Abstract
Context
Autism is an etiologically heterogeneous neurodevelopmental disorder for which there is no known unifying etiology or pathogenesis. Many conditions of atypical development can lead to autism, including fragile X syndrome (FXS), which is presently the most common known single gene cause of autism.
Objective
To examine whole-brain morphometric patterns that discriminate young boys with FXS from those with idiopathic autism (iAUT), as well as control participants.
Design
Cross sectional, in-vivo neuroimaging study.
Setting
Academic medical centers.
Patients
Young boys (n=165, 1.57-4.15 years) diagnosed as FXS or iAUT as well as typically developing (TD) and idiopathic developmentally delayed (DD) controls.
Main Outcome measures
Univariate voxel-based morphometric (VBM) analyses, VBM multivariate pattern classification (linear support vector machine) and clustering analyses (self organizing map).
Results
We found that frontal and temporal grey and white matter regions often implicated in social cognition, including the medial prefrontal cortex, orbitofrontal cortex, superior temporal region, temporal pole, amygdala, insula, and dorsal cingulum, were aberrant in FXS and iAUT as compared to controls. However, these differences were in opposite directions for FXS and iAUT relative to controls; in general, greater volume was seen in iAUT compared to controls, who in turn had greater volume than FXS. Multivariate analysis showed that the overall pattern of brain structure in iAUT generally resembled that of the controls more than FXS, both with and without AUT (FXS+A, FXS-A, respectively).
Conclusions
Our findings demonstrate that FXS and iAUT are associated with distinct neuroanatomical patterns, and further underscores the neurobiological heterogeneity of iAUT.
INTRODUCTION
Currently, lists of inclusion and exclusion criteria form the basis of all DSM-based diagnoses. One prevalent developmental disorder, autism, is characterized by a suite of altered behaviors, including difficulties with social interactions and impairments in language, as well as repetitive and restrictive interests 1. Interestingly, many individuals with fragile X syndrome (FXS), a condition arising from mutations of a specific gene on the X-chromosome, also exhibit behaviors on the autism spectrum, making FXS the most common known single-gene cause of autism. Because of the broad similarity in behavioral phenotype, researchers have hoped that a characterization of the morphological brain changes in FXS may lead to a helpful neuroanatomical model for idiopathic autism (iAUT) as well. However, aberrant behaviors are likely the result of a complex interplay of brain changes, and the correspondence between behavior and brain change may not necessarily be one-to-one. That is, a behavior that looks similar to an outside observer may potentially be caused by any of a number of different brain states. In a similar vein, there is still little evidence supporting the idea that the similarly aberrant behaviors exhibited by those with FXS and iAUT are the result of similar brain changes. Thus, it is possible that the behaviors exhibited by FXS and iAUT, though similar on the surface, are the result of differing morophological brain changes. Though we are operating within the framework outlined above, please note that the utility and validity of the diagnostic taxonomy of autism, and the (dis)similarities between symptoms of autism seen in FXS and iAUT are currently a topic of active discussion 2.
Two recent studies have directly compared the brains of individuals with FXS and iAUT. One study performed by our group examined grey matter volumes (GMV) of a small number of a priori selected subcortical and mesial temporal brain regions of interest (ROIs) in the same sample as our current study: a large sample of very young boys with FXS and iAUT, as well as typically developing (TD) boys, and those with idiopathic developmental delay (DD). This previous study found that the amygdale-caudate profile distinguished individuals with iAUT from those with FXS (both with and without AUT). Specifically, those with iAUT were found to have a larger amygdala, while FXS exhibited a larger caudate 3. In another study, voxel-based morphometry (VBM) of GMV was performed in a small number of FXS, iAUT and TD control adults (total n = 30) 4. FXS, compared to iAUT and controls, exhibited greater dorsolateral prefrontal cortex (DLPFC) and caudate volumes, and reduced volumes in the left postcentral, middle temporal and right fusiform gyri. As compared to FXS and controls, iAUT had smaller cerebellar volumes.
Though these results are intriguing, the current study extends the previous findings in four novel ways: 1) we examine both GMV and white matter volume (WMV) in a large number of very young children with FXS, iAUT, TD and DD children, which is important as WM differences are thought to play an important role in AUT 5, 2) we examine the whole brain, relative to previous studies that have typically restricted their analyses using volumetric measures or small volume correction (SVC) to a priori hypothesized regions, 3) we examine morphometric patterns in which FXS and iAUT are on opposite extremes of controls, i.e., FXS > controls > iAUT and iAUT > controls > FXS; findings from this analysis are particularly novel, as they demonstrate that diametrically differing neuroanatomical patterns can lead to similar symptoms, i.e., ‘two-sides of the same coin’ 3, and 4) we combine univariate VBM and multivariate supervised, as well as unsupervised, machine learning algorithms to identify fine-grained patterns that differentiate between groups 6, 7. We find that results from univariate and multivariate analyses are largely complementary; univariate analysis examines between-group differences in voxel intensities (volumes) one voxel at a time, whereas multivariate pattern classification analysis (MVPA) identifies patterns of voxel intensities that are different (or discriminate) between groups and does not require individual voxels to be different 8-10.
We hypothesized that if iAUT and FXS are indeed neuroanatomically distinct, as some studies are beginning to suggest, there should be little overlap in the abnormal brain morphometric patterns that distinguish iAUT or FXS from TD and DD controls, and the discrimination accuracy using MVPA between iAUT and FXS should be high. If on the other hand, FXS is a representative neuroanatomical model for iAUT, then discrimination between iAUT and FXS using morphometric pattern classification algorithms would be poor, and there should be considerable overlap in the spatial patterns of brain abnormalities found in both iAUT and FXS, as compared to TD and DD controls. Further, as is increasingly suggested by studies in myriad disciplines 11, iAUT may be comprised of many, currently unidentified, subgroups with diverse etiologies and disease pathways. If this is the case and iAUT is indeed etiologically heterogeneous, one may hypothesize that iAUT as a group will be more similar to TD and DD controls, also neurobiologically heterogenous as groups, as opposed to individuals comprising the FXS group who share the same genetic risk factor for aberrant neurodevelopment.
