Abstract
Objective
This study modeled the predictive power of unit/patient characteristics, nurse workload, nurse expertise, and hospital-acquired pressure ulcer (HAPU) preventive clinical processes of care on unit-level prevalence of HAPUs.
Data Sources
Seven hundred and eighty-nine medical-surgical units (215 hospitals) in 2009.
Study Design
Using unit-level data, HAPUs were modeled with Poisson regression with zero-inflation (due to low prevalence of HAPUs) with significant covariates as predictors.
Data Collection/Extraction Methods
Hospitals submitted data on NQF endorsed ongoing performance measures to CALNOC registry.
Principal Findings
Fewer HAPUs were predicted by a combination of unit/patient characteristics (shorter length of stay, fewer patients at-risk, fewer male patients), RN workload (more hours of care, greater patient [bed] turnover), RN expertise (more years of experience, fewer contract staff hours), and processes of care (more risk assessment completed).
Conclusions
Unit/patient characteristics were potent HAPU predictors yet generally are not modifiable. RN workload, nurse expertise, and processes of care (risk assessment/interventions) are significant predictors that can be addressed to reduce HAPU. Support strategies may be needed for units where experienced full-time nurses are not available for HAPU prevention. Further research is warranted to test these finding in the context of higher HAPU prevalence.
Keywords: Nursing, quality of care/patient safety (measurement), acute inpatient care, modeling
Hospital acquired pressure ulcers (HAPUs) remain a serious iatrogenic problem threatening patient safety and increasing hospital costs. The Centers for Medicaid and Medicare Services (CMS) elimination of reimbursement for treatment of Stage III and IV HAPUs as hospital-acquired conditions has challenged hospitals to reduce HAPUs, while quality organizations such as the National Quality Forum (NQF) have deemed Stage III and IV HAPUs among the “Never 27” adverse events (Wachter, Foster, and Dudley 2008). State-mandated pressure ulcer reporting of Stage III or IV HAPUs also emphasizes policy makers' interest in HAPUs (Alquist 2006; California Senate Bill 1301 2006). In response, hospitals are examining processes of care to ensure that early skin assessment and pressure ulcer risk screening are performed to confirm that pressure ulcers present on admission are not reported as hospital acquired and that high-risk patients receive early intervention.
HAPUs are nursing-sensitive indicators of patient care quality (National Quality Forum 2004; The Joint Commission [TJC] 2009), and nurses are pivotal to prevention and amelioration. California's mandated nurse staffing ratios reduced the number of patients assigned per licensed nurse, and increased the nursing hours per patient day (HPPD) (Burnes Bolton, Aydin et al. 2007), yet to date, mandated nurse–patient ratios have produced inconsistent HAPU results (Donaldson and Shapiro 2010; Cook et al. 2012). HAPU prevalence in U.S. acute care hospitals ranges from zero (Bales 2009) to 15.8 percent (Jenkins 2010), but limited data are available on how lower rates are achieved (National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel [NPUAP/EPUAP] 2009; Preventing Pressure Ulcers in Hospitals 2011).
Conceptually, fewer HAPUs should occur when nurses provide more hours of care, are full-time employees of the hospital, have higher education, are certified in their field, and have more years of practice. Studies have documented the positive impact of higher nursing education, expertise, and full-time employment on other patient outcomes (Estabrooks et al. 2010; Aiken et al. 2011; Manojlovich et al. 2011).
The relationship between patients and direct care staff who impact HAPUs is, however, imprecise, conceptually problematic, and operationally difficult (Clarke and Donaldson 2008). The inconsistent relationships of these variables to HAPU prevalence may be a reflection of data source variation (Aiken, Clarke, and Sloane 2002; Cho et al. 2003; Unruh 2003; Burnes Bolton, Donaldson et al. 2007; Cook et al. 2012; Blegen et al. 2013). Classic quality studies have used large hospital-wide administrative datasets (American Nurses Association [ANA] 1997; Kovner and Gergen 1998; ANA 2000; Aiken, Clarke, and Sloane 2002; Aiken et al. 2002; Needleman et al. 2002). While nurses are employed by hospitals, they practice in individual patient care units where staffing, workload, and environment vary widely (Mark et al. 2004; Duffield et al. 2009). Researchers recommend unit-level analyses, rather than hospital, to understand nursing's impact on patient outcomes (Mark et al. 2004; Lake and Cheung 2006; Duffield et al. 2009).
Thus, the present study was funded to develop a predictive model for acute care medical-surgical units addressing effects of unit-level nurse workload, staff nurse characteristics, and selected risk assessment and intervention processes of care on nursing sensitive patient outcomes, including HAPUs (Donaldson, Aydin, and Fridman 2010). Clarke and Donaldson (2008) provided a general conceptual model, positing that quality and safety outcomes are predicted by a combination of patient population needs, staff workload and qualifications, and the quantity and quality of nursing care provided. A comprehensive set of variables that capture national voluntary consensus standards for nursing sensitive care (National Quality Forum 2004; TJC 2009) and robust analytic procedures were combined to identify predictors of HAPU prevention at the unit level to identify factors that could be leveraged to reduce HAPUs.
Aim
This study modeled the predictive power of measures representing patient characteristics, nurse workload, nurse expertise, and HAPU preventive clinical processes of care on HAPU prevalence.
