Abstract
Study Design Technical report.
Objective To provide a technical description of the transfer of the brachialis to the anterior interosseous nerve (AIN) for the treatment of tetraplegia after a cervical spinal cord injury (SCI).
Methods In this technical report, the authors present a case illustration of an ideal surgical candidate for a brachialis-to-AIN transfer: a 21-year-old patient with a complete C7 spinal cord injury and failure of any hand motor recovery. The authors provide detailed description including images and video showing how to perform the brachialis-to-AIN transfer.
Results The brachialis nerve and AIN fascicles can be successfully isolated using visual inspection and motor mapping. Then, careful dissection and microsurgical coaptation can be used for a successful anterior interosseous reinnervation.
Conclusion The nerve transfer techniques for reinnervation have been described predominantly for the treatment of brachial plexus injuries. The majority of the nerve transfer techniques have focused on the upper brachial plexus or distal nerves of the lower brachial plexus. More recently, nerve transfers have reemerged as a potential reinnervation strategy for select patients with cervical SCI. The brachialis-to-AIN transfer technique offers a potential means for restoration of intrinsic hand function in patients with SCI.
Keywords: brachialis, anterior interosseous nerve, nerve transfer, spinal cord injury
Introduction
Spinal cord injury (SCI) is major public health problem that affects nearly 40 per one million Americans per year.1 SCI typically afflicts patients < 40 years of age and therefore has a major societal and economic impact on disability and work-years lost.1 Although most SCI research has focused on neuroprotection and neuroregeneration, recent applications of peripheral nerve reinnervation strategies for SCI show significant promise.2 3 4
Reinnervation by nerve transfers have traditionally been performed for brachial plexus injuries. The nerve transfers using an expendable nearby motor nerve to reinnervate a denervated nerve have resulted in more rapid and improved functional recovery than the traditional nerve graft reconstruction following brachial plexus and distal peripheral nerve injuries.5 6 7 8 9 10 11 12 13 14 15 16 Peripheral nerve transfers have also focused on either upper trunk reinnervation or distal lower trunk reinnervation.7 17 18 19
Recent advances have identified that (1) nerve transfer techniques may be a suitable treatment modality for select patients with cervical SCI,3 4 6 20 21 and (2) proximal lower trunk reinnervation strategies may allow reinnervation of the hand function.3 5 6 18 22 Over the last two decades, multiple authors have reported their experience using the nerve to the brachialis muscle as a donor to the nearby anterior interosseous motor group within the median nerve in the arm.3 11 14 22 23 In patients with low cervical SCI, transfer of the brachialis to the anterior interosseous nerve (AIN) offers hope for improving intrinsic hand function, a crucial component for pinch. Improvement in hand function is a meritorious goal as 77% of quadriplegics report that recovery of hand function would improve their quality of life.24 As previously reported, the brachialis-to-AIN transfer to can facilitate hand function and self-feeding in the patient with SCI.3 Here, we provide a technical description of the brachialis-to-AIN transfer operation in detail with text, an illustration, photographs, and a video.
Illustrative Case
A 21-year-old man who sustained a complete C7 American Spinal Injury Association–A SCI 8 months previously (Fig. 1A) presented for nerve transfer evaluation. Preoperatively, the patient showed full strength in his biceps, triceps, and wrist extension but only minimal finger movements in his hands. Since his SCI, the patient had failed to make any functional hand recovery and required assistance with basic tasks such as feeding. A preoperative electromyogram and nerve conduction study showed normal motor activity above C7, no measureable compound muscle action potential to the median or ulnar nerve at the wrist, the presence of fibrillations, no motor unit potentials to the flexor pollicis longus (FPL) or flexor digitorum profundus (FDP), and intact sensory nerve conduction.
Fig. 1.