METHODS
Participants
Participants for this study were recruited by collaborating research teams at the Stanford University School of Medicine (SU) and the University of North Carolina-Chapel Hill (UNC). The study protocols were approved by the human subjects committees at SU and UNC, and consent was obtained. TD (n = 31, mean age 2.55 years + standard deviation 0.60) and DD (n = 19, 2.96 years + 0.50) children were recruited through local intervention programs, preschools, childcare facilities, community media, and state run agencies (e.g. Regional Center system in California, and Child Development Service Agencies in North Carolina). Children with FXS (n = 52, 2.90 years + 0.63) were recruited through registry databases maintained by Stanford and UNC, in addition to postings to the National Fragile X Foundation website and quarterly newsletter, and mailings to other regional fragile X organizations. Children with iAUT (n = 63, 2.77 years + 0.41) were recruited from clinics specializing in pervasive developmental disorders in North Carolina and community clinics and service agencies for the Stanford site (see Table 1, eTable 1 and eFigure 1 for profiles of demographics, cognitive abilities and brain tissue volumes). FXS and iAUT participants were tested with the Autism Diagnostic Interview Revised (ADI-R) 12 and Autism Diagnostic Observation Scale (ADOS) 13-15. Children were included in the iAUT group if they had received a clinical diagnosis of autism and met all criteria on the ADI-R and/or the ADOS-G. Participants were excluded from the study if they were born pre-term (< 34 weeks), had a low birth weight (< 2000 grams), showed evidence of a genetic condition or syndrome other than FXS, exhibited sensory impairments or had any serious medical or neurological condition that affected growth or development (e.g. seizure disorder, diabetes, congenital heart disease). Further, the FXS group was divided into subgroups based on their scores on the ADOS and ADI at the time of their scan. Those children who met full criteria for autism on both the ADOS and the ADI were placed in the FXS with autism (FXS+A) group. Children who did not meet full criteria on these two measures were placed in the FXS without autism (FXS-A) group. Details regarding demographic information and distribution of sample between recruitment sites can be found in Table 1 and eTable 2. There were no significant differences between sites in any of the cognitive measurements for each diagnostic group (all p’s > 0.05).
Table 1.
Demographic information. Abbreviations: TD = typically developing; DD = developmental delay of unknown origin; FXS = fragile X syndrome; iAUT = idiopathic autism.
| TD | DD | FX | iAUT | ANOVA | Post-Hoc | |||
|---|---|---|---|---|---|---|---|---|
| F | P | P | ||||||
| SU:UNC a | N | 11:20 | 11:8 | 28:24 | 17:46 | 11.32 | 0.01 | |
| Age | N | 31 | 19 | 52 | 63 | FX > TD*, DD > TD* |
||
| Mean | 2.55 | 2.96 | 2.90 | 2.77 | 3.65 | 0.014 | ||
| SD | 0.60 | 0.50 | 0.63 | 0.41 | ||||
| Mullen | N | 31 | 19 | 52 | 63 | FX < TD***, iAUT < TD***, DD < TD*** |
||
| Mean | 109.55 | 55.47 | 54.94 | 54.10 | 207.13 | <0.001 | ||
| SD | 17.24 | 7.53 | 9.14 | 9.41 | ||||
| RBS | N | 16 | 14 | 37 | 16 | FX > TD***, iAUT > TD***, > DD* |
||
| Mean | 3.13 | 13.07 | 18.70 | 26.25 | 12.69 | <0.001 | ||
| SD | 4.27 | 12.42 | 11.08 | 14.36 | ||||
|
ADI Rep
Total |
N | 50 | 63 | |||||
| Mean | 3.12 | 4.84 | 30.39 | <0.001 | ||||
| SD | 1.49 | 1.76 | ||||||
|
ADI Social
Total |
N | 50 | 63 | |||||
| Mean | 9.18 | 18.62 | 125.53 | <0.001 | ||||
| SD | 4.92 | 4.04 | ||||||
|
ADI Comm.
Verbal |
N | 5 | 5 | |||||
| Mean | 6.80 | 12.80 | 5.56 | <0.05 | ||||
| SD | 5.07 | 2.59 | ||||||
|
ADI Comm.
Nonverbal |
N | 45 | 58 | |||||
| Mean | 9.09 | 11.59 | 18.23 | <0.001 | ||||
| SD | 3.74 | 2.12 | ||||||
|
ADI Sum
[Verbal] |
N | 5 | 5 | |||||
| Mean | 18.20 | 35.60 | 11.09 | 0.01 | ||||
| SD | 10.92 | 4.16 | ||||||
|
ADI Sum
[Nonverbal] |
N | 44 | 58 | |||||
| Mean | 26.43 | 39.52 | 80.63 | <0.001 | ||||
| SD | 8.90 | 5.78 | ||||||
|
Adjusted
ADI Sum |
N | 49 | 63 | |||||
| Mean | 0.93 | 1.43 | 81.53 | <0.001 | ||||
| SD | 0.36 | 0.23 | ||||||
|
ADOS
Soc/Com Total |
N | 52 | 54 | |||||
| Mean | 10.19 | 18.00 | 80.05 | <0.001 | ||||
| SD | 5.71 | 2.87 | ||||||
|
ADOS
Severity Measure |
N | 52 | 53 | |||||
| Mean | 4.10 | 7.62 | 85.12 | <0.001 | ||||
| SD | 2.38 | 1.43 | ||||||
| FMRP | N | 50 | ||||||
| Mean | 5.83 | |||||||
| SD | 3.94 | |||||||
Mullen: Mullen Composite Standard Score, RBS: Repetitive Behavior Score Overall Total Score, ADI: Autism Diagnostic Interview, Sum of Socialization, Communication (Verbal or Non-verbal), ADOS: Autism Diagnostic Observation Scale, Repetitive and Stereotype
Pearson Chi-square performed. No significant difference in any of the measures between sites (SU and UNC) for each disgnostic group.
For further information regarding our methods related to genotyping, cognitive measures and neuropsychiatric assessments, as well as MRI scanning, preprocessing procedures and cross-site validation of imaging parameters, please see the associated Supplemental eText.