Methods
Sample
The study was conducted using data from CALNOC, a nursing-sensitive benchmarking registry with an established history of quality measurement and reporting (Aydin et al. 2004, 2008). The convenience sample included 789 medical-surgical units from 215 hospitals. Hospital demographics were generally representative of acute care hospitals in this geographic area, although for-profit hospitals were slightly underrepresented. Eighty-five percent of the hospitals were in California, with 15 percent in Washington and Oregon. With the exception of registered nurse (RN) education data, all CALNOC hospitals regularly submit data for benchmarking on variables included in the models. RN education variables were obtained using CALNOC's RN Education Survey. The grant funded recruitment of CALNOC hospitals to survey their nurses in addition to ongoing data submissions. Nurses from 144 units from 45 hospitals completed the RN survey between January 2009 and April 2010.
Measures
Four sets of unit-level predictor variables were studied: unit/patient characteristics, nurse workload, RN expertise, and HAPU clinical processes of care. Variables are listed in Table1. Outcome variables were any stage of HAPU and stage II or greater (HAPU-II+). Unit/patient characteristics were aggregated from patients in the study. Variables included number of patients; mean patient age; percent male or female patients; percent medical or surgical patients; average length of stay (LOS); and percent of patients at-risk for pressure ulcers. The denominator for percentages was all patients included in the prevalence study. Nurse workload variables were calculated from productive hours worked by nurses with direct patient care responsibilities, excluding education, sick leave, and vacations. Patient days included midnight census (or equivalent) plus hours for short-stay patients divided by 24. Total hours of nursing care per patient day (HPPD) included total hours (RN + Licensed Vocational Nurses [LVN] + unlicensed) and licensed hours (RN + LVN). Skill mix included the number of RN, LVN, or unlicensed care hours as percent of total care hours. Patient (bed) turnover was an indicator of workload intensity generated by admissions, discharges, and transfers (ADT), calculated as total ADT as a percent of total patient days. RN voluntary turnover is the number of RN voluntary separations as percent of total full-time and part-time RNs on the unit per month.
Table 1.
Descriptive Statistics for Study Variables
| Mean | SD | Inter-Quartile Range | |
|---|---|---|---|
| Unit/patient characteristics (N = 789 units) | |||
| Number of patients per prevalence study | 22.09 | 9.45 | 11.75 |
| Mean age of patients on unit on prevalence study day | 62.41 | 6.38 | 8.29 |
| Percent male versus female patients on prevalence study day | 46.61 | 12.29 | 10.17 |
| Percent medical versus surgical patients/prevalence study day | 70.04 | 28.97 | 43.50 |
| Average length of stay (LOS) on prevalence study day | 7.09 | 4.20 | 3.41 |
| Percent of patients at risk at time of study | 36.79 | 17.67 | 23.39 |
| RN voluntary turnover as percent of total RNs* | 1.40 | 13.94 | 0.73 |
| Nurse workload (N = 691 units) | |||
| Total hours per patient day (HPPD)* | 9.85 | 1.90 | 1.85 |
| Licensed hours per patient day (HPPD)* | 7.53 | 1.57 | 1.47 |
| Skill mix | |||
| Percent RN hours* | 73.76 | 10.41 | 11.43 |
| Percent LVN hours* | 3.05 | 5.46 | 4.38 |
| Percent unlicensed hours* | 23.20 | 9.30 | 10.86 |
| Patient (bed) turnover as percent of total patient days | 57.59 | 22.53 | 23.01 |
| RN expertise (N = 144 units) | |||
| RN experience in years | 10.97 | 2.90 | 3.29 |
| Percent certified | 23.79 | 14.10 | 16.74 |
| Percent BSN or higher | 52.91 | 16.81 | 21.16 |
| Mean age | 39.92 | 3.48 | 4.80 |
| Percent contracted hours (N = 691 units)* | 3.28 | 5.23 | 3.55 |
| HAPU clinical process (N = 789 units) | |||
| Percent of patients with risk assessment within 24 hours of admission | 98.23 | 3.18 | 2.27 |
| Percent of patients with skin assessment within 24 hours of admission | 97.91 | 3.75 | 2.51 |
| Percent of patients at risk with protocol at time of study | 89.12 | 16.55 | 15.63 |
| HAPU outcome variables (N = 789 units) | |||
| Percent of patients with HAPU (any stage) | 2.94 | 3.37 | 4.33 |
| Percent of patients with HAPU-II+* | 1.71 | 2.34 | 2.44 |
Note: RN Voluntary Turnover excludes transfers within the organization, separations due to death, disability, illness, pregnancy, relocation, military service, education, retirement, promotions, performance or discipline, cutbacks due to mergers, cyclical layoffs, or other permanent reductions in force.
NQF-15 measure.
To measure RN expertise, four variables were calculated from the RN survey: years of nursing experience; percent with certification in a functional or clinical area; percent with a baccalaureate or higher degree in nursing; and age. The denominator for percentages was all valid responses to the survey question. Contracted hours was the percent of direct care hours worked by contract or agency nurses or the nonemployee RN, LVN, and unlicensed care hours, submitted with nurse workload variables.
Clinical process variables related to HAPU were obtained from direct observation and chart review computed as percentages of all patients included in the study. Data included pressure ulcer risk assessment documented within 24 hours of admission (admission risk assessment); skin assessment documented within 24 hours of admission (evaluation of the patient's skin with an emphasis on major pressure points), and specific prevention protocols or interventions implemented at the time of the study for patients with HAPU risk.
Pressure ulcer stages were defined using the nationally accepted standard definitions (NPUAP/EPUAP 2009). Outcome measures were prevalence of HAPU at any stage (stages I-IV, unstageable, and deep tissue injury [DTI]) and HAPU stage II or greater (stages II–IV and unstageable, without DTI).
Procedures
CALNOC staff provided hospital training to support data capture and strengthen reliability. Although this study used only de-identified data, CALNOC maintained Institutional Review Board approval at Cedars-Sinai Medical Center and the University of California San Francisco.