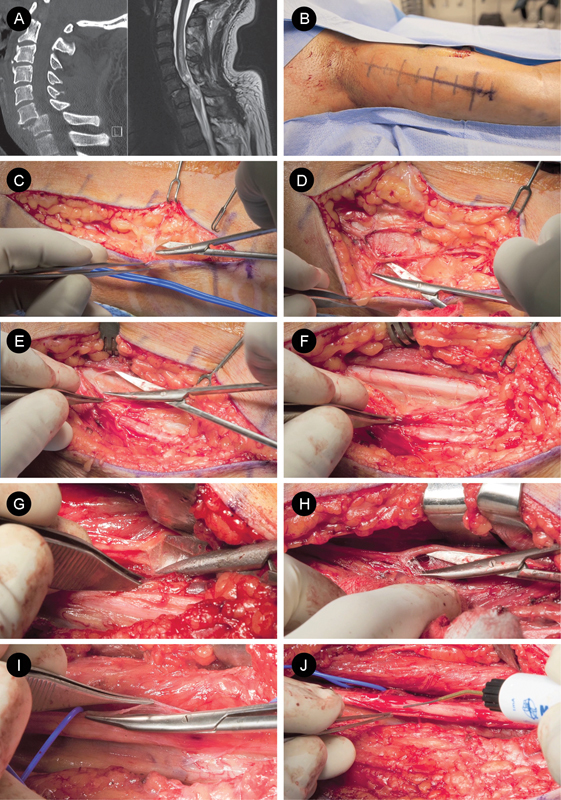
Transfer of brachialis nerve to anterior interosseous nerve (AIN) for C7 spinal cord injury (SCI): approach and dissection. (A) Sagittal cervical spine computed tomography scan and magnetic resonance imaging of a patient with a C7 SCI, an ideal candidate for a brachialis-to-AIN transfer. (B) Skin incision in the bicipital cleft and (C, D) careful subcutaneous dissection allow for identification and preservation of the medial antebrachial cutaneous nerve. (E) The median nerve is exposed and (F) dissected proximally and distally. (G) The musculocutaneous nerve is exposed deep to the biceps muscle and lateral to the median nerve. (H) Once identified, the musculocutaneous branches, biceps brachii, brachialis, and lateral antebrachial cutaneous nerve nerves are identified. (I) The epineurium of the median nerve is sharply incised and (J) nerve fascicles are visually inspected and stimulated using a handheld nerve stimulator (Vari-Stim III, Medtronic).
Surgical Technique
Positioning and Skin Incision
After induction of general endotracheal anesthesia, the patient is positioned supine and the pressure points are appropriately padded. The arm is abducted, supinated, and placed on an arm board. In a thin person, the humerus and median nerve may be palpable. A generous skin incision is marked in the bicipital cleft (Fig. 1B). The entire arm including the axilla are prepped and draped in the usual sterile fashion. The surgeon is seated between the arm and the torso and the assistant is seated between the arm and the head. A no. 10 blade scalpel is used to make skin incision down to the level of the subcutaneous tissue (Fig. 1C).
Dissection and Visual Identification of Median and Musculocutaneous Nerves
Sharp dissection and bipolar cautery are used to divide the subcutaneous tissue while mobilizing and preserving the medial antebrachial cutaneous nerve (Fig. 1C, D). The median nerve is palpated, and the deeper connective tissues are dissected until the median nerve can be visually identified (Fig. 1E). The median nerve is exposed proximally and distally (Fig. 1F) and is then protected. The musculocutaneous nerve is then palpated deep to the biceps muscle and lateral to the median nerve. Once palpated, the musculocutaneous nerve is exposed and visually inspected (Fig. 1G, H).
Neurolysis and Motor Mapping of Median and Musculocutaneous Nerves
Neurolysis of the musculocutaneous nerve (Fig. 1H) reveals the three branches: biceps brachii nerve, brachialis nerve, and lateral antebrachial cutaneous nerve (LABC). The biceps brachii nerve branches off the musculocutaneous nerve first, ∼13 cm from the acromion.25 The brachialis nerve emerges from the main trunk more distally, ∼17 cm from the acromion.25 The LABC continues distally to provide cutaneous sensation to the lateral forearm. When the proximal musculocutaneous nerve is examined in cross section, the fasciculi of the biceps brachii nerve, brachialis nerve, and LABC are located from lateral to medial, respectively.25 Following the dissection and visual identification, the biceps brachii nerve, brachialis nerve, and LABC are marked with vessel loops and protected.
Attention is then returned to the median nerve. The epineurium is sharply incised parallel to the nerve fibers (Fig. 1I), and the fascicles are inspected. The anatomical location of the median nerve fascicles are correlated with intraoperative mapping data using a handheld nerve stimulator (Vari-Stim III, Medtronic, Minneapolis, MN, United States; Fig. 1J). The nerve fascicles reside in canonical anatomical locations within the median nerve (Fig. 2). The fascicles for the nerve to the pronator teres are typically located in the anterior portion median nerve (Fig. 2; Fig. 3A, yellow loop) and stimulation of this fascicle may elicit arm pronation (see online video). Fascicles in the anterior-medial portion of the median nerve typically innervate the palmaris longus (PL) and the flexor carpi radialis (FCR; Fig. 2, inset). Stimulation of the FCR/PL fascicle may produce flexion and abduction of the hand. Fascicles in the posterior-medial median nerve supply the AIN (Fig. 2, inset); in contrast to brachial plexus injuries, depending on the time of denervation, stimulation may lead to activity in the FDP muscle of the index and middle fingers, FPL muscle of the thumb, and pronator quadratus of the forearm (see online video). The sensory component of the median nerve occupies the lateral half of the nerve (Fig. 2, inset) and is usually easily separable from the motor components. Once the AIN is identified by visual inspection and/or motor mapping, it is dissected proximally and protected with a vessel loop (Fig. 3B, red). Care is taken during the remainder of the operation to protect all the identified median nerve fascicles.