Univariate Analyses of MRIs Using Generalized Linear Models
Regional GMV and WMV differences between FXS, iAUT and controls (TD and DD combined) were examined using whole-brain ANCOVA, covarying out age, site and total GMV (TGMV) or TWMV for GM and WM analyses, respectively. We used two control groups as TD represents typical development and DD allows us to better match for overall cognitive functioning (i.e., lower overall IQ) as well as for the putative widespread neural effects associated with the presence of a significant developmental disorder. The two control samples (TD and DD) were initially grouped together because of the overall small N, and the results obtained were subsequently examined separately for TD and DD groups. The main analyses of interest were the comparisons between FXS and iAUT, FXS and controls, and iAUT and controls. In all VBM analyses, images were thresholded with a joint expected probability threshold of p < 0.01 (height) and p < 0.01 (family-wise error [FWE] corrected for spatial extent), corrected for non-stationary cluster extent threshold (non-isotropic smoothness) 16. Volumes of these significant regions were then extracted and compared separating TD and DD controls, and FXS-A and FXS+A children.
Images containing spatial information regarding significant regions were then combined to create overlap maps. These maps display voxels that illustrate relationships between groups, such as regions that significantly differentiate between FXS and TD/DD controls, as well as between FXS and iAUT. We also display maps that indicate differences between FXS and controls, as well as iAUT and controls. These regions were extracted individually and correlated with total Repetitive Behavior Scale17 (RBS), adjusted ADI sum (corrected for the number of items given to each child), ADOS composite score, ADOS severity score 13 and all ADI and ADOS subtests for FXS and iAUT separately.
Multivariate Pattern Classification Analyses of MRIs Using Linear SVM
We performed linear support vector machine (SVM) analyses in order to identify regions where spatially distributed patterns of GMV and WMV differences were particularly useful in discriminating between groups of participants (e.g. between FXS and iAUT brains) 9, 10. Linear SVM is a machine-learning approach that attempts to classify items (in this case, GMV and WMV maps) based on a linear separation in (highly) multi-dimensional space 8. The output of an SVM classification includes statistical measures of classification accuracy. In this manner, we can assess the differences/similarities of two groups of brains, based on how accurately/poorly they can be discriminated with SVM.
Before carrying out SVM analyses, each individual’s spatially normalized, modulated but unsmoothed GMV and WMV images were resampled to 4x4x4 mm voxels and converted to matrices followed by calculation of the residuals taking age, site and TGMV or TWMV into account and normalizing the matrix such that mean = 0, SD = 1. SVM analysis between FXS+A and iAUT was also performed on behavioral data alone to examine whether these two groups could be distinguished in this manner. Behavioral measures included all subtests and/or composite scores of ADI, ADOS, Mullen Scales of Early Learning 18, and Vineland Adaptive Behavioral Scales 19. Behavioral scores for these measures were available in most subjects. However, when needed, missing values were replaced by the mean average of their diagnostic group (data from 2 children with iAUT and none from FXS were missing for Vineland. See Table 1 for other measures.). When different modules were given and standardized scores were not available, adjusted scores were calculated correcting for the number of items. Wherever indicated in the results as ‘whole-brain SVM,’ principal components analysis (PCA) was performed to reduce the number of dimensions to N eigenvectors where N was the minimum number of components that accounted for at least 70% of the variance. On some occasions, feature reduction using recursive-feature elimination (RFE) 20, 21 was performed (indicated as ‘RFE-SVM’), where the bottom 30% of the voxels based on the absolute value of their weights were iteratively excluded until the performance started degrading.
The matrices with vectors for n-1 participants (i.e. all participants except for one, out of a matrix comprising two groups of participants) were input as a training dataset to train a linear support vector pattern classifier (with fixed regularization parameter C = 1) to correctly identify GMV, WMV or behavioral patterns of the nth participant. This process of training a classifier and testing on the nth subject was repeated n times until all participants were used as test-data once. Unbalanced sample-size for the classes was corrected using weighted SVM. Prediction accuracy, sensitivity, specificity and positive predictive values were calculated.
Analyses were performed with an in-house matlab-based (Mathworks, Natick, MA) MVPA toolbox, which adopted libsvm (Chih-Chung Chang and Chih-Jen Lin, LIBSVM: a library for support vector machines, 2001. Software available at http://www.csie.ntu.edu.tw/~cjlin/libsvm). SVM analyses were used to classify FXS from iAUT, FXS+A from iAUT, FXS from TD/DD, iAUT from TD/DD and TD from DD. In addition, we performed SVM analysis of FXS and TD/DD and applied the resulting classifier to iAUT to determine whether this group would appear more similar to TD/DD or FXS. Further, we repeated analyses including only brain voxels from the bilateral caudate and cerebellar vermis regions to determine if SVM classification would be altered when the only voxels used for classification were those from brain regions that have been reported to be morphometrically similar between FXS and iAUT 22. To perform this limited voxel analysis, we coregistered bilateral caudate and vermis regions from the Automated Anatomical Atlas Label 23 to the custom template and extracted GMV values from all subjects’ images as described above. Classification accuracies were statistically compared using permutation analyses (i.e., classes were randomly permuted and analyses were repeated 2000 times to obtain the distribution of data).
Finally, we used self organizing maps (SOM; from the Neural Network toolbox, Matlab R2009b) to visualize and convert complex relations between high-dimensional features (voxels) into simple geometric relations 21. The goal was to examine the brain-based representations of iAUT in relation to FXS and controls. The default setting was used to train a 2-by-2 two-dimensional map of 4 neurons (clusters). Prior to training, the number of features (voxels) was reduced using RFE-SVM 20, 21; this process selected voxels that jointly discriminated TD/DD and FXS; note that since the main goal of this analysis was to examine the spatial relationship between iAUT and other groups, this procedure does not bias the results.
RESULTS
Behavioral Results
Between FXS and iAUT (Table 1), the ADI and ADOS measures of social, communication and repetitive behavior indicated greater behavioral problems in iAUT as compared to FXS. However, repetitive behavior as measured by the RBS, and IQ were not significantly different between the two groups. When FXS+A and FXS-A were compared (eTable 1), all behavioral measures, including repetitive behavior and social and communication skills (but not IQ) showed significant between-group differences. As expected, FXS+A showed more severe problems in these domains than did FXS-A. Scores for FXS+A and iAUT (eTable 1) were fairly similar across domains. While ADI measures of social function were significantly more impaired in iAUT than in FXS+A, the ADOS social and communication scores and ADI communication measures were not significantly different between these groups. Repetitive behavior and IQ were also not significantly different between FXS+A and iAUT. See Table 1 and eTable 1 for details.