CALNOC data collection and validation methods (Aydin et al. 2004, 2008) and pressure ulcer prevalence study methodology (Stotts et al. 2013) are published. Study data were submitted by the hospitals using either scannable data collection forms or spreadsheets with embedded data checking prior to submission and upon upload at CALNOC.
Analyses
Data were aggregated over the year to provide one observation per unit for each variable by averaging monthly or quarterly data. Most hospitals conducted prevalence studies quarterly with all variables collected for each study. Nurse workload variables were comprised of monthly data for each unit. Each unit conducted the RN survey once during the study period. Error checks included unit identifiers, expected ranges for staffing measures, variable outliers, and completeness. Variation over quarters was checked for variables measured repeatedly over the study period to ascertain that minimal information would be lost by annual averaging. Summary statistics for the HAPU outcome variables and model covariates were examined.
The low prevalence of HAPUs created an excess number of units without HAPU and right skew of the distributions. This required discrete zero-inflated Poisson (ZIP) regression to model the annual rate of HAPUs on each unit. This model assumes two mechanisms that give rise to units without HAPUs over the year: (1) a Poisson distribution that predicts events (HAPU) with unit-dependent mean rates (including some zeroes), and (2) a binary distribution with unit-dependent probabilities of no HAPU events. The observed “number of patients with HAPU” was assumed by the statistical criteria to be created by a mixture of these two processes. Combining these mechanisms makes a model that can fit data containing an “inflated” number of units without HAPUs. The associations of study covariates with each of these separate mechanisms were assessed using both Poisson and logistic regression simultaneous equations with the number of patients assessed and additional significant covariates as predictors (Cameron and Trivedi 1998; Atkins and Gallop 2007).
Correlations among covariates were assessed prior to modeling to detect high values and guide the modeling with respect to co-linearity and potentially “exchangeable” covariates. The Poisson regressions included the number of patients assessed (rate denominator) and a standard set of predictors deemed necessary to control for potential differences in patient, unit, and hospital characteristics. This standard set of predictors included average patient age, percent of medical patients, average number of patients in HAPU prevalence studies (a measure of unit size), hospital ownership type (government, corporate system, for-profit, and other not-for-profit), and teaching status. Prevalence studies include all patients on the unit on the day of the study. Teaching status was defined using membership in UHC (2010) and/or the Council of Teaching Hospitals (COTH), supplemented by data from American Hospital Association Annual Survey (AHA 2003) on medical school affiliation and number of full time physician residents. All teaching hospitals were members of UHC, COTH, and/or had medical school affiliation with at least 90 full-time residents.
Additional unit characteristics in the pool of covariates included number of discharges per month and average patient LOS at time of study. Additional hospital characteristics were hospital average daily census and setting (urban or rural). Each of these additional covariates was independently tested for significance after controlling for the standard set of covariates. Only those that tested significant for an outcome were considered for final models. Significant covariates were added one at a time in order of significance (forward selection) to construct the final multiple regression models. High correlations among multiple workload, risk assessment, and skin assessment variables prevented all covariates from being introduced simultaneously in a single equation. Those selected were slightly more predictive. However, for the ZIP model, the same or correlated covariates were used as concurrent predictors in the Poisson and logistic regression equations. Interactions of significant RN workload, expertise, and HAPU processes of care main effects were also tested.
Estimated changes in outcome for a one-standard deviation change in covariates and the difference in outcome for different levels of categorical covariates were calculated. Results were transformed back to a linear scale for interpretability where necessary. Curves with predicted HAPU-Any Stage and HAPU-II+ prevalence for selected predictors provided a visual tool to assess estimated impact of changes in predictors on outcomes.
Findings
The 789 medical surgical units from 215 CALNOC hospitals included mostly small sized hospitals (33 percent: <100 beds; 39 percent: 100–199 beds) and some moderate and large (17 percent: 200–299 beds; 11 percent: 300+ beds). Most hospitals were not-for-profit (85 percent), with only a few for-profit (6 percent) or government (9 percent). Most were urban (90 percent) and a few (10 percent) were teaching hospitals. A total of 66,327 patients were observed from, on average, 3.67 (SD 2.32) units participating in HAPU prevalence studies per hospital (ranging from 1 to 14 units).
Descriptive statistics are summarized in Table1. On average, there were 22 patients in each prevalence study with a 1-week LOS; over 70 percent were medical; patients received over 9 hours of care per day, typically provided by RNs. Patient (bed) turnover was high, almost 60 percent of patients daily. RNs averaged about 40 years old, with nearly 11 years of experience, and over half were baccalaureate prepared. Nearly all patients had skin assessment and pressure ulcer risk assessment performed at admission and most at-risk patients had a prevention protocol in place. The prevalence of HAPU-Any Stage was 2.9 percent and HAPU-II+ was 1.7 percent.
RN survey data were obtained from a 144 unit subsample. When compared with units not providing these data, these units were significantly more likely to come from larger academic medical centers, had slightly younger patients on average (60.8 years vs. 62.8), fewer medical patients (64.4 vs. 71.3 percent), lower patient (bed) turnover (53.1 percent vs. 59.1 percent), lower licensed HPPD (7.2 hours vs. 7.6), and lower percent contracted hours (2.5 vs. 3.5). No other variables showed meaningful differences between samples.
High correlations were found between related predictors in the following measures (p < .05): (1) staffing: licensed hours and total HPPD (r = 0.82); (2) clinical processes: patients with admission risk assessment and patients at risk with prevention protocol implemented at the time of study (r = 0.85); and (3) RN education: years of nursing experience and age (r = 0.76). There was a small negative correlation (r = −0.269) between LOS at time of survey and patient (bed) turnover (a measure of both increased nursing workload—the “churn” of patients and LOS).
