Abstract
Study Design Case report.
Objective Multifocal epithelioid hemangioendothelioma (EHE) of the spine is a rare disorder. We describe a novel, multimodal treatment of a painful osteolytic lumbar lesion secondary to EHE. The minimally invasive treatment results in an excellent patient outcome with decreased morbidity compared to traditional techniques.
Methods A previously healthy young adult presented with a painful osteolytic lesion at the L2 vertebrae. Imaging revealed multifocal spinal lesions consistent with a history of EHE. Core needle biopsy confirmed the diagnosis. Preoperative cryoablation of L2 was followed by a staged surgery, which included a partial L2 corpectomy, tumor resection, bone grafting, and vertebral reconstruction using a minimally invasive technique. This treatment was followed by prolonged therapy with interferon and bisphosphonate.
Results At 3.5 years' follow-up, the patient has maintained his vertebral body height, has not required a fusion, and has had no recurrence of disease.
Conclusion Multimodal treatment consisting of tumor cryoablation, partial corpectomy, allograft reconstruction of the vertebrae, and adjuvant interferon and bisphosphonate can result in good outcomes for well-contained EHE tumors of the spine.
Keywords: multifocal spinal epithelioid hemangioendothelioma, cryoablation, MIS, intravertebral polyethylene mesh bag
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor with behavior intermediate between hemangioma and angiosarcoma.1 Primary bone EHE accounts for less than 1% of malignant bone tumors, and spinal involvement is especially rare.2
Because spinal EHE is a rare condition, described treatments vary and range from observation to wide resection with adjuvant chemotherapy or radiotherapy.1 2 We report a unique case of multifocal EHE of the spine resulting in a painful osteolytic lesion at L2, which was treated with cryoablation, minimally invasive partial corpectomy with reconstruction of L2, and adjuvant bisphosphonate and interferon therapy.
Case Report
An 18-year-old man who had undergone previous treatment for EHE of the pelvis presented with severe, worsening midlumbar pain of several months' duration secondary to a painful osteolytic lesion at L2. Magnetic resonance imaging (MRI) of the entire spine demonstrated multiple lesions consistent with multifocal EHE at L2, T9, and C7. Computed tomography (CT) showed a large lytic lesion at L2, suspicious for anterior collapse (Fig. 1). A biopsy confirmed the diagnosis of multifocal EHE (Fig. 2). The T9 and C7 lesions did not demonstrate any destructive lytic features. The patient also had bilateral pars defects with grade I L5/S1 spondylolisthesis.
Fig. 1.
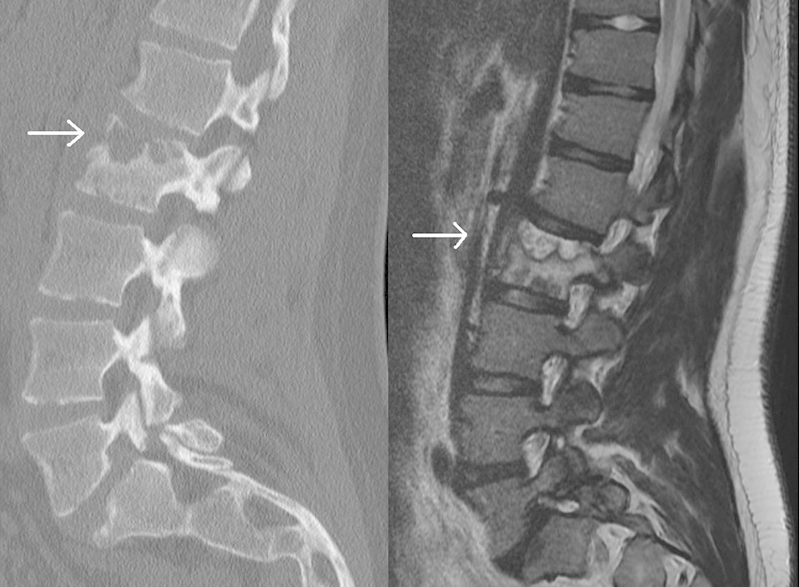
Sagittal computed tomography (left) and sagittal T2-weighted magnetic resonance imaging (right) demonstrate a lesion causing lysis of the left outer cortex in the superior end plate of L2.