Fig. 2.
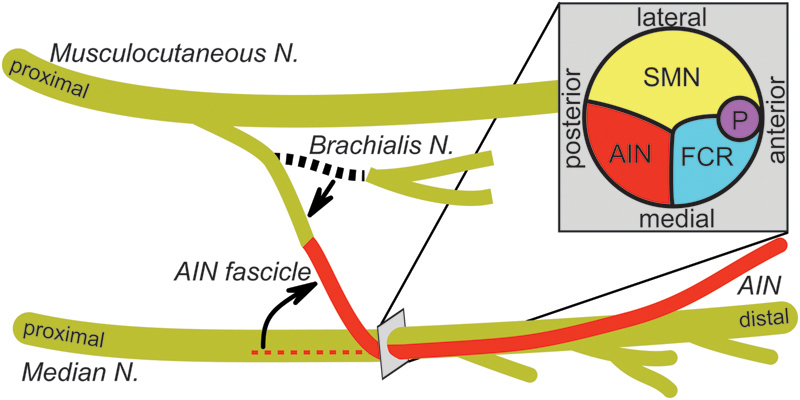
Illustration of transfer of brachialis nerve to anterior interosseous nerve (AIN) for C7 spinal cord injury (SCI): anatomy and anastomosis. Illustration shows the nerve anatomy for a brachialis-to-AIN transfer. The donor brachialis nerve arises from the medial portion of the musculocutaneous nerve. Distally, the musculocutaneous nerve becomes the lateral antebrachial cutaneous nerve. The recipient AIN branches from the lateral portion of the median nerve in the forearm, distal to the palmaris longus (PL) and flexor carpi radialis (FCR) nerve branch and proximal to the flexor digitorum superficialis nerve branch. The AIN fascicle is dissected proximally within the median nerve to allow a tension-free repair. The AIN fascicle is then transected proximally and translocated laterally. The brachialis is traced from the musculocutaneous nerve and then dissected distally. After transection, the brachialis nerve is translocated laterally to meet the AIN and allow a tension-free repair. (Inset) A cross-sectional view of the median nerve is shown to demonstrate that the AIN fascicle located in the posteromedial region (AIN, red). Adjacent to the AIN fascicle are the FCR/PL fascicles (FCR, blue), sensory fascicles (SMN, yellow) and the pronator teres fascicles (P, purple).
Fig. 3.
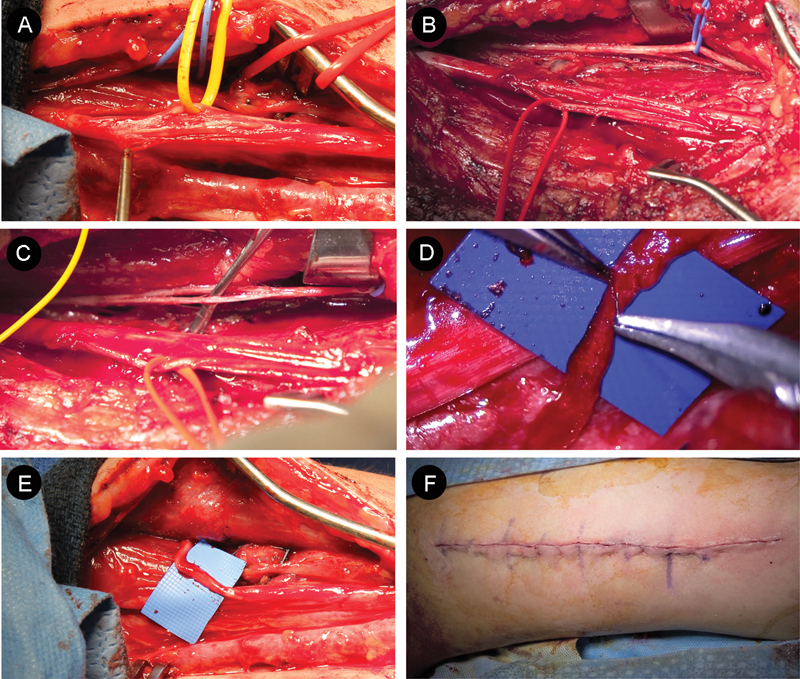
Transfer of brachialis nerve to anterior interosseous nerve (AIN) for C7 spinal cord injury: microdissection and anastomosis. (A) Nerve to the pronator teres fascicles (yellow loop) is located in the anterior portion of the median nerve. (B) AIN (red) and brachial nerve (blue) are identified and marked with vessel loops. (C) The brachialis branch is dissected distally to allow a tension-free repair. (D) Using a surgical microscope, the brachial nerve donor and AIN recipient are anastomosed, end-to-end. (E) Care is taken to ensure a tension-free repair. (F) The wound is closed in layers, a dressing is applied, and a sling is used to keep the elbow in a flexed and supinated position.