Study Models
Two statistical models examined the predictive power of study variables on both HAPU-Any Stage and again on HAPU-II+. Model 1 included all units, while Model 2 included only the subsample of units with RN survey data. Figure1 provides an overview of statistically significant findings from both models, showing the direction of each variable that predicted a reduction in HAPU. Table2 provides the statistically significant predictors and details on the magnitude and direction of significant covariate effects for one standard deviation increase in each covariate for the fitted models. Sample sizes are the number of units with complete data for all variables included in each model. The estimated percent change in HAPU for one standard deviation increase in the respective predictor variable (Poisson regression findings) and the odds ratios (ORs) for the covariates' effects on the probability of belonging to units without HAPU events (logistic regression findings) are detailed in the table.
Figure 1.
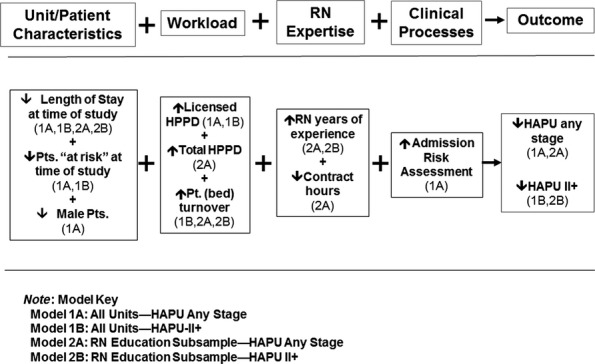
Overview of Significant Model Findings
Table 2.
Multivariable Zero-Inflated Poisson Regression Model Summaries
| Model and Variable | Parameter Estimate | Standard Error | p-value | Change in Outcome per 1 SD Increase in Predictor (%)* |
|---|---|---|---|---|
| Model 1: All units†,‡ | ||||
| (A) Dependent variable = Number of HAPU-any stage N = 656 units | ||||
| Poisson equation covariates | ||||
| LOS time of study | 0.049696 | 0.005062 | <.0001 | 23.2 |
| Licensed HPPD | −0.089453 | 0.021995 | <.0001 | −13.1 |
| % Risk assess within 24 hours of admission | −0.020248 | 0.007991 | .0113 | −6.2 |
| Logistic equation covariates¶ | % units without HAPU-any stage = 25.0% | |||
| % Patients “at risk” at study | −0.026990 | 0.012461 | .0303 | ↑17.7% pts. “at risk” →17.1% without HAPU-any stage (OR = 0.62) |
| % Male patients | −0.045280 | 0.019379 | .0195 | ↑12.3% Male pts. →16.0% without HAPU-any stage (OR = 0.57) |
| (B) Dependent variable = Number of HAPU-II+N=513 units | ||||
| Poisson equation covariates | ||||
| % Patients “at risk” at study | 0.009867 | 0.002831 | .0005 | 19.0 |
| Licensed HPPD | −0.090562 | 0.031979 | .0046 | −13.2 |
| Pt. (bed) turnover | −0.010134 | 0.002662 | .0001 | −20.4 |
| Logistic equation covariates§ | % units without HAPU-II+ = 38.0% | |||
| LOS time of study | −0.194533 | 0.082874 | .0189 | ↑4.2 in days LOS=21.2% without HAPU II+ (OR = 0.44) |
| Model 2: RN education subsample†,¶,** | ||||
| (A) Dependent variable = Number of HAPU-any stage—N = 135 units | ||||
| Poisson equation covariates | ||||
| LOS time of study | 0.049148 | 0.007451 | <.0001 | 22.9 |
| Total HPPD | −0.107995 | 0.050902 | .0339 | −18.6 |
| Patient (bed) turnover | −0.013028 | 0.003774 | .0006 | −25.4 |
| RN experience | −0.058446 | 0.021388 | .0063 | −15.6 |
| % Contract hours | 0.027882 | 0.013855 | .0442 | 15.7 |
| (B) Dependent variable = Number of HAPU-II+—N = 135 units | ||||
| Poisson equation covariates | ||||
| LOS time of study | 0.047619 | 0.008825 | <.0001 | 22.1 |
| Patient (bed) turnover | −0.018689 | 0.005034 | .0002 | −34.3 |
| RN experience | −0.062537 | 0.027533 | .0231 | −16.6 |
Note: All Ns are the number of units with valid data for variables included in the model.
Measures % change in mean outcome count for changes in significant covariates.
A “standard set” of control variables added to all models and not specified in the table included average patient age, percent of medical patients, average number of patients in HAPU prevalence studies (a measure of unit size), hospital ownership type (government, corporate system, for-profit, and other not-for-profit), and teaching status.
Additional unit and hospital characteristic not included in models 1A and B, but tested as covariates and found not significant included: total hours of nursing care per patient day; number of RN, LVN, unlicensed, or contracted care hours as percent of total care hours; RN voluntary turnover; hospital urban/rural setting; skin assessment documented within 24 hours of admission and specific prevention protocols or interventions implemented at the time of the study for patients with HAPU risk.
Measures change in the probability of belonging to units without HAPU-Any Stage or HAPU-II+ for changes in significant covariates.
There were no significant covariates in the logistic equation for this model.
Additional unit and hospital characteristic not included in models 2A and B, but tested as covariates and found not significant included: percent male or female patients; licensed hours of nursing care per patient day; number of RN, LVN, or unlicensed care hours as percent of total care hours; RN voluntary turnover; hospital urban/rural setting; percent of patients at-risk for pressure ulcers; pressure ulcer risk assessment documented within 24 hours of admission; skin assessment documented with 24 hours of admission and specific prevention protocols or interventions implemented at the time of the study for patients with HAPU risk. Nonsignificant RN expertise covariates included percent with certification in a functional or clinical area; percent with a baccalaureate or higher degree in nursing, and age.