Univariate VBM Results
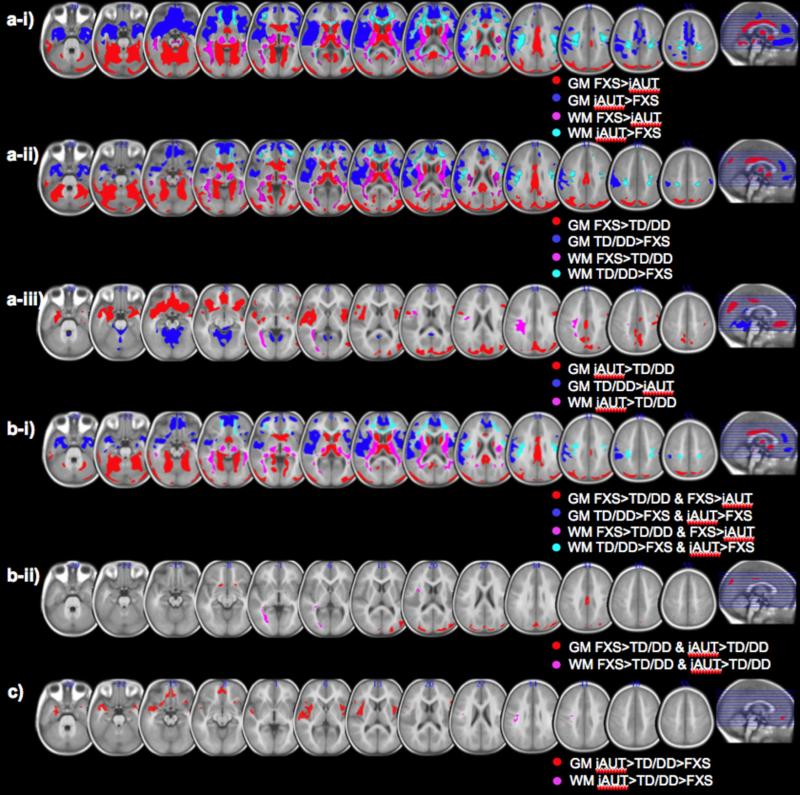
Between-group differences in regional GMV and WMV corrected for TGMV and TWMV, respectively, as well as age and site (SU and UNC), are reported in eTable 1 and eFigure 1 as well as in a series of figures (Figure 1, eFigures 2, 3 and 4) and tables (Tables 2 and 3): iAUT vs. TD/DD in Figure 1a-iii and eFigure 2, FXS vs. TD/DD in Figure 1a-ii and eFigure 3, and FXS vs. iAUT in Figure 1a-i and eFigure 4. Analyses contrasting FXS vs. iAUT as well as FXS vs. TD/DD show that the morphometric pattern that differentiates FXS from iAUT is qualitatively similar to the pattern that discriminates FXS from TD/DD controls (Figure 1b-i), implying similar morphometric brain structure across the iAUT and TD/DD groups. Regions comprising this morphometric pattern included significantly greater bilateral caudate, thalamus, hypothalamus, parieto-occipital, lingual / fusiform, cerebellar and cingulate GM and perisylvian and temporal WM regions, and significantly reduced orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), amygdala, insular and sylvian GM, and frontal and sensorimotor WM regions in FXS as compared to iAUT and to TD/DD controls.
Figure 1. Differences in regional brain volumes between groups.
a. Regions that show significant differences in regional grey matter volume (GMV) and white matter volume (WMV) between fragile X syndrome (FXS) and idiopathic autism (iAUT) (a-i), FXS and typically developing (TD) as well as idiopathic developmentally delayed (DD) controls (a-ii), and iAUT and TD/DD controls (a-iii). b-i. Brain regions that show similar regional brain volumes for iAUT and TD/DD controls compared to FXS. (GMV: red, WMV: violet), and for TD/DD and iAUT compared to FXS (GMV: blue, WMV: cyan). b-ii. Brain regions that show similar regional brain volumes for FXS and iAUT compared to TD/DD controls. (GMV: red, WMV: violet). Overlaid on custom T1 template. c. Brain regions that show opposite regional volume patterns for FXS and iAUT. Left side shows right hemisphere. Statistical threshold is set at p = 0.01 family-wise error (FWE) cluster-level corrected.
Table 2.
Grey matter regions that show significant between-group differences in the univariate VBM analyses. (Capital letters denote regions of interest depicted in eFigures 2ab, 3ab and 4ab).
| GRAY MATTER VOLUME | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | BA | Talairach Coordinates | T | P (corr) | Cluster | ||
| x | y | z | |||||
| iAUT >> TD/DD | |||||||
| A Bilateral cuneus, bilateral posterior cingulate gyrus (Parietal and Occipital lobes) |
19 | −19 | −85 | 34 | 4.57 | <0.001 | 21060 |
| 22 | −90 | 27 | 4.26 | ||||
| 2 | −23 | 41 | 4.19 | ||||
| B Bilateral right inferior frontal gyrus, superior temporal gyrus, parahippocampal gyrus, (Frontal, Temporal, Limbic lobes) |
38/47 | 23 | 21 | −16 | 4.55 | <0.001 | 48448 |
| 33 | 4 | −17 | 4.45 | ||||
| 32 | 15 | −10 | 4.2 | ||||
|
| |||||||
| TD/DD >> iAUT | |||||||
| C Bilateral cerebellar (culmen), fusiform and lingual gyri | 12 | −51 | −3 | 4.93 | <0.001 | 11630 | |
| −6 | −36 | −18 | 3.98 | ||||
| −13 | −51 | 0 | 3.89 | ||||
|
| |||||||
| FXS >> TD/DD | |||||||
| F Bilateral caudate body, bilateral anterior cingulate, bilateral middle cingulate, bilateral posterior cingulate |
15 | 1 | 20 | 10.78 | <0.001 | 161001 | |
| 12 | 7 | 15 | 10.57 | ||||
| −14 | 6 | 17 | 8.68 | ||||
|
| |||||||
| TD/DD >> FXS | |||||||
| G Right orbitofrontal cortex, insula, claustrum, superior parietal (Frontal, temporal and parietal lobe) |
13 | 39 | −3 | 19 | 8.06 | <0.001 | 98288 |
| 32 | 5 | 16 | 7.94 | ||||
| 29 | 15 | 10 | 7.89 | ||||
| H Left superior temporal, insula, superior parietal cortex (Frontal, temporal and parietal) |
13 | −40 | −3 | 20 | 7.77 | <0.001 | 52800 |
| −30 | 14 | 13 | 7.45 | ||||
| −31 | 7 | 16 | 7.26 | ||||
Table 3.