Attention is then returned to the dissected musculocutaneous nerve branches for motor mapping (Fig. 3B, blue). Stimulation of the nerve to the biceps brachii muscle elicits a strong elbow flexion, and brachialis stimulation produces a weaker flexion (see online video). The brachialis branch is dissected distally to allow a tension-free repair (Fig. 3C). Once the dissection is complete, the distal brachialis nerve donor and the proximal recipient AIN are sharply incised and translocated toward one another (Fig. 2).
Microsurgical Nerve Transfer
The donor and recipient nerve fascicles are gently placed on Silastic (Dow Corning Corporation, Midland, MI, United States) and opposed to one another, end to end. A tension-free repair is crucial. Using an operative microscope, a microsurgical nerve transfer is performed (Fig. 3D; Video 1). The donor and recipient nerve ends are approximated with two interrupted 9–0 nylon sutures. The sutures are placed 180 degrees apart. After inspecting the tension-free coaptation (Fig. 3E), the transfer is then augmented with fibrin glue.
Video 1
Transfer of brachialis nerve to anterior interosseous nerve for C7 spinal cord injury: operative technique. Online content including video sequences viewable at: www.thieme-connect.com/products/ejournals/html/10.1055/s-0034-1396760.
Closure and Dressing
The wound is copiously irrigated with antibiotic-laden solution, and a subcutaneous drain and pain pump are placed. After closing the wound with deep-dermal interrupted 3–0 Vicryl sutures (Ethicon, Inc., Somerville, NJ, United States) and a running subcuticular Monocryl suture (Ethicon, Inc.) (Fig. 3F), the arm is gently bandaged and secured in a flexed and supinated position within a sling. The patient is awakened after surgery and discharged home on the same day. The pain pump and drain are removed the following day, and the patient wears a sling for 1 week.
Clinical Outcome of Illustrative Case
The patient's recovery from bilateral surgery was uncomplicated. He continued to participate in regular outpatient physical therapy and home exercises through a specialized SCI rehabilitation program. As part of a multidisciplinary treatment plan, hand physical therapy has included patient-tailored and repetitive movements of isolated shoulder, elbow, wrist, and hand muscle groups. Conventional hand physical therapy included passive range of motion, isolated muscle group movements, hand putty exercises, low-load handgrip exercises, active functional exercises, assisted exercises, and resistance training. The traditional therapy has been enriched by advanced technologies. In addition, the patient has participated in goal-directed exercises to improve activities of daily living. After 3 months of outpatient physical therapy, the patient demonstrated progress on the left side. This patient demonstrated very early reinnervation by 3 months in the left hand, with Medical Research Council Scale for Muscle Strength 2 to 3/5 function of the first- and second-digit FDP and FPL. Recovery of the right side was more modest, in line with what we would expect at this time.
Discussion
The development of the brachialis-to-AIN transfer for SCI has been made possible by (1) the important advances in peripheral nerve surgery and (2) the application of peripheral nerve surgery techniques for patients with SCI. Here, we provide a detailed technical report for the brachialis-to-AIN transfer.
Novel Methods in Peripheral Nerve Surgery
Several advances in brachial plexus and peripheral nerve surgery have led to the brachialis-to-AIN transfer. First, direct nerve transfers using expendable nearby motor nerves lead to a quicker and greater improvement in the functional recovery than the traditional nerve graft reconstruction.3 4 5 7 11 12 15 16 17 18 19 22 23 Recent work has revealed that the brachialis nerve is an expendable branch for elbow flexion.8 14 Ray et al previously reported a series of patients treated with brachialis-to-AIN transfer with good clinical results.22 Using these same principles, we have treated several patients with SCI with the brachialis-to-AIN transfer, providing good reinnervation of the FDP and FPL.
Application of Brachialis-to-AIN Transfer in SCI
Application of peripheral nerve transfers for SCI is not new.3 4 11 26 27 28 29 30 31 32 33 34 Most clinical applications have utilized intercostal nerve roots proximal to the SCI to reinnervate lumbar plexus nerve. The nerve transfer strategies for the treatment of cervical SCI are relatively sparse, especially for restoration of hand function. In addition to nerve transfers, patients with SCI may benefit from upper extremity tendon transfers, which are frequently guided by the International Classification of Surgery of the Hand in Tetraplegia (Table 1).35 36 37 Therefore, all nerve transfers should be approached in a multidisciplinary fashion to maximize the potential for future tendon transfers, if needed.