In Model 1A, longer LOS at the time of the prevalence study predicted increased HAPU-Any Stage, while higher licensed HPPD and more patients assessed at admission predicted fewer HAPU-Any Stage. Of these, the strongest predictor was shorter LOS, with one standard deviation increase in LOS predicting an estimated 23 percent more HAPU (an average increase from 2.94 percent to 3.62 percent of patients with HAPU, everything else held constant). Logistic regression findings showed that having one SD more patients “at risk” (17.7 percent) would decrease the percent of units without HAPU from 25 percent to 17 percent. More male patients would decrease the percent of units without HAPU from 25 to 16 percent.
In Model 1B, more patients “at risk” predicted higher HAPU-II+, while higher licensed HPPD and higher patient (bed) turnover were predictors of fewer HAPU-II+. The logistic regression showed longer LOS at the time of the study was associated with a decrease in the percent of units without HAPU II+ from 38 to 21 percent.
Model 2 shows results for the subsample of units participating in the RN survey. Model 2A revealed that increased LOS and percent contracted hours predicted more HAPU-Any Stage, while increased total HPPD, patient (bed) turnover, and years of RN experience predicted fewer HAPU. In Model 2B, HAPU-II+ predictors included LOS, patient (bed) turnover, and years of RN experience. No significant interactions were found between workload, expertise, and processes of care main effects.
To assess the estimated combined effect of changes in predictor variables and explore possible tradeoffs on the outcomes, prevalence estimates for a hypothetical spectrum of unit characteristics were estimated based on the predictive models. Figure2 examines effects of covariates for Model 1. First we examined workload and clinical processes (Licensed HPPD, patient risk status, and % of patients with admission risk assessment), looking at the effect on HAPU-Any Stage (Model 1A) of varying the number of licensed HPPD, the percent of patients at risk at the time of the study, and percent of patients with admission risk assessment. Each graph was built around the mean and standard deviation for the study sample. Overall, HAPU-Any Stage decreased as the number of licensed HPPD increased. Since the percent of patients with admission risk assessment was relatively high for all hospitals, the effect of this covariate was extremely small (<1 percent) (i.e., lines for predicted values are very close together).
Figure 2.
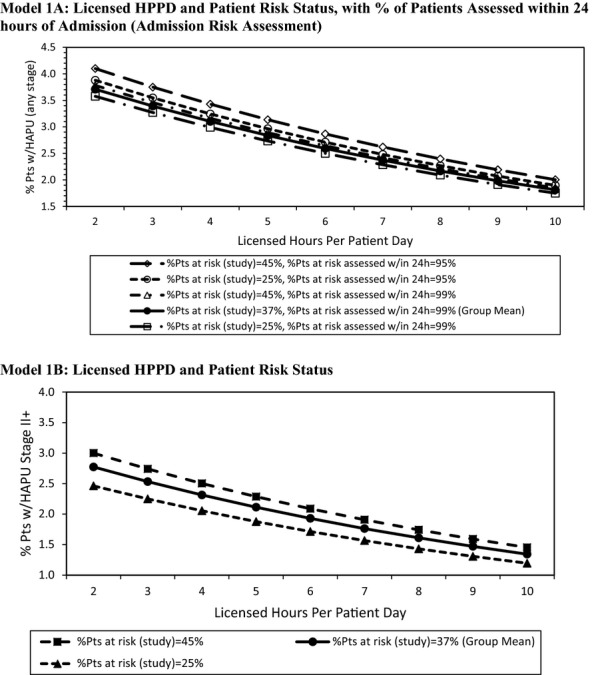
Predicted HAPU Any Stage and HAPU II+ by Key Model Predictors
Notes Mean licensed hours per patient day = 7.53. Mean HAPU-any stage = 2.3. Mean HAPU II+ = 1.4. Solid curve indicates sample unit average (group mean). Continuous and categorical model predictors not depicted were set to the overall sample averages or proportions, respectively.
The effect of varying the number of licensed HPPD and the percent of patients at-risk at the time of the prevalence study (25 and 45 percent) on HAPU-II+ (Model 1B) are displayed in Figure2. The analysis revealed that with a higher percentage at risk, there were more HAPU-II+. This finding suggests that a unit could compensate for a larger percentage of patients at risk by increasing their licensed nursing hours and, depending on the number of additional licensed nursing hours, could expect the same or a stable HAPU-II+ rate. For example, having 25 percent at risk of HAPU at 2 hours of licensed care results in a prevalence of about 2.5 percent, which is the same as the 45 percent at risk who are receiving nearly 5 hours of care.
The impacts of workload and RN expertise (Model 2A) are examined in Figure3, showing the effect of varying the number of total nursing HPPD and the related curves on years of RN experience and use of contract staff on HAPU-Any Stage. The percent of patients with HAPUs decreased as total HPPD increased, the years of RN experience increased, and percent of hours provided by contract staff decreased. Thus, at five total HPPD, the contrast is clear—with 6 years of experience and 10 percent of hours provided by contact staff, HAPUs approached 5.2 percent versus 2.2 percent when RNs have 16 years of experience and no contract staff hours are used. When RNs with greater years of experience were not available, HAPU rates were lower on units staffed by employee RNs, when compared to units with a combination of regular staff plus contract or agency nurses.
Figure 3.