Fig. 2.
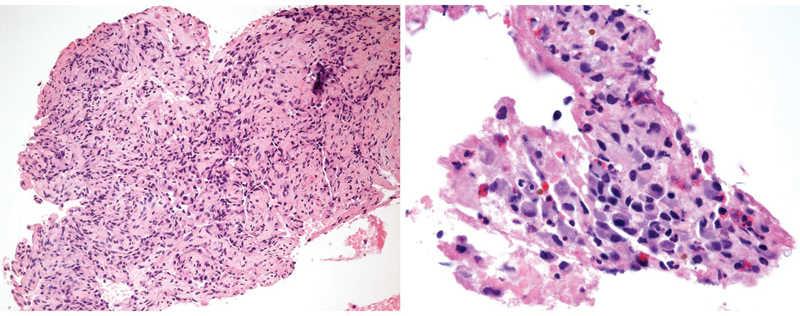
Low- (left) and high-power magnification (right) of hematoxylin and eosin–stained slides demonstrating a proliferation of benign epithelioid endothelial cells consistent with epithelioid hemangioma of bone.
The patient was initially treated for 3 months with thoracic-lumbar-sacral orthosis bracing, which failed as the patient's pain progressively worsened. A subsequent MRI revealed an enlarged lesion in the L2 vertebra (Fig. 1). Given the patient's young age and L5/S1 spondylolisthesis, the decision was made to avoid L2 corpectomy and fusion. Instead, a novel approach was utilized with percutaneous CT-guided cryoablation of the L2 lesion followed by intralesional curettage and bone grafting utilizing a minimally invasive technique.
The patient underwent percutaneous cryoablation of the cystic lesion at L2 after first undergoing embolization. Using CT guidance and general anesthesia in a radiology suite with a full operative setup, an 11-gauge needle was advanced into the lesions using a transpedicular approach. The needle served as a biopsy needle as well as made a pilot hole for the cryoprobe. Using CT fluoroscopic guidance and coaxial technique, two percutaneous cryoprobes were inserted into the lesion using a transpedicular and lateral paraspinal approach. The lesions were cryoablated at 20% power for 10 minutes, passively thawed for 5 minutes, and actively reablated for 10 minutes, while monitoring the ice every 2 minutes with limited axial CT scans (Fig. 3). Complete cryoablation of the tumor was performed by withdrawing one of the percutaneous cryoprobes to obtain more lateral coverage and repeating the process. The following day, the patient returned to the radiology suite. The L2 pedicle was cannulated under biplane fluoroscopy. Using sequentially larger cannulas, a working cannula was established in the L2 vertebrae. Curettage was performed on the lytic lesion using articulated instruments. After debulking the lesion circumferentially, a polyethylene mesh bag was deployed into the defect and packed with allograft bone (Fig. 4). The patient tolerated the procedure well without any neurologic complications. Postoperatively, he began a 2-year course of Zometa (zoledronate; Novartis Pharmaceuticals, Basel, Switzerland) and interferon α 2b to prevent recurrence and progression of his multifocal EHE.
Fig. 3.
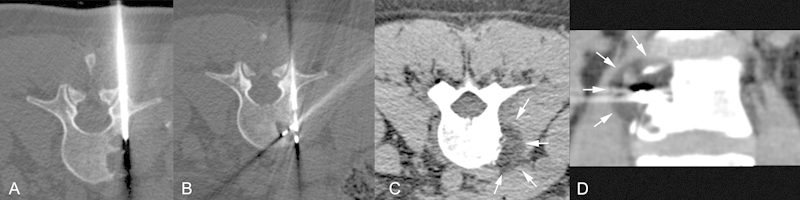
(A) Computed tomography (CT)-guided percutaneous biopsy utilizing an 11-gauge needle. (B) 2 Perc 15 Cryoablation Probes placed in the lesion. (C, D) Axial CT and coronal reformats performed 10 minutes after the start of the cryoablation demonstrate the ice as a circular hypodensity (white arrows at the margin of the ice).
Fig. 4.