White matter regions that show significant between-group differences in the univariate VBM analyses. (Capital letters denote regions of interest depicted in eFigures 2c, 3cd and 4cd).
| WHITE MATTER VOLUME | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | BA | Talairach Coordinates | T | P (corr) | Cluster | ||
| x | y | z | |||||
| iAUT >> TD/DD | |||||||
| D Region near right middle occipital gyrus | 35 | −54 | 3 | 4.09 | <0.01 | 4033 | |
| 26 | −79 | 9 | 3.79 | ||||
| 38 | −43 | 5 | 3.65 | ||||
| E Region near right frontal lobe and right insula | 38 | 1 | 21 | 4.11 | <0.01 | 6197 | |
| 40 | −25 | 33 | 3.52 | ||||
| 24 | −36 | 31 | 3.27 | ||||
| * Also found left ROIs mirroring each of these, that failed to reach sig at p=0.03 |
|||||||
|
| |||||||
| TD/DD >> iAUT | |||||||
| n.s. | |||||||
|
| |||||||
| FXS >> TD/DD | |||||||
| I Region near left superior temporal gyrus and left insula | −30 | 11 | 14 | 7.8 | <0.001 | 23461 | |
| −38 | −3 | 21 | 7.3 | ||||
| −40 | −51 | 23 | 7.11 | ||||
| J Region near right frontal lobe, right insula and right medial frontal |
31 | 6 | 15 | 8.87 | <0.001 | 26926 | |
| 29 | 13 | 11 | 8.64 | ||||
| 32 | −13 | 18 | 6.86 | ||||
|
| |||||||
| TD/DD >> FXS | |||||||
| K Region near left frontal white matter (near superior frontal gyrus), left basal ganglia (caudate, putamen) |
−22 | 18 | 11 | 6.78 | <0.001 | 9625 | |
| −18 | 21 | −4 | 6.66 | ||||
| −21 | 12 | 17 | 6.12 | ||||
| L Region near left precentral gyrus and left postcentral gyrus |
−31 | −29 | 51 | 6.27 | <0.01 | 4162 | |
| −13 | −17 | 58 | 5.43 | ||||
| −14 | −3 | 58 | 4.68 | ||||
| M Region near left precentral gyrus and inferior frontal gyrus |
−43 | −3 | 27 | 7.98 | =0.001 | 5554 | |
| −33 | −13 | 44 | 4.46 | ||||
| −48 | −16 | 34 | 4.25 | ||||
| N Region near right medial frontal gyrus, right superior frontal gyrus, and right anterior cingulate gyrus |
24 | 42 | 10 | 5.12 | <0.01 | 4675 | |
| 17 | 59 | 5 | 5.07 | ||||
| 19 | 42 | −8 | 5.01 | ||||
| O Region near right precentral gyrus and inferior frontal gyrus |
47 | −3 | 26 | 7.62 | <0.001 | 11828 | |
| 30 | −30 | 50 | 6.42 | ||||
| 34 | −6 | 37 | 6.33 | ||||
While some brain regions showed significant differences in regional volumes between iAUT and TD/DD, these differences were primarily driven by dissimilarity between iAUT and TD rather than between iAUT and DD (eFigure 2d,e). This is in contrast to brain regions that showed significantly different GMV and WMV between FXS and TD/DD, where FXS was significantly different from both TD and DD groups (eFigure 3e,f). Brain regions differentiating iAUT from TD/DD included significantly greater OFC, mPFC, amygdala, insula, inferior frontal, parahippocampal, superior temporal sulcus (STS), temporal pole (TP), parieto-occipital and right temporo-parietal GM regions and frontal, sensorimotor and temporal WM regions, and significantly reduced cerebellar and occipital GM regions for iAUT. Notably, there were several brain regions that showed FXS and iAUT to be on the opposite extreme relative to controls; i.e., significantly reduced in FXS and increased in iAUT compared to controls including bilateral STS, TP, OFC, mPFC, amygdala, insula and dorsal cingulum (Figure 1c).
We also examined a subset of FXS children with a diagnosis of autism (FXS+A) (eFigure 2d,e, eFigure 3e,f, eFigure 4e,f). The pattern of differences between FXS and iAUT (i.e., brain regions that showed and did not show significant differences between these groups) did not change when FXS+A was compared to iAUT (except for the right dorsal WM in eFigure 2c, ROI e, see eFigure 2e). Finally, we performed regression analyses between the regions detected in these univariate analyses and all domain and total scores listed in Table 1. There were no significant correlations (Bonferroni corrected).
Multivariate Pattern Classification
Support vector machine (SVM) analysis
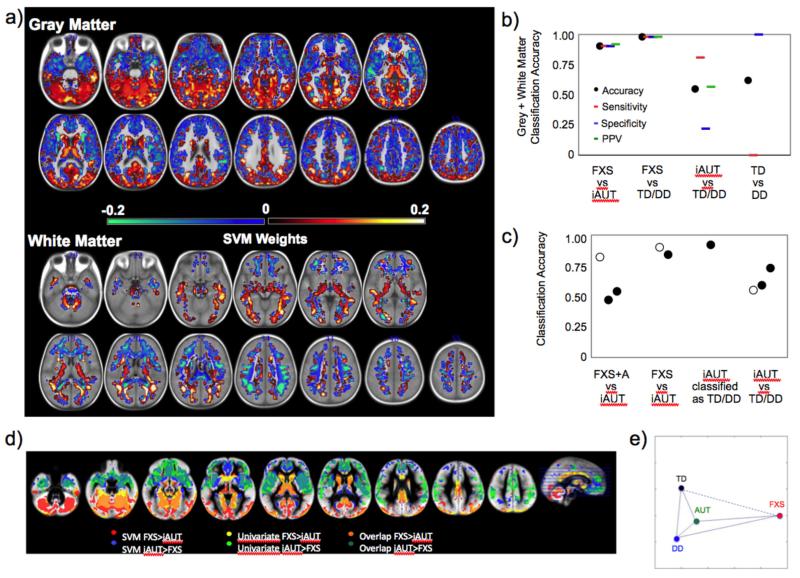
We used a linear SVM algorithm with a leave-one-out cross-validation procedure to examine how accurately the four participant groups could be distinguished based on spatial patterns of brain morphometry (Figure 2). Results using GM voxels only, WM voxels only and GM and WM voxels combined were very similar and not significantly different from each other, therefore the results from GM+WM are reported here. Discriminability between FXS and iAUT was high using whole-brain SVM (accuracy = 90%). Maps derived from univariate and multivariate analyses showed similar patterns for both approaches (Figure 2d). These results indicate that the brains of individuals with FXS and iAUT exhibit dissociable morphometric features in both GM and WM. Even when a subset of FXS individuals who met criteria for autism (FXS+A; see eTable 2 for demographics) was compared with iAUT, the classification accuracy remained high with whole-brain SVM (82%) and significantly greater than chance (p < 0.001), and not-significantly different between the FXS (entire group) vs. iAUT classification (p > 0.1). High discrimination accuracy between FXS and iAUT was observed despite low and nonsignificant classification accuracy between these two groups using all available behavioral measures (47%, p > 0.1; using RFE-SVM 54%, p > 0.1).