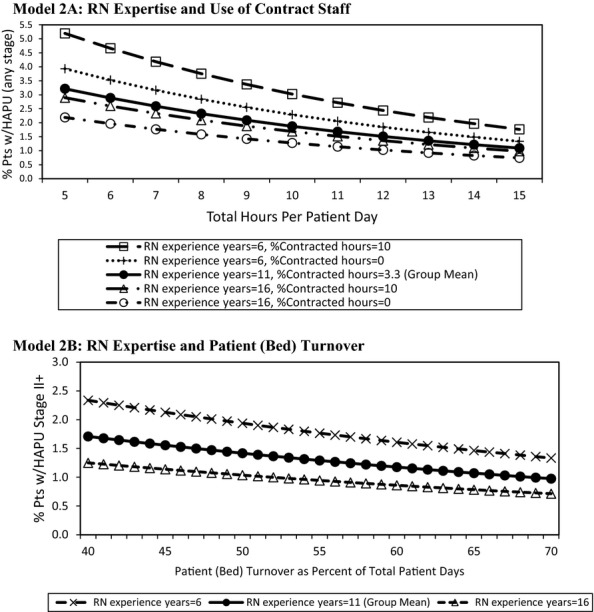
Predicted HAPU Any Stage and HAPU II+ by Key Model Predictors
Notes. Mean total hours per patient day = 9.85; mean patient (bed) turnover as percent of total patient days = 57.6. Mean HAPU-any stage = 2.3. Mean HAPU II+ = 1.4. Solid curve indicates sample unit average levels (group mean). Continuous and categorical model predictors not depicted were set to the overall sample averages or proportions, respectively.
Figure3 examines workload and RN expertise findings from Model 2B (RN expertise and patient [bed] turnover), showing that as patient (bed) turnover increased, the prevalence of HAPU-II+ decreased slightly. Yet LOS alone was not a sufficient explanation for HAPU rates. When care was provided by more experienced nurses, there were fewer HAPU-II+. When staffing with nurses with either more or less experience was compared as patient (bed) turnover increased, fewer HAPU-II+ were seen with staffing by nurses with greater experience. The approximate 1 percent reduction in HAPU-II+ seen when comparing the most to the least experienced nurses (with the average 55 percent patient [bed] turnover) narrowed only slightly as the patient (bed) turnover varied from 40 to 70 percent.
Discussion
Multiple factors related to structure and processes of care converged to prevent HAPU, including a combination of patient risk status, LOS, nurse staffing, activity on the unit (patient [bed] turnover), RN years of experience, percent of hours provided by contract staff, and patient risk assessment. Even with low HAPU prevalence rates (<3 percent) and high rates of admission risk assessment (>98 percent), differences in patients and in structure and processes of care were identified that impacted HAPU prevalence.
In general, the same or highly correlated predictors were significant. Longer LOS appeared in all analyses, while staffing and patient (bed) turnover each appeared in three of the four sets in Table2. RN experience appeared in both subsample analyses. Sample size was also important. The sample for Model 1 was much larger than the Model 2 subsample, decreasing predictive power for Model 2. HAPU-II+ are rarer events, making Models 1B and 2B less powerful than Models 1A and 2A. Model 1A is the most powerful. Model 2B is the least powerful with a smaller sample predicting a rarer event, yet it confirms the predictive importance of RN years of experience found in Model 2A. The added RN education variables in Model 2 may also replace or affect other covariates in the model.
Study models confirm that unit/patient characteristics were potent predictors of lower HAPU. Our data on shorter LOS were consistent with others who have reported the association between LOS and HAPU (Russo, Steiner, and Spector 2008; Lyder et al. 2012). The models also indicated that units with fewer male patients were more likely to have no HAPU. A recent systematic review of 15 studies with gender included in multivariable modeling identified three studies showing males at increased risk. The authors offered no explanation for the disparate findings and concluded that there is minimal evidence to suggest gender is a risk factor for HAPU (Coleman et al. 2013). Why males may more often develop ulcers is not known and bears further study.
Unit/patient characteristics are generally not modifiable. However, RN workload, experience, and admission risk assessment were significant predictors that can be strategically manipulated to reduce HAPU. The combination of increased levels of admission risk assessment and fewer patients at risk for pressure ulcers at the time of the study predicted fewer HAPUs. We note that as the proportion of patients at-risk increased, the number of HAPU-II+ increased. Increasing nursing hours and/or licensed hours could maintain or reduce the number of HAPU-II+; however, to date, the literature linking HAPU prevalence and nurse staffing has produced mixed results. Several studies reported no relationship between higher levels of staffing and HAPU development (Aiken, Clarke, and Sloane 2002; Needleman et al. 2002; Unruh 2003; Mark et al. 2004; Donaldson et al. 2005; Burnes Bolton, Aydin et al. 2007; Duffield et al. 2011). In contrast, others (Cho et al. 2003; Duffield et al. 2011) found that more nursing hours predict more HAPUs. Unruh and Zhang (2012) found mixed results that seem related to the workload measure used.
Patient (bed) turnover captures workload created by a combination of admissions, discharges, and transfers. High turnover results in increased demands for care, but it is also a proxy for shorter LOS. In contrast to our findings associating higher patient (bed) turnover with lower HAPU, recent work by Park et al. (2012) found that when patient turnover increased, the beneficial effect of RN staffing on failure-to-rescue was reduced. Needleman et al. (2011) found exposure to a high turnover shift in noncritical care units was related to increased patient mortality (15 percent). And CALNOC's predictive model of medication administration accuracy found that higher patient (bed) turnover predicted more errors in medication administration safe practices that were, in turn, highly correlated with medication errors (Donaldson, Aydin, and Fridman 2014).