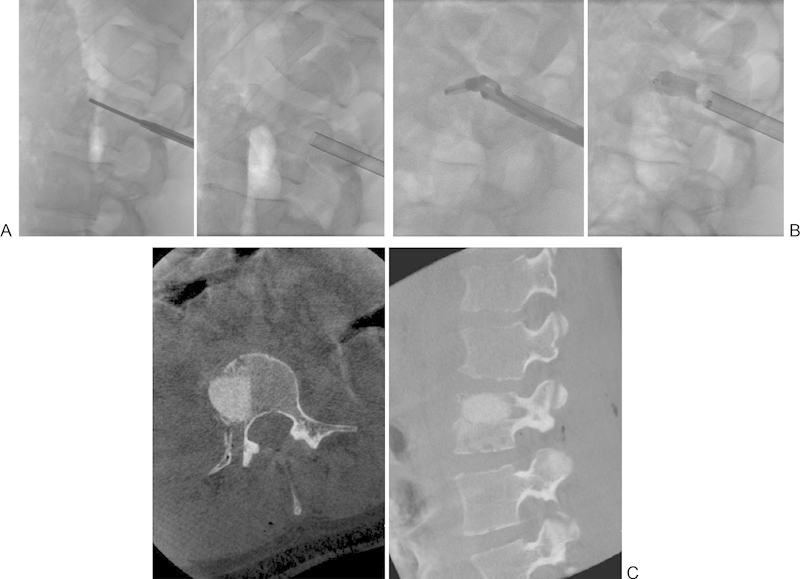
(A) Establishment of transpedicular working cannula using biplanar fluoroscopy and minimally invasive technique. (B) Fluoroscopic images of articulated instruments used for curettage (left) and placement of allograft filled mesh bag (right). (C) Intraoperative axial (left) and sagittal (right) flat panel computed tomography scans demonstrating the position of the mesh bag into the vertebral bony defect.
At his most recent follow-up 3.5 years after surgery, the patient is pain-free with a normal neurologic exam. MRI has shown no further progression or regression of the lesions at C7 and T9. CT demonstrated incorporation of his allograft into the surrounding bone at L2 with no signs of instability (Fig. 5). The patient will receive annual repeat imaging for 5 years following surgery to monitor his disease.
Fig. 5.
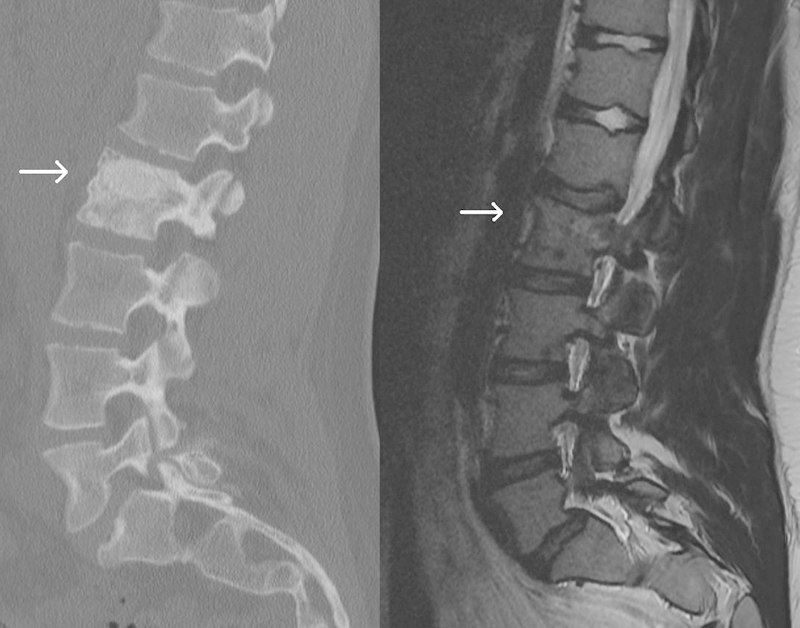
Sagittal computed tomography (left) and T2 magnetic resonance imaging (right) at 18 months postoperatively demonstrating some incorporation of the allograft, maintenance of vertebral height, and no further progression of the lesion.