Figure 2. Pattern classification results.
a. Whole-brain representation of pattern classification results from FXS vs. iAUT using GM and WM voxels. Warm colors represent voxels with positive weight for the classification FXS vs. iAUT (FXS > iAUT) and cool colors represent negative weights (iAUT > FXS). Left side shows right hemisphere. b. Support vector machine (SVM) between-group classification accuracy using a combination of all GM and WM as features. Black dots indicate accuracy, red dashes indicate sensitivity, blue dashes indicate specificity and green dashes indicate positive predictive value (PPV). c. SVM classification accuracy for various control analyses. For FXS+A vs iAUT we show accuracy for whole brain with dimensionality reduction using PCA (open circle), all behavior (first filled circle), and behavior using recursive feature elimination (RFE) (second filled circle). For FXS vs iAUT we show accuracy for whole brain with dimensionality reduction using PCA (open circle), and using only the caudate and cerebellar vermis as features (filled circle). For iAUT classified as TD/DD using the classifier from FXS vs TD/DD we show accuracy for whole brain with dimensionality reduction using PCA (filled circle). For iAUT vs TD/DD we show accuracy for whole brain with dimensionality reduction using PCA (open circle), only those areas significant in univariate VBM analyses (first filled circle), and whole brain using RFE (second filled circle). d. Overlay of univariate and SVM analyses from the FXS vs. iAUT contrast for GM (SVM weights thresholded based on p = 0.05 permutation-based correction). e. Brain-based representations of the four groups (TD, DD, FXS and iAUT) using a self-organizing map (SOM). Solid lines: Euclidian distance > 1.
We also performed SVM classification using only brain regions that have been reported to show similar morphometric abnormalities in FXS and some studies of iAUT (i.e., the caudate and cerebellar vermis 22). This analysis should maximize similarities between FXS and iAUT, thereby minimizing the ability to distinguish between the two groups. However, even using this subset of brain regions, classification accuracy between FXS and iAUT remained quite high (84%), and was not significantly different from FXS vs. TD/DD classification (87%, p > 0.1; note that classification accuracy was 98% between FXS and TD/DD controls when the whole brain was used; also reported in 7). In contrast, classification accuracy between the two control groups (TD vs. DD) was low (62% accuracy using whole brain; p > 0.1).
Further, when the classifier (model) derived from the FXS vs. TD/DD classification was applied to iAUT, 92% of the children were classified as TD/DD controls, suggesting that the brain regions that best distinguish FXS from TD/DD can also be used to reliably distinguish FXS from iAUT. In other words, these multivariate analysis techniques demonstrate that, as compared to controls, young boys with FXS represent a more unique and homogeneous group with respect to neuroanatomy than do boys with iAUT.
Classification accuracy discriminating iAUT from the TD/DD group using whole-brain SVM was 55% (not significantly greater than chance, p > 0.1). Even when restricting the voxels to those that were significant from univariate analysis, classification analysis was 59% (not significantly greater than chance, p > 0.1). When RFE-SVM was performed, classification accuracy between iAUT and TD/DD improved from 55% to 73% (significantly greater than chance, p < 0.001). However, this accuracy was still significantly lower than that derived from the FXS vs. iAUT or FXS vs. TD/DD classification analyses, p’s < 0.001). This finding implies that the joint information carried by a small number of voxels (20,224mm3) rather than information from the whole-brain can discriminate iAUT vs. controls (though, in this case, the performance of the classifier is less accurate than that derived for FXS vs. controls).
Self-organizing maps (SOM) analysis
To further visualize the relation between the discriminative patterns characterizing the four groups (TD, DD, iAUT and FXS), we used a technique known as SOM, which converts complex relations between high-dimensional items into simple geometric relations, adopting the method used by Formisano and colleagues 21 (Figure 2e). This brain-based representation also demonstrates the relative neuroanatomical resemblance (proximity) of iAUT to TD and DD, as compared to FXS.
COMMENT
We examined neuroanatomical profiles of boys between the ages of 1-4 years old, diagnosed with iAUT and FXS, two neurodevelopmental disorders that have, at the descriptive level, overlapping behavioral phenotypes. However, iAUT is an etiologically heterogeneous and behaviorally defined neurodevelopmental disorder that involves deficits in social interaction and communication as well as rigid and repetitive patterns of behavior. FXS, on the other hand, is a specific, genetically-defined disorder caused by the silencing of the fragile X mental retardation 1 (FMR1) gene 22. Many of the traits observed in those with FXS overlap with symptoms of iAUT, such as poor social interaction, qualitative abnormalities of communication and stereotyped behavior; researchers have estimated that autistic spectrum disorders (ASDs) can be diagnosed in as many as 60% of those with FXS 22, 24. The overlap in behavioral/cognitive symptoms reported in some studies has motivated some researchers to suggest overlapping neurobiological mechanisms underlying these two disorders 22, 24. Indeed, prior research has suggested that there may be similar morphometric brain abnormalities in the caudate, the posterior vermis of the cerebellum, 22 and in the connectivity between frontal and anterior temporal regions and their long-distance reciprocal and parietal connections 5.