This is this first study to link patient (bed) turnover to HAPUs. HAPUs were predicted separately by both LOS and patient turnover, in opposite directions. The weak negative correlation between the two measures indicated they were measuring related, but different concepts. Longer LOS is clinically related to HAPU risk for individual patients, while patient turnover describes the level of activity on the unit. On units with greater turnover, patients spend less time on the unit, leaving nurses less time for assessment and prevention. However, in the case of HAPU, higher turnover is associated with fewer HAPU, indicating that the “churn” on the unit does not impede HAPU prevention activities. Patient acuity overall may be variably associated with turnover also, as it is plausible that rapidly discharged patients are less ill and those transferred to critical care are more ill. Clearly, more research is needed on the impacts of patient (bed) turnover on HAPU since research on other outcomes shows it to be associated with poorer rather than better patient outcomes.
Several other predictors reflecting nursing expertise were not significant in our models. The study was conducted during a significant economic downturn when RN voluntary turnover was low and unrelated to HAPU findings. Having a BSN or higher or professional certification was also not significantly related to HAPU prevalence. While Blegen et al. (2013) found that hospitals with a greater percentage of RNs with BSN or higher had fewer HAPUs, the difference may be explained in part by the Blegen et al. data showing more RNs with a BSN. In addition, those certified in wound care have higher pressure ulcer knowledge than those without certification (Zulkowski, Ayello, and Wexler 2007); there was not a statistical difference in those who were certified in other areas and those who were not certified, although those certified scored higher.
Finally, the findings from this study demonstrate the importance of nursing experience, with lower HAPU rates predicted by a combination of more experienced staff and fewer contract nurses, mitigating the effects of unit/patient characteristics on HAPU. Experienced nurses differ from advanced beginners and competent nurses in their ability to communicate, organize care, work around interruptions, and anticipate patient needs (Burger et al. 2010). When nurses change environments as occurs with the contract nurses, they lack experience in the clinical setting and may not be as efficient in their care (e.g., obtaining supplies, knowing who to communicate with); they also may not have consistent patient assignments that would afford time to fully understand their patients' needs. Our data also showed that when more experienced nurses are not available, using the hospital's permanent employees, rather than contract staff not on the hospital's payroll, kept HAPUs low.
The significance of nursing experience as a predictor must also be viewed in light of trends in the nursing workforce, including the overall aging of the workforce as well as recent increases in the number of nurses ages 23–26. Such changes point to the importance of retaining experienced nurses, ensuring that enough are available to mentor younger nurses and offset the risk of adverse patient outcomes (Buerhaus 2008; Auerbach, Buerhaus, and Staiger 2011).
While HAPU rates at the time of this study were low, CALNOC data show that they have decreased over time. HAPU-Any Stage was 7.45 percent for CALNOC medical-surgical units in 2002 (Burnes Bolton, Donaldson et al. 2007). Significant reductions in HAPU and HAPU II+ occurred from 2003 to 2010 in 78 hospitals drawn from the same registry participants (Stotts et al. 2013). More recently, the median percent of patients with HAPU II+ in adult acute care for 268 participating hospitals (including the 215 hospitals in this study) decreased from 3.3 percent in 2007 to 0.5 percent in 2012 (CALNOC 2013). We believe that the study's low rates were due to the high rate of skin and pressure ulcer risk assessment that triggered implementation of prevention protocols, performed on nearly all (89 percent) of the at-risk patients. Such reliable processes of care closely reflect national/international standards for pressure ulcer care (NPUAP/EPUAP 2009).
Limitations
The study hospitals comprise a convenience sample of hospitals participating in ongoing CALNOC benchmarking of quality outcomes and may have been intrinsically different from hospitals not involved in ongoing performance benchmarking. The units providing the RN education survey data for this subsample were significantly more likely to come from larger academic medical centers, limiting the generalizability of these models to units in similar settings. Measurement of HAPUs with the existing staging system also was a recognized limitation, that is, lack of sensitivity in identifying HAPUs in patients with darkly pigmented skin (NPUAP/EPUAP, 2009).
Conclusion
Unit-level data from this study reveal multiple factors predicting fewer HAPUs, including a combination of unit/patient characteristics, RN workload, RN expertise, and clinical processes. Importantly, the predictive models show that RN workload, expertise, and clinical processes of care (risk assessment) can be manipulated to mitigate HAPU prevalence. Leaders can use these data to reduce the HAPU threats to patient care quality, costs, and outcomes. The importance of early skin and risk assessment in preventive care cannot be over-emphasized. These data also point to the potential need for support strategies for units where full-time experienced nurses may not be available for HAPU prevention. Further research is warranted to test these finding in the context of higher HAPU prevalence.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was made possible by a Robert Wood Johnson Foundation, Interdisciplinary Nursing Quality Research Initiative (RWJF INQRI) grant entitled: “Impact of Medical Surgical Acute Care Microsystem Nurse Characteristics and Practices on Patient Outcomes,” Nancy Donaldson and Carolyn Aydin Co-Principal Investigators. The study was conducted by the CALNOC Research Team in collaboration with the University of California San Francisco School of Nursing and Cedars-Sinai Medical Center.
Disclosures: None.
Disclaimers: None.
Supporting Information
Appendix SA1: Author Matrix.