Discussion
EHE is a rare tumor with a histopathology that is intermediate between angiosarcoma and hemangioma. Distant metastasis rates are estimated to be between 20 and 30%, with local recurrence rates as high as 13% in some series.3 4 However, given the intermediate behavior of the tumor, patients with metastatic EHE can survive long term.4 5 EHE has a strong tendency to present with multifocal lesions with over 50% of cases presenting with multicentric disease.6 In two large case series of patients with EHE of bone, local pain was the most common symptom.6 7 Spinal EHE typically presents with localized neck or back pain as in our patient's case.1 4 However, cases of spinal EHE presenting as myelopathy, paresthesias, and paraplegia have been described.1 8 9
The imaging characteristics of spinal EHE are heterogenous and typically nondiagnostic.10 11 The tumors can be well marginated by sclerosis or poorly defined with extensive lysis and cortical destruction.1 4 Given the high frequency of multifocal disease, screening whole-body imaging such as a bone scan may be indicated following diagnosis of EHE. The clinical course of spinal EHE is quite variable as are the treatment options reported in the literature. Ma et al reported on five cases of spinal EHE, all of which presented with local pain and partial vertebral body collapse. Wide resection and postoperative radiation were performed in four of the five cases, with no evidence of recurrent disease at an average of 47 months' follow-up. A limited laminectomy and cytoreductive surgery were performed in one case with an extensive thoracic lesion and paraplegia.1 Aquilina et al described the longest reported follow-up of spinal EHE (11 years) for a thoracic lesion treated with vertebrectomy and postoperative chemoradiation without any evidence of disease progression.4
We describe a novel technique of percutaneous cryoablation followed by minimally invasive reconstruction of a large, lytic EHE lesion utilizing a mesh bag filled with allograft bone introduced through a transpedicular cannula. This may avoid the need for spinal fusion in select patients. Of the case reports and series in the literature, most describe treatment of spinal EHE with wide resection and sometimes subsequent adjuvant radiotherapy. Although preoperative embolization has been recommended for these tumors prior to surgical intervention,1 12 to the authors' knowledge this is the first description of cryoablation being used in the treatment of spinal EHE. Cryoablation has been used successfully in the treatment of giant cell tumors.13 Mirra et al described complete resolution of a large cervical giant cell tumor after treatment with cryotherapy and adjuvant radiation.14 Cryoablation is also used with percutaneous cementoplasty for ablation and consolidation of metastatic spinal lesions.15 16 Traditional surgical techniques for large lesions, such as the L2 lesion in our case, require corpectomy and fusion of the junctional segments. Given our patient's young age and spondylolisthesis, minimally invasive reconstruction had the advantage of avoiding a multilevel lumbar fusion. Assuming that the cryoablation killed the tumor cells, we were able to curette the lesion out to healthy bone. This left an irregular defect in the vertebral body requiring the mesh bag implant, which conformed to the shape of the void. Although local curettage has been used successfully in the treatment of spinal EHE tumors,17 this is the only description to the authors' knowledge of utilizing curettage for the treatment of spinal EHE through minimally invasive technique.
Intravertebral polyethylene mesh bags have been used successfully to treat vertebral compression fractures and to restore anterior column height.18 19 The mesh bag implants are designed to fill vertebral body defects, such as those created by a tumor, with bone graft.19 These implants have the advantage of being deployed through a small tube, which allows for minimally invasive or even percutaneous placement.20 We utilized this minimally invasive technique to introduce articulated curettes into the lesion prior to deploying the mesh bag. Mesh bag implants provide good conformity to the vertebral defect, which allows for less extensive bony resection in the case of benign or metastatic spinal tumors. These implants also keep the graft material contained as opposed to packing the lesion with bone graft alone. This technique produces a more biologic reconstruction in younger patients by avoiding wider bony resection, fusion, and cement augmentation. Minimally invasive operations may also be an attractive option in patients who are poor candidates for more invasive procedures by reducing blood loss, operative time, and postoperative recovery.