In this study, we show novel evidence that voxel-by-voxel brain volumes of boys with FXS and iAUT are on opposite extremes relative to controls for some GM and WM regions. Further, we demonstrate that morphometric spatial patterns are significantly different between FXS (and FXS+A) and iAUT, even at this very young age, using both univariate analysis as well as supervised and unsupervised machine learning methods. These distinct neuroanatomical patterns are present even though multivariate pattern classification analysis using diagnostic-behavioral data could not differentiate between FXS+A and iAUT. Another recent study 25 also found neuroanatomical differences between AUT and FXS+A even though the two groups were behaviorally indistinguishable. Specifically, the group with AUT was found to have thinner cortex in the left ACC and bilateral medial PFC, as compared to the group with FXS+A.
Several frontal and temporal GM and WM regions, including the mPFC, OFC, STS, and TP, as well as subcortical structures such as the amygdala, insula and dorsal cingulum, showed patterns of volumetric differences that were on the opposite extremes for FXS (and FXS+A) and iAUT relative to controls, such that iAUT > controls > FXS (Figure 1c). This is somewhat different from the findings of our previous ROI-based volumetric study in the same population, in which we found greater amygdala volume in iAUT relative to both controls and FXS, but no difference between controls and FXS 3. In this previous study, we also found that caudate volume was increased in both FXS and iAUT compared to controls. Another study that examined VBM GM and conjunction analysis found regions where iAUT (or FXS) volumes were significantly different from both FXS (or iAUT) and control adults 4. Thus, no previous studies have observed brain regions that show a pattern in which FXS and iAUT lie on opposite extremes relative to controls. This new finding is quite interesting, as it suggests that these two disorders are, neuroanatomically, ‘two-sides of the same coin’ 3 for some brain regions,
Using multivariate pattern classification analyses of GM and WM, our results show that at least at this young age, the abnormal spatial patterns found in iAUT and FXS (and FXS+A) are strikingly distinct from one another. This was true even when we consider brain regions (caudate and cerebellar vermis) that have been proposed to be similarly aberrant for both disorders, and when we considered only FXS+A. It is interesting that despite the robust classification power of MVPA for neuroanatomical data, FXS+A could not be distinguished from iAUT using multivariate approaches of behavioral data. Those with FXS (and FXS+A) exhibit much more obvious brain differences from our control groups than did those with iAUT. This was evidenced by significantly stronger classification accuracy between FXS (and FXS+A) and controls compared to iAUT and controls, and relatively weaker statistical difference between iAUT and controls as compared to FXS and controls.
While univariate analysis revealed several brain regions that were significantly different between iAUT and controls, our whole-brain SVM could not reliably differentiate between iAUT and controls. However, even when SVM was restricted to voxels/features that showed significant effects in univariate analysis, classification accuracy remained relatively low. These results suggest that morphometric patterns have very little discriminative power between iAUT and controls.
It is possible that particular neuroanatomical differences shared by FXS and iAUT are related to specific aberrant behaviors exhibited by both of these groups. For example, in adults with ASD, neuroimaging data indicate that particular brain regions, including the mPFC, temporo-parietal junction (TPJ), STS and TP, may be linked to deficits in social cognition 11, 26, 27. The fronto-insular cortex (FI; right > left) and anterior cingulate cortex (ACC) are thought to be involved in intuitive judgments required by complex situations such as social interactions, and have been suggested to play a critical role in ASD 28. The caudate and cerebellar vermis on the other hand, may be correlated with repetitive behavior symptoms 29-31. Supplementary correlation analyses with social, communication, language and repetitive behavior, and regional GMV and WMV identified from univariate analyses did not show significant correlations in our sample of FXS or iAUT. Just as multivariate analyses, such as SVM, may be more accurate group classifiers, future studies using multivariate regression analyses to detect brain-behavior associations, such as LASSO and Support Vector Regression 32, may find these techniques to be more sensitive to the morphometric patterns that characterize specific behavioral phenotypes.
Interestingly, our results revealed that frontal and temporal regions implicated in social cognition, specifically the mPFC/ACC, FI, STS, and TP, as well as the amygdala, do show divergent patterns of abnormality in iAUT versus the patterns observed in our groups of FXS or FXS+A; that is, these social processing regions are significantly larger in iAUT and are smaller in FXS, when compared to TD/DD controls (Figure 1). This dissociation was also observed in the dorsal frontoparietal white matter tracts, which is interesting in light of the developmental disconnection hypothesis of autism 5. These findings may partly explain recent evidence suggesting that the profile of social and communicative symptomatology in FXS and iAUT are different 2 and do not support the hypothesis that overlapping neurobiological mechanisms underlie these two disorders. While beyond the scope of the current paper, the dynamic nature of classification systems for autism over time (e.g. 33) may also be a confounding factor in comparing iAUT to other developmental disorders such as FXS.
While the results in the current study were quite striking, there are important limitations that should be addressed in future investigations. For example, measures such as ADI and ADOS are optimized to identify individuals with iAUT and may not be optimal to use in specific, more homogeneous populations such as FXS. Further, examination of more specific behavioral phenotypes, such as social cognition, may be more fruitful in pursuing this line of research. Finally, additional studies are needed to compare and contrast the trajectories of cognitive and behavioral development in children with FXS and iAUT. Such studies should relate these trajectories to profiles of neuroanatomical development in order to better model brain-behavior relationships associated with age of onset of symptoms, the occurrence of developmental regression, and social developmental milestones.
The results of the current study, generated with both univariate VBM and multivariate SVM techniques, suggest that iAUT and FXS exhibit distinct neuroanatomical profiles relative to one another. Our results also indicate that iAUT is more likely to exhibit patterns similar to controls, likely due to the neurobiological heterogeneity of these groups. That is, individuals are defined as being TD, DD or iAUT based on behavioral measures, whereas a diagnosis of FXS is established via a specific genetic difference shared by all members of the FXS group. It has been suggested that various ASD-associated genetic syndromes such as FXS, Angelman syndrome and Rett syndrome, may converge on common biological pathways or brain circuits that give rise to ASD 34. However, our analyses of high resolution imaging data from male toddlers with FXS and iAUT showed striking differences in brain morphometry at a very early age, even though we restricted our sample to males only, and repeated our analyses using a subset of FXS participants who met the behavioral criteria for autism (FXS+A) in order to increase phenotypic similarity between our FXS and iAUT groups. It may be useful in the future to contrast individuals with homogeneous genetic conditions with and without ASD-like behavioral features (e.g. FXS+A vs. FXS children without AUT, FXS-A). Though significant differences were not found between FXS+A and FXS-A in the present study (except for autistic symptoms), significant differences may be found within other ASD-associated genetic disorders.