References
- Aiken LH, Clarke SP. Sloane D. Hospital Staffing, Organizational Support, and Quality of Care: Cross-National Findings. International Journal of Quality Health Care. 2002;14:5–13. doi: 10.1093/intqhc/14.1.5. [DOI] [PubMed] [Google Scholar]
- Aiken LH, Clarke SP, Sloane DM, Sochalski J. Silber JH. Hospital Nurse Staffing and Patient Mortality, Nurse Burnout, and Job Dissatisfaction. Journal of the American Medical Association. 2002;288(16):1987–93. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- Aiken LH, Cimiotti JP, Sloane DM, Smith HL, Flynn L. Neff DF. Effects of Nurse Staffing and Nurse Education on Patient Deaths in Hospitals with Different Nurse Work Environments. Medical Care. 2011;49(12):1047–53. doi: 10.1097/MLR.0b013e3182330b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquist SB. 1301, An Act to Add Sections 1279.1, 1279.2, 1279.3, and 1280.4 to the Health and Safety Code, Relating to Health Facilities. Sacramento, CA: California Legislature; 2006. [accessed on January 5, 2007]. Available at ftp://www.leginfo.ca.gov/pub/senate-journal/sen-journal-0x-20060831-5495.PDF. [Google Scholar]
- American Hospital Association. American Annual Survey Database: Fiscal Year 2003. Chicago, IL: American Hospital Association; 2003. [Google Scholar]
- American Nurses Association. Implementing Nursing's Report Card: A Study of RN Staffing, Length of Stay and Patient Outcomes. Washington, DC: American Nurses Publishing; 1997. [Google Scholar]
- American Nurses Association. Nurse Staffing and Patient Outcomes in the Inpatient Hospital Setting (Executive Summary) Washington, DC: American Nurses Publishing; 2000. [Google Scholar]
- Atkins DC. Gallop RJ. Rethinking How Family Researchers Model Infrequent Outcomes: A Tutorial on Count Regression and Zero-Inflated Models. Journal of Family Psychology. 2007;21(4):726–35. doi: 10.1037/0893-3200.21.4.726. [DOI] [PubMed] [Google Scholar]
- Auerbach DL, Buerhaus PI. Staiger DO. Registered Nurse Supply Grows Faster Than Projected Amid Surge in New Entrants Age 23-26. Health Affairs. 2011;30(12):2286–92. doi: 10.1377/hlthaff.2011.0588. [DOI] [PubMed] [Google Scholar]
- Aydin CE, Burnes Bolton L, Donaldson N, Brown DS, Buffum M, Elashoff JD. Sandhu M. Creating and Analyzing a Statewide Nursing Quality Measurement Database. Journal of Nursing Scholarship. 2004;36(4):371–8. doi: 10.1111/j.1547-5069.2004.04066.x. [DOI] [PubMed] [Google Scholar]
- Aydin CE, Burnes Bolton L, Donaldson N, Brown DS. Mukerji A. Beyond Nursing Quality Measurement: The Nation's First Regional Nursing Virtual Dashboard. In: Grady ML, editor; Henriksen K, Battles JB, Keyes MA, editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 1: Assessment) Rockville, MD: Agency for Healthcare Research and Quality; 2008. pp. 217–34. [Google Scholar]
- Bales I. Padwojski A. Reaching for the Moon: Achieving Zero Pressure Ulcer Prevalence. Journal of Wound Care. 2009;18(4):137–44. doi: 10.12968/jowc.2009.18.4.41605. [DOI] [PubMed] [Google Scholar]
- Blegen MA, Goode CJ, Park SH, Vaughn T. Spetz J. Baccalaureate Education in Nursing and Patient Outcomes. Journal of Nursing Administration. 2013;43(2):89–94. doi: 10.1097/NNA.0b013e31827f2028. [DOI] [PubMed] [Google Scholar]
- Buerhaus PI. Current and Future State of the US Nursing Workforce. Journal of the American Medical Association. 2008;300(20):2422–4. doi: 10.1001/jama.2008.729. [DOI] [PubMed] [Google Scholar]
- Burger JL, Parker K, Cason L, Hauck S, Kaetzel D, O'Nan C. White A. Responses to Work Complexity: The Novice to Expert Effect. Western Journal of Nursing Research. 2010;32(4):497–510. doi: 10.1177/0193945909355149. [DOI] [PubMed] [Google Scholar]
- Burnes Bolton L, Aydin C, Donaldson N, Brown DS, Sandhu M, Fridman M. Aronow HU. Mandated Nurse Staffing Ratios in California: A Comparison of Staffing and Nursing-Sensitive Outcomes Pre- and Post Regulation. Policy, Politics and Nursing Practice. 2007;8(4):238–50. doi: 10.1177/1527154407312737. [DOI] [PubMed] [Google Scholar]
- Burnes Bolton L, Donaldson NE, Rutledge DN, Bennett C. Brown DS. The Impact of Nursing Interventions: Overview of Effective Interventions, Outcomes, Measures, and Priorities for Future Research. Medical Care Research and Review. 2007;64(2 Suppl):123S–43S. doi: 10.1177/1077558707299248. [DOI] [PubMed] [Google Scholar]
- California Senate Bill 1301. 2006. Chapter 647, Statutes of 2007 with Regard to Reporting of Pressure Ulcers (Section 1279.1 (b)(4)(F) of the Health and Safety Code) [accessed on August 14, 2009]. Accessed at http://www.cdph.ca.gov/certlic/facilities/Documents/LNC-AFL-08-09.pdf.
- CALNOC. 2013. CALNOC Performance Outcome Index, Trend Comparison Reports: 2007-2012 [accessed on August 13, 2013]. Available at http://www.calnoc.org.
- Cameron AC. Trivedi PK. Regression Analysis of Count Data. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- Cho SH, Ketefian S, Barkauskas VH. Smith DG. The Effects of Nurse Staffing on Adverse Events, Morbidity, Mortality, and Medical Costs. Nursing Research. 2003;52(2):71–9. doi: 10.1097/00006199-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Clarke S