Postoperatively, the patient was placed on a 2-year course of Zometa (zoledronate) and interferon α 2b per pediatric oncology recommendations. Bisphosphonates have been shown to be effective at controlling bony destruction secondary to both metastatic and primary tumors of the spine. This relates to both their antiresorptive and likely antitumor properties.21 22 23 Other studies have shown bisphosphonates to be an effective monotherapy for both giant cell tumors and osteolytic EHE.24 25 Recent studies have demonstrated the effectiveness of denosumab (Prolia, Amgen, Inc., Thousand Oaks, California, United States), an anti-RANKL antibody, in the treatment of recurrent or unresectable giant cell tumors.26 27 It has also been shown to be superior to zoledronate in the treatment of metastatic disease.28 More studies are needed to determine its potential in the treatment of EHE.
The approach presented in this case avoids the morbidity of wide resection and the complications associated with postoperative radiotherapy. It is difficult to know which technique had the largest treatment effect in this multimodal approach, which included embolization, cryoablation, curettage with bone grafting, and adjuvant pharmacologic therapy. This approach may be warranted in other locally aggressive lesions such as giant cell tumor.
In conclusion, we describe a novel treatment for aggressive EHE of the spine. In our case, percutaneous cryoablation was performed followed by cannulation of the involved pedicle and use of specialized articulated curettes to achieve intralesional resection. Allograft bone was delivered into the defect using a specialized mesh bag implant. This allowed for a biologic reconstruction of the vertebra through a minimally invasive technique without the need for fusion. The remaining systemic disease was controlled using bisphosphonate and interferon therapy supplemented by cryoablation of a growing T2 lesion. This is a useful technique in select cases of primary or multifocal spinal EHE.
Footnotes
Disclosures Arjun S. Sebastian, none Marcus J. Adair, none Jonathan M. Morris, none Mustafa H. Khan, none Carola A. S. Arndt, none Ahmad Nassr, none
References
- 1.Ma J, Wang L, Mo W, Yang X, Xiao J. Epithelioid hemangioendotheliomas of the spine: clinical characters with middle and long-term follow-up under surgical treatments. Eur Spine J. 2011;20(8):1371–1376. doi: 10.1007/s00586-011-1798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerry G, Marx O, Kraus D. et al. Multifocal epithelioid hemangioendothelioma derived from the spine region: case report and literature review. Case Rep Oncol. 2012;5(1):91–98. doi: 10.1159/000336947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss S W, Enzinger F M. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50(5):970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Aquilina K, Lim C, Kamel M H, Marks C J, O'Sullivan M G, Keohane C. Epithelioid hemangioendothelioma of the spine. Report of two cases. J Neurosurg Spine. 2005;3(5):393–399. doi: 10.3171/spi.2005.3.5.0393. [DOI] [PubMed] [Google Scholar]
- 5.Hurley T R, Whisler W W, Clasen R A. et al. Recurrent intracranial epithelioid hemangioendothelioma associated with multicentric disease of liver and heart: case report. Neurosurgery. 1994;35(1):148–151. doi: 10.1227/00006123-199407000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Kleer C G, Unni K K, McLeod R A. Epithelioid hemangioendothelioma of bone. Am J Surg Pathol. 1996;20(11):1301–1311. doi: 10.1097/00000478-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Campanacci M, Boriani S, Giunti A. Hemangioendothelioma of bone: a study of 29 cases. Cancer. 1980;46(4):804–814. doi: 10.1002/1097-0142(19800815)46:4<804::aid-cncr2820460427>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Brennan J W, Midha R, Ang L C, Perez-Ordonez B. Epithelioid hemangioendothelioma of the spine presenting as cervical myelopathy: case report. Neurosurgery. 2001;48(5):1166–1169. doi: 10.1097/00006123-200105000-00045. [DOI] [PubMed] [Google Scholar]