On a related note, it may also be interesting to examine the detailed genetic, cognitive and environmental profiles of children with FXS (or FXS+A) who were misclassified as iAUT (or vice versa) based on structural MRI, a quantitative endophenotype (eTable 3). The 12 individuals who were misclassified in our dataset did not exhibit any notable demographic or behavioral characteristics that distinguished them from other FXS using univariate analysis and none of the misclassified FXS had a diagnosis of AUT (i.e., none of the misclassified FXS were FXS+A). Nonetheless, with a larger sample and detailed multivariate analyses of demographic, behavior, genetic make-up and behavior changes over time, this route may provide invaluable information for future targets for iAUT research.
We demonstrate that FXS and iAUT are expressed as differing morphometric brain patterns. Further, this study has yielded intriguing evidence of the early brain phenotype in FXS. Our data may provide important clues regarding the altered neurodevelopmental pathways created by chronic diminished expression of the FMR1 gene from a very early age. This work is particularly important for allowing researchers to establish a specific disease template in young humans in a manner comparable to research being performed in animal models of this disease (e.g. fly, mouse). The creation of an early and accurate human brain phenotype for FXS in humans will significantly improve our capability to detect whether new disease-specific treatments can significantly alter the FXS phenotype in affected individuals.
Supplementary Material
ACKNOWLEDGMENT
This study was funded by MH64708 (A.L.R., J.P.), MH61696 (J.P.), HD03110-36 (J.P.), MH050047 (A.L.R.), and the Canel Family fund. F.H. was funded by NARSAD Young Investigator Award, the Stanford Child Health Spectrum and NICHD HD054720. We sincerely thank all of the families and the Department of Radiology staff and faculty of the Lucile Packard Children’s Hospital at Stanford who made the study possible. Chad Chappell, MA, Nancy Garrett, BA, Michael Graves, MChe, Cindy Hagan, BA, Cindy Johnston, MS, Arianna Martin, BA, Matthew Mosconi, PhD, Rachel Smith, BA, Cristiana Vattuone, BA, Christa Watson, BA, and Anh Weber, PhD, were involved in data collection.
REFERENCES
- 1.Association AP. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Fourth Edition (Text Revision) Author; Washington DC: 2000. [Google Scholar]
- 2.Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in Fragile X Syndrome: A Category Mistake? J Am Acad Child Adolesc Psychiatry. 2010 Sep;49(9):921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazlett HC, Poe M, Lightbody AA, et al. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndome and autism. Jourlan of Neurodevelopmental Disorders. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson LB, Tregellas JR, Hagerman RJ, Rogers SJ, Rojas DC. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Res. 2009 Nov 30;174(2):138–145. doi: 10.1016/j.pscychresns.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007 Feb;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Ecker C, Rocha-Rego V, Johnston P, et al. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage. 2010 Jan 1;49(1):44–56. doi: 10.1016/j.neuroimage.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008 Sep;65(9):1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burges C. A tutorial on support vector machines for pattern recognition. Data Min. Knowl. Discov. 1998;2(2):144–152. [Google Scholar]
- 9.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat Rev Neurosci. 2006 Jul;7(7):523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 10.Mourao-Miranda J, Reynaud E, McGlone F, Calvert G, Brammer M. The impact of temporal compression and space selection on SVM analysis of single-subject and multi-subject fMRI data. Neuroimage. 2006 Dec;33(4):1055–1065. doi: 10.1016/j.neuroimage.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006 Oct;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 12.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994 Oct;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 13.Gotham K, Pickles A, Lord C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. J Autism Dev Disord. 2008 Dec 12; doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000 Jun;30(3):205–223. [PubMed] [Google Scholar]
- 15.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule-WPS (ADOS-WPS) Western Psychological Services; Los Angeles, CA: 1999. [Google Scholar]
- 16.Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004 Jun;22(2):676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007 May;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- 18.Mullen EM. Mullen Scales of Early Learning AGS Edition. American Guidance Service, Inc.; Circle Pines, MN: 1995. [Google Scholar]
- 19.Sparrow SS, Balla DA, Cicche HV. Vineland Adaptive Behavior Scales-Interview Edition Survey Form Manual. American Guidance Service, Inc.; Circle Pines: 1984. [Google Scholar]
- 20.De Martino F, Valente G, Staeren N, Ashburner J, Goebel R, Formisano E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage. 2008 Oct 15;43(1):44–58. doi: 10.1016/j.neuroimage.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 21.Formisano E, De Martino F, Bonte M, Goebel R. “Who” is saying “what”? Brain-based decoding of human voice and speech. Science. 2008 Nov 7;322(5903):970–973. doi: 10.1126/science.1164318. [DOI] [PubMed] [Google Scholar]
- 22.Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006 Oct;9(10):1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- 23.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 24.Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr. 2006 Feb;27(1):63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Meguid N, Fahim C, Yoon U, et al. Brain morphology in autism and fragile X syndrome correlates with social IQ: first report from the Canadian-Swiss-Egyptian Neurodevelopmental Study. J Child Neurol. 2010 May;25(5):599–608. doi: 10.1177/0883073809341670. [DOI] [PubMed] [Google Scholar]
- 26.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008 Mar;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006 Apr;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 28.Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005 Aug;9(8):367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007 Aug 1;62(3):262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 30.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001 Apr 15;49(8):655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 31.Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruitet J, McFarland DJ, Wolpaw JR. A comparison of regression techniques for a two-dimensional sensorimotor rhythm-based brain-computer interface. J Neural Eng. 2010 Feb;7(1):16003. doi: 10.1088/1741-2560/7/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels AM, Rosenberg RE, Law JK, Lord C, Kaufmann WE, Law PA. Stability of Initial Autism Spectrum Disorder Diagnoses in Community Settings. J Autism Dev Disord. 2010 May 15; doi: 10.1007/s10803-010-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008 May;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.