants and inhibition by non-nucleoside analogs. J Biol Chem. 1993 Aug 5;268(22):16571–16577. [PubMed] [Google Scholar]
- Balzarini J., Karlsson A., Pérez-Pérez M. J., Vrang L., Walbers J., Zhang H., Oberg B., Vandamme A. M., Camarasa M. J., De Clercq E. HIV-1-specific reverse transcriptase inhibitors show differential activity against HIV-1 mutant strains containing different amino acid substitutions in the reverse transcriptase. Virology. 1993 Jan;192(1):246–253. doi: 10.1006/viro.1993.1027. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Pérez-Pérez M. J., San-Félix A., Schols D., Perno C. F., Vandamme A. M., Camarasa M. J., De Clercq E. 2',5'-Bis-O-(tert-butyldimethylsilyl)-3'-spiro-5''-(4''-amino-1'',2''- oxathiole-2'',2'-dioxide)pyrimidine (TSAO) nucleoside analogues: highlyselective inhibitors of human immunodeficiency virus type 1 that are targeted at the viral reverse transcriptase. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4392–4396. doi: 10.1073/pnas.89.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. L., Currens M. J., McMahon J. B., Boyd M. R., Hughes S. H. Analysis of nonnucleoside drug-resistant variants of human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1993 Apr;67(4):2412–2420. doi: 10.1128/jvi.67.4.2412-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A. T., Krukowski A., Erickson J. W. Slow-cooling protocols for crystallographic refinement by simulated annealing. Acta Crystallogr A. 1990 Jul 1;46(Pt 7):585–593. doi: 10.1107/s0108767390002355. [DOI] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Cohen K. A., Hopkins J., Ingraham R. H., Pargellis C., Wu J. C., Palladino D. E., Kinkade P., Warren T. C., Rogers S., Adams J. Characterization of the binding site for nevirapine (BI-RG-587), a nonnucleoside inhibitor of human immunodeficiency virus type-1 reverse transcriptase. J Biol Chem. 1991 Aug 5;266(22):14670–14674. [PubMed] [Google Scholar]
- Debyser Z., De Vreese K., Knops-Gerrits P. P., Baekelandt V., Bhikhabhai R., Strandberg B., Pauwels R., Anné J., Desmyter J., De Clercq E. Kinetics of different human immunodeficiency virus type 1 reverse transcriptases resistant to human immunodeficiency virus type 1-specific reverse transcriptase inhibitors. Mol Pharmacol. 1993 Apr;43(4):521–526. [PubMed] [Google Scholar]
- Dueweke T. J., Pushkarskaya T., Poppe S. M., Swaney S. M., Zhao J. Q., Chen I. S., Stevenson M., Tarpley W. G. A mutation in reverse transcriptase of bis(heteroaryl)piperazine-resistant human immunodeficiency virus type 1 that confers increased sensitivity to other nonnucleoside inhibitors. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4713–4717. doi: 10.1073/pnas.90.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. E., Nunberg J. H., O'Brien J. A., Quintero J. C., Schleif W. A., Freund K. F., Gaul S. L., Saari W. S., Wai J. S., Hoffman J. M. Pyridinone derivatives: specific human immunodeficiency virus type 1 reverse transcriptase inhibitors with antiviral activity. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6863–6867. doi: 10.1073/pnas.88.15.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave K. D., Proudfoot J. R., Grozinger K. G., Cullen E., Kapadia S. R., Patel U. R., Fuchs V. U., Mauldin S. C., Vitous J., Behnke M. L. Novel non-nucleoside inhibitors of HIV-1 reverse transcriptase. 1. Tricyclic pyridobenzo- and dipyridodiazepinones. J Med Chem. 1991 Jul;34(7):2231–2241. doi: 10.1021/jm00111a045. [DOI] [PubMed] [Google Scholar]
- Jacobo-Molina A., Ding J., Nanni R. G., Clark A. D., Jr, Lu X., Tantillo C., Williams R. L., Kamer G., Ferris A. L., Clark P. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Steitz T. A. Reverse transcriptase of human immunodeficiency virus can use either human tRNA(3Lys) or Escherichia coli tRNA(2Gln) as a primer in an in vitro primer-utilization assay. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9652–9656. doi: 10.1073/pnas.89.20.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- Kopp E. B., Miglietta J. J., Shrutkowski A. G., Shih C. K., Grob P. M., Skoog M. T. Steady state kinetics and inhibition of HIV-1 reverse transcriptase by a non-nucleoside dipyridodiazepinone, BI-RG-587, using a heteropolymeric template. Nucleic Acids Res. 1991 Jun 11;19(11):3035–3039. doi: 10.1093/nar/19.11.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A. 3'-Azido-3'-deoxythymidine resistance suppressed by a mutation conferring human immunodeficiency virus type 1 resistance to nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 1992 Dec;36(12):2664–2669. doi: 10.1128/aac.36.12.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Purifoy D. J. Infectious potential of human immunodeficiency virus type 1 reverse transcriptase mutants with altered inhibitor sensitivity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4803–4807. doi: 10.1073/pnas.86.13.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors J. W., Dutschman G. E., Im G. J., Tramontano E., Winkler S. R., Cheng Y. C. In vitro selection and molecular characterization of human immunodeficiency virus-1 resistant to non-nucleoside inhibitors of reverse transcriptase. Mol Pharmacol. 1992 Mar;41(3):446–451. [PubMed] [Google Scholar]
- Merluzzi V. J., Hargrave K. D., Labadia M., Grozinger K., Skoog M., Wu J. C., Shih C. K., Eckner K., Hattox S., Adams J. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990 Dec 7;250(4986):1411–1413. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Yarchoan R., Broder S. Molecular targets for AIDS therapy. Science. 1990 Sep 28;249(4976):1533–1544. doi: 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- Miyasaka T., Tanaka H., Baba M., Hayakawa H., Walker R. T., Balzarini J., De Clercq E. A novel lead for specific anti-HIV-1 agents: 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine. J Med Chem. 1989 Dec;32(12):2507–2509. doi: 10.1021/jm00132a002. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Schleif W. A., Boots E. J., O'Brien J. A., Quintero J. C., Hoffman J. M., Emini E. A., Goldman M. E. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J Virol. 1991 Sep;65(9):4887–4892. doi: 10.1128/jvi.65.9.4887-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Andries K., Desmyter J., Schols D., Kukla M. J., Breslin H. J., Raeymaeckers A., Van Gelder J., Woestenborghs R., Heykants J. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990 Feb 1;343(6257):470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- Richman D., Shih C. K., Lowy I., Rose J., Prodanovich P., Goff S., Griffin J. Human immunodeficiency virus type 1 mutants resistant to nonnucleoside inhibitors of reverse transcriptase arise in tissue culture. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11241–11245. doi: 10.1073/pnas.88.24.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D. L., Busso M., Tan C. K., Reusser F., Palmer J. R., Poppe S. M., Aristoff P. A., Downey K. M., So A. G., Resnick L. Nonnucleoside reverse transcriptase inhibitors that potently and specifically block human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8806–8810. doi: 10.1073/pnas.88.19.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana V. V., Emini E. A., Gotlib L., Graham D. J., Lineberger D. W., Long W. J., Schlabach A. J., Wolfgang J. A., Condra J. H. Functional analysis of HIV-1 reverse transcriptase amino acids involved in resistance to multiple nonnucleoside inhibitors. J Biol Chem. 1992 Sep 5;267(25):17526–17530. [PubMed] [Google Scholar]
- Schäfer W., Friebe W. G., Leinert H., Mertens A., Poll T., von der Saal W., Zilch H., Nuber B., Ziegler M. L. Non-nucleoside inhibitors of HIV-1 reverse transcriptase: molecular modeling and X-ray structure investigations. J Med Chem. 1993 Mar 19;36(6):726–732. doi: 10.1021/jm00058a009. [DOI] [PubMed] [Google Scholar]
- Shih C. K., Rose J. M., Hansen G. L., Wu J. C., Bacolla A., Griffin J. A. Chimeric human immunodeficiency virus type 1/type 2 reverse transcriptases display reversed sensitivity to nonnucleoside analog inhibitors. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9878–9882. doi: 10.1073/pnas.88.21.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Martin J. L., Tudor-Williams G., Bach M. C., Vavro C. L., King D. M., Kellam P., Kemp S. D., Larder B. A. Resistance to ddI and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science. 1991 Sep 27;253(5027):1557–1559. doi: 10.1126/science.1716788. [DOI] [PubMed] [Google Scholar]
- Wu J. C., Warren T. C., Adams J., Proudfoot J., Skiles J., Raghavan P., Perry C., Potocki I., Farina P. R., Grob P. M. A novel dipyridodiazepinone inhibitor of HIV-1 reverse transcriptase acts through a nonsubstrate binding site. Biochemistry. 1991 Feb 26;30(8):2022–2026. doi: 10.1021/bi00222a003. [DOI] [PubMed] [Google Scholar]
- Zhang K. Y. SQUASH - combining constraints for macromolecular phase refinement and extension. Acta Crystallogr D Biol Crystallogr. 1993 Jan 1;49(Pt 1):213–222. doi: 10.1107/S0907444992007911. [DOI] [PubMed] [Google Scholar]