Table 1. Nerve the IC of hand surgery in tetraplegic patients and AISA scores.
| IC motor group | Key muscle | AISA level | Key muscle |
|---|---|---|---|
| 0 | Biceps | C5 | Biceps |
| 1 | Brachioradialis | ||
| 1 | Brachioradialis | C6 | ECRL |
| 2 | ECRL | ||
| 3 | ECRB | ||
| 4 | PT | ||
| 4 | PT | C7 | Triceps |
| 5 | FCR | ||
| 6 | ED | ||
| 7 | EPL | ||
| 8 | FDS | C8 | FDP |
| 9 | FDS and FDP |
Abbreviations: ASIA, American Spinal Injury Association; ECRB, extensor carpi radialis brevis; ECRL, extensor carpi radialis longus; ED, extensor digitorum; EPL, extensor pollicis longus; FCR, flexor carpi radialis; FDP, flexor digitorum profundus; FDS, flexor digitorum superficialis; IC, International Classification; PT, pronator teres.
Although not all-inclusive, Table 2 illustrates recently reported nerve transfers to restore hand function. Transfer of the axillary nerve to triceps nerve and of the teres minor nerve to triceps brachii nerve offer reinnervation strategies for triceps function in patients with C6 SCI (Table 2).6 34 38 The wrist and hand function can be restored with a handful of methods including transfers of the supinator to the posterior interosseous, the distal extensor carpi radialis to the FPL, and the axillary to the radial nerve (Table 2).3 20 34 39 40 After showing efficacy for patients with brachial plexus injury,22 we have a growing experience utilizing brachialis-to-AIN transfers to improve the upper extremity function in tetraplegic patients (Table 2).
Table 2. Nerve transfers to restore upper extremity function after SCI.
| Level of injury | Nerve transfer | Anticipated recovery |
|---|---|---|
| C5 | Brachialis to extensor carpi radialis40 | Wrist extension |
| C6 | Axillary to triceps34 38 | Elbow extension: triceps |
| C6 | Teres minor to triceps brachii6 | Elbow extension: triceps |
| C6 | Axillary to radial34 | Wrist and finger extension |
| C7 | Distal extensor carpi radialis to flexor pollicis longus39 | Finger and thumb flexion: FPL |
| C7 | Brachialis to AIN3 | Finger and thumb flexion: FDP and FPL |
| C7 | Supinator to posterior interosseous20 | Thumb and finger extension |
Abbreviations: AIN, anterior interosseous nerve; FDP, flexor digitorum profundus; FPL, flexor pollicis longus; SCI, spinal cord injury.
Recovery of hand function after the nerve transfer for SCI depends on distance from the end organ, time, and therapy. After initial recovery from surgery, physical therapy is vital for motor re-education after nerve transfers.41 Therapy initially focuses on passive range of motion, repetitive movements of target muscles, and use of exercise putty and gripping activities to activate nerve-transfer recipient muscles. This is followed by conventional assisted and resistance exercises, which are often enriched with splinting and advanced technologies. Specific motor re-education strategies for brachialis-to-AIN transfers are still under investigation. However, they follow similar principles and exercises described for median-to-radial nerve transfer re-education methods that facilitate recovery of the FDP and FPL.42 43 This therapy includes patient education, scar/edema management, repetitive muscle exercises, custom splints, cocontraction exercises, the use of specialized technical equipment, wrist flexion against resistance, and ball, putty, and coin exercises.42 43 The patient illustrated in this case demonstrated improvement in left hand function 3 months after surgery, which is earlier than one would anticipate. Based on previous reports, we expect the early phases of recovery to occur by 6 months to 1 year and improvement over the next 4 years.3 22 Hence, we anticipate additional recovery over the next several years and will continue to monitor recovery with detailed physical examinations. Future studies will be necessary to further define recovery time course.
Technical Nuances and Consideration for Brachialis-to-AIN Transfer in the Patient with SCI
As reviewed elsewhere,4 there are several general considerations regarding peripheral nerve transfers for patients with SCI. The pathophysiology underlying the paralysis from SCI and peripheral nerve injuries are different. For SCI, there are three spinal cord segments of interest: the spinal cord segments (1) rostral to the SCI, (2) within the SCI and (3) caudal to the SCI. The region rostral to the injury has normal upper motor neurons (UMNs) and lower motor neurons (LMNs). The damaged spinal cord segment may have impaired UMNs and LMNs due to injuries in the corticospinal tracts and the ventral horn. The spinal cord region below the injury may have damaged inputs from UMNs but intact LMNs. This pathophysiological nuance impacts on the preferred timing of surgery, intraoperative findings, and potential for recovery. Ideally the donor should arise from a functional but expendable nerve branch arising rostral to the SCI segment. If the LMNs of the recipient branch are severely damaged, the axons will degenerate over time. Therefore, these territories must be treated like a brachial plexus injury, and it is ideal to perform the reinnervation surgery soon after the SCI. One consideration in peripheral nerve transfer is that reinnervation should ideally be performed as close to the target muscle as possible to reduce the lead time before functional reanimation and to prevent distal denervation and motor end plate changes. The donor should have a large number of motor neurons, and the donor and recipient should be similar in size. Furthermore, the nerve repairs should ideally be done directly without a graft, and there should be a tension-free anastomosis.