- 9.Gokhan G A, Akyuz M, Gurer I E, Tuncer R. Epithelioid hemangioendothelioma derived from the spine region: case report and review of the literature. Wien Klin Wochenschr. 2006;118(11–12):358–361. doi: 10.1007/s00508-006-0582-5. [DOI] [PubMed] [Google Scholar]
- 10.Adler B, Naheedy J, Yeager N, Nicol K, Klamar J. Multifocal epithelioid hemangioendothelioma in a 16-year-old boy. Pediatr Radiol. 2005;35(10):1014–1018. doi: 10.1007/s00247-005-1492-9. [DOI] [PubMed] [Google Scholar]
- 11.Bölke E, Gripp S, Peiper M. et al. Multifocal epithelioid hemangioendothelioma: case report of a clinical chamaeleon. Eur J Med Res. 2006;11(11):462–466. [PubMed] [Google Scholar]
- 12.Sybert D R, Steffee A D, Keppler L, Biscup R S, Enker P. Seven-year follow-up of vertebral excision and reconstruction for malignant hemangioendothelioma of bone. Spine (Phila Pa 1976) 1995;20(7):841–844. doi: 10.1097/00007632-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt J J, Grogan T J. Giant cell tumor of bone. Clin Orthop Relat Res. 1986;(204):45–58. [PubMed] [Google Scholar]
- 14.Mirra J M, Rand F, Rand R, Calcaterra T, Dawson E. Giant-cell tumor of the second cervical vertebra treated by cryosurgery and irradiation. Clin Orthop Relat Res. 1981;(154):228–233. [PubMed] [Google Scholar]
- 15.Heran M K. Preoperative embolization of spinal metastatic disease: rationale and technical considerations. Semin Musculoskelet Radiol. 2011;15(2):135–142. doi: 10.1055/s-0031-1275596. [DOI] [PubMed] [Google Scholar]
- 16.Munk P L, Murphy K J, Gangi A, Liu D M. Fire and ice: percutaneous ablative therapies and cement injection in management of metastatic disease of the spine. Semin Musculoskelet Radiol. 2011;15(2):125–134. doi: 10.1055/s-0031-1275595. [DOI] [PubMed] [Google Scholar]
- 17.Tsuneyoshi M, Dorfman H D, Bauer T W. Epithelioid hemangioendothelioma of bone. A clinicopathologic, ultrastructural, and immunohistochemical study. Am J Surg Pathol. 1986;10(11):754–764. doi: 10.1097/00000478-198611000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Inamasu J, Guiot B H, Uribe J S. Flexion-distraction injury of the L1 vertebra treated with short-segment posterior fixation and Optimesh. J Clin Neurosci. 2008;15(2):214–218. doi: 10.1016/j.jocn.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Chiu J C, Maziad A M. Post-traumatic vertebral compression fracture treated with minimally invasive biologic vertebral augmentation for reconstruction. Surg Technol Int. 2011;XXI:268–277. [PubMed] [Google Scholar]
- 20.Zheng X, Chaudhari R, Wu C, Mehbod A A, Erkan S, Transfeldt E E. Biomechanical evaluation of an expandable meshed bag augmented with pedicle or facet screws for percutaneous lumbar interbody fusion. Spine J. 2010;10(11):987–993. doi: 10.1016/j.spinee.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Green J R, Clézardin P. Mechanisms of bisphosphonate effects on osteoclasts, tumor cell growth, and metastasis. Am J Clin Oncol. 2002;25(6) 01:S3–S9. doi: 10.1097/00000421-200212001-00002. [DOI] [PubMed] [Google Scholar]
- 22.Mackiewicz-Wysocka M, Pankowska M, Wysocki P J. Progress in the treatment of bone metastases in cancer patients. Expert Opin Investig Drugs. 2012;21(6):785–795. doi: 10.1517/13543784.2012.679928. [DOI] [PubMed] [Google Scholar]
- 23.Guise T A. Antitumor effects of bisphosphonates: promising preclinical evidence. Cancer Treat Rev. 2008;34 01:S19–S24. doi: 10.1016/j.ctrv.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Gille O, Oliveira BdeA, Guerin P, Lepreux S, Richez C, Vital J M. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine (Phila Pa 1976) 2012;37(6):E396–E399. doi: 10.1097/BRS.0b013e31823ed70d. [DOI] [PubMed] [Google Scholar]
- 25.Coppo P, Lassoued S, Billey T, Lassoued K. Successful treatment of osteolytic epithelioid hemangioendothelioma with pamidronate. Clin Exp Rheumatol. 2005;23(3):400–401. [PubMed] [Google Scholar]
- 26.Thomas D, Henshaw R, Skubitz K. et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 27.Thomas D M. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24(4):397–403. doi: 10.1097/CCO.0b013e328354c129. [DOI] [PubMed] [Google Scholar]
- 28.Stopeck A T, Lipton A, Body J J. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]