The timing of the surgery is a point of discussion when considering the nerve transfers for SCI. Fundamentally, the nerve transfers for SCI should be performed after the anticipated time window necessary for a spontaneous recovery. Some authors typically advocate waiting at least 6 months for spontaneous recovery.6 Indeed, the chance for a meaningful recovery falls dramatically after 6 months but is still present for up to 2 years.4 44 Nerve transfer to LMNs below the level of injury is not time-dependent and can theoretically be delayed until all hope of recovery is lost. However, LMNs at the level or in the zone of injury begin to degenerate immediately after injury and behave as a typical peripheral nerve injury. If the intended nerve transfer recipient is fed by roots in the zone of injury, waiting comes with a cost of myotome denervation and progressive motor end plate fibrosis. Myotome denervation reduces the chances for a good functional outcome after nerve transfer, and one can expect poor functional recovery if nerve transfers are performed late into the zone of injury with both upper and LMN dysfunction.3 4 11 Although the donor nerve is a functionally expendable branch (e.g., elbow flexion is maintained after brachialis procurement8 14), one must also consider the status of the recipient nerve: Would the recipient nerve recover spontaneously with conservative therapy alone? Many suggest that 6 months is a sufficient window to allow recovery in the vast majority of patients.6 45 46 But this balance of waiting for spontaneous recovery and surgical intervention is further complicated by the potential zones of partial preservation, seen in some patients with SCI.47 48 When taken together, a thoughtful patient-tailored balance of timing and risks/benefits must be considered by the surgeon and patient.
There are several specific considerations with brachialis-to-AIN transfers for SCI. First, the isolation of the AIN requires knowledge of the cross-sectional anatomy of the median nerve. The anatomical identification and confirmation of the AIN and brachialis nerves with direct intraoperative simulation are essential for an accurate and successful transfer. For patients with an SCI, intact LMNs will lead to larger fascicles and a motor response to intraoperative stimulation. An ideal SCI candidate for a brachialis-to-AIN transfer has disrupted UMNs but intact AIN functional stimulation (i.e., intact LMNs).
Conclusion
Nerve transfers represent a step forward in improved independent function in select patients with cervical SCIs.3 4 5 6 Restoration of the hand function following an SCI can markedly improve a patient's independence and subjective quality of life.2 3 4 5 6 24 We have previously shown that brachialis-to-AIN transfer can facilitate hand function after an SCI and can allow a patient to feed independently.3 As shown in this technical report, the brachialis-to-AIN transfer is a straightforward procedure that can be utilized by neurosurgeons who seek to facilitate hand function in select patients with SCI.
Funding
This work has been supported by the Department of Neurosurgery at Washington University and grant funding from the National Institutes of Health (T32NS007205, A.H.H.).
Footnotes
Disclosures Ammar H. Hawasli, none Jodie Chang, none Matthew R. Reynolds, none Wilson Z. Ray, Consultant: DePuy Synthes, Ulrich
References
- 1.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 2.Anderson K D, Fridén J, Lieber R L. Acceptable benefits and risks associated with surgically improving arm function in individuals living with cervical spinal cord injury. Spinal Cord. 2009;47(4):334–338. doi: 10.1038/sc.2008.148. [DOI] [PubMed] [Google Scholar]
- 3.Mackinnon S E, Yee A, Ray W Z. Nerve transfers for the restoration of hand function after spinal cord injury. J Neurosurg. 2012;117(1):176–185. doi: 10.3171/2012.3.JNS12328. [DOI] [PubMed] [Google Scholar]
- 4.Senjaya F, Midha R. Nerve transfer strategies for spinal cord injury. World Neurosurg. 2013;80(6):e319–e326. doi: 10.1016/j.wneu.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bertelli J A, Ghizoni M F. Results and current approach for brachial plexus reconstruction. J Brachial Plex Peripher Nerve Inj. 2011;6(1):2. doi: 10.1186/1749-7221-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertelli J A, Ghizoni M F, Tacca C P. Transfer of the teres minor motor branch for triceps reinnervation in tetraplegia. J Neurosurg. 2011;114(5):1457–1460. doi: 10.3171/2010.12.JNS101519. [DOI] [PubMed] [Google Scholar]
- 7.Brown J M, Mackinnon S E. Nerve transfers in the forearm and hand. Hand Clin. 2008;24(4):319–340. doi: 10.1016/j.hcl.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Carlsen B T, Kircher M F, Spinner R J, Bishop A T, Shin A Y. Comparison of single versus double nerve transfers for elbow flexion after brachial plexus injury. Plast Reconstr Surg. 2011;127(1):269–276. doi: 10.1097/PRS.0b013e3181f95be7. [DOI] [PubMed] [Google Scholar]
- 9.Colbert S H, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand (NY) 2006;1(2):71–77. doi: 10.1007/s11552-006-9004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P, Malungpaishrope K. Combined nerve transfers for C5 and C6 brachial plexus avulsion injury. J Hand Surg Am. 2006;31(2):183–189. doi: 10.1016/j.jhsa.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Mackinnon S. New York, NY: Thieme Medical Publishers; 1988. Surgery of the Peripheral Nerve. [Google Scholar]
- 12.Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16(5):E5. doi: 10.3171/foc.2004.16.5.6. [DOI] [PubMed] [Google Scholar]
- 13.Murphy R K, Ray W Z, Mackinnon S E. Repair of a median nerve transection injury using multiple nerve transfers, with long-term functional recovery. J Neurosurg. 2012;117(5):886–889. doi: 10.3171/2012.8.JNS111356. [DOI] [PubMed] [Google Scholar]
- 14.Oberlin C, Ameur N E, Teboul F, Beaulieu J Y, Vacher C. Restoration of elbow flexion in brachial plexus injury by transfer of ulnar nerve fascicles to the nerve to the biceps muscle. Tech Hand Up Extrem Surg. 2002;6(2):86–90. doi: 10.1097/00130911-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Ray W Z, Pet M A, Yee A, Mackinnon S E. Double fascicular nerve transfer to the biceps and brachialis muscles after brachial plexus injury: clinical outcomes in a series of 29 cases. J Neurosurg. 2011;114(6):1520–1528. doi: 10.3171/2011.1.JNS10810. [DOI] [PubMed] [Google Scholar]
- 16.Tung T H, Novak C B, Mackinnon S E. Nerve transfers to the biceps and brachialis branches to improve elbow flexion strength after brachial plexus injuries. J Neurosurg. 2003;98(2):313–318. doi: 10.3171/jns.2003.98.2.0313. [DOI] [PubMed] [Google Scholar]
- 17.Brown J M, Shah M N, Mackinnon S E. Distal nerve transfers: a biology-based rationale. Neurosurg Focus. 2009;26(2):E12. doi: 10.3171/FOC.2009.26.2.E12. [DOI] [PubMed] [Google Scholar]
- 18.Brown J M Yee A Mackinnon S E Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: technical nuances Neurosurgery 2009655966–977., discussion 977–978 [DOI] [PubMed] [Google Scholar]
- 19.Ray W Z, Mackinnon S E. Clinical outcomes following median to radial nerve transfers. J Hand Surg Am. 2011;36(2):201–208. doi: 10.1016/j.jhsa.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertelli J A, Tacca C P, Ghizoni M F, Kechele P R, Santos M A. Transfer of supinator motor branches to the posterior interosseous nerve to reconstruct thumb and finger extension in tetraplegia: case report. J Hand Surg Am. 2010;35(10):1647–1651. doi: 10.1016/j.jhsa.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Kale S S, Glaus S W, Yee A. et al. Reverse end-to-side nerve transfer: from animal model to clinical use. J Hand Surg Am. 2011;36(10):1631–163900. doi: 10.1016/j.jhsa.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Ray W Z, Yarbrough C K, Yee A, Mackinnon S E. Clinical outcomes following brachialis to anterior interosseous nerve transfers. J Neurosurg. 2012;117(3):604–609. doi: 10.3171/2012.6.JNS111332. [DOI] [PubMed] [Google Scholar]
- 23.Palazzi S, Palazzi J L, Caceres J P. Neurotization with the brachialis muscle motor nerve. Microsurgery. 2006;26(4):330–333. doi: 10.1002/micr.20247. [DOI] [PubMed] [Google Scholar]
- 24.Snoek G J, IJzerman M J, Hermens H J, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–532. doi: 10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- 25.Chiarapattanakom P, Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Anatomy and internal topography of the musculocutaneous nerve: the nerves to the biceps and brachialis muscle. J Hand Surg Am. 1998;23(2):250–255. doi: 10.1016/S0363-5023(98)80122-6. [DOI] [PubMed] [Google Scholar]
- 26.Dai K R, Yu C T, Wu R S, Zhang X F, Yuan J X, Sun Y H. Intercostal-lumbar-spinal nerve anastomoses for cord transection. A preliminary investigation. J Reconstr Microsurg. 1985;1(3):223–226. doi: 10.1055/s-2007-1007078. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Hou C, Chen A. Reconstructed bladder innervation above the level of spinal cord injury to produce urination by abdomen-to-bladder reflex contractions. J Neurosurg Spine. 2011;14(6):799–802. doi: 10.3171/2011.2.SPINE10685. [DOI] [PubMed] [Google Scholar]
- 28.Lin H Hou C Chen A Xu Z Innervation of reconstructed bladder above the level of spinal cord injury for inducing micturition by contractions of the abdomen-to-bladder reflex arc Neurosurgery 2010665948–952., discussion 952 [PubMed] [Google Scholar]
- 29.Lin H, Hou C, Zhen X, Xu Z. Clinical study of reconstructed bladder innervation below the level of spinal cord injury to produce urination by Achilles tendon-to-bladder reflex contractions. J Neurosurg Spine. 2009;10(5):452–457. doi: 10.3171/2009.1.SPINE08540. [DOI] [PubMed] [Google Scholar]
- 30.Livshits A, Catz A, Folman Y. et al. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord. 2004;42(4):211–217. doi: 10.1038/sj.sc.3101574. [DOI] [PubMed] [Google Scholar]
- 31.Yang M L, Li J J, Zhang S C. et al. Functional restoration of the paralyzed diaphragm in high cervical quadriplegia via phrenic nerve neurotization utilizing the functional spinal accessory nerve. J Neurosurg Spine. 2011;15(2):190–194. doi: 10.3171/2011.3.SPINE10911. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Johnston L, Zhang Z. et al. Restoration of stepping-forward and ambulatory function in patients with paraplegia: rerouting of vascularized intercostal nerves to lumbar nerve roots using selected interfascicular anastomosis. Surg Technol Int. 2003;11:244–248. [PubMed] [Google Scholar]
- 33.Zhang S, Wang Y, Johnston L. Restoration of function in complete spinal cord injury using peripheral nerve rerouting: a summary of procedures. Surg Technol Int. 2008;17:287–291. [PubMed] [Google Scholar]
- 34.Brown J M. Nerve transfers in tetraplegia I: background and technique. Surg Neurol Int. 2011;2:121. doi: 10.4103/2152-7806.84392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allieu Y. General indications for functional surgery of the hand in tetraplegic patients. Hand Clin. 2002;18(3):413–421. doi: 10.1016/s0749-0712(02)00021-5. [DOI] [PubMed] [Google Scholar]
- 36.Fridén J, Reinholdt C. Current concepts in reconstruction of hand function in tetraplegia. Scand J Surg. 2008;97(4):341–346. doi: 10.1177/145749690809700411. [DOI] [PubMed] [Google Scholar]
- 37.Hentz V R, LeClercq C. Philadelphia, PA: Saunders Company; 2002. Surgical Rehabilitation of the Upper Limb in Tetraplegia. [Google Scholar]
- 38.Davidge K M Kahn L C Novak C B et al. The deltoid to triceps nerve transfer: a novel approach to early salvage of elbow extension in tetraplegia Presented at: 2014 Annual American Society for Peripheral Nerve Meeting; January 10–12, 2014; Kauai, Hawaii
- 39.Bertelli J A Mendes Lehm V L Tacca C P Winkelmann Duarte E C Ghizoni M F Duarte H Transfer of the distal terminal motor branch of the extensor carpi radialis brevis to the nerve of the flexor pollicis longus: an anatomic study and clinical application in a tetraplegic patient Neurosurgery 20127041011–1016., discussion 1016 [DOI] [PubMed] [Google Scholar]
- 40.Fridén J, Gohritz A. Brachialis-to-extensor carpi radialis longus selective nerve transfer to restore wrist extension in tetraplegia: case report. J Hand Surg Am. 2012;37(8):1606–1608. doi: 10.1016/j.jhsa.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Novak C B Rehabilitation following motor nerve transfers Hand Clin 2008244417–423., vi [DOI] [PubMed] [Google Scholar]
- 42.Davidge K M, Yee A, Kahn L C, Mackinnon S E. Median to radial nerve transfers for restoration of wrist, finger, and thumb extension. J Hand Surg Am. 2013;38(9):1812–1827. doi: 10.1016/j.jhsa.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 43.Davidge K M Kahn L C Junkins N et al. Optimizing post-surgical nerve transfer motor re-education in tetraplegia Presented at: 2014 American Association for Hand Surgery Annual Meeting; January 8–14, 2014; Kauai, Hawaii
- 44.Waters R L, Sie I H, Gellman H, Tognella M. Functional hand surgery following tetraplegia. Arch Phys Med Rehabil. 1996;77(1):86–94. doi: 10.1016/s0003-9993(96)90227-0. [DOI] [PubMed] [Google Scholar]
- 45.Waters R L, Adkins R H, Yakura J S. Definition of complete spinal cord injury. Paraplegia. 1991;29(9):573–581. doi: 10.1038/sc.1991.85. [DOI] [PubMed] [Google Scholar]
- 46.Waters R L, Adkins R H, Yakura J S, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993;74(3):242–247. [PubMed] [Google Scholar]
- 47.Bunge R P, Puckett W R, Hiester E D. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv Neurol. 1997;72:305–315. [PubMed] [Google Scholar]
- 48.Liverman C T, Altevogt B M, Joy J E, Washington, DC: The National Academies Press; 2005. Spinal Cord Injury: Progress, Promise and Priorities. [Google Scholar]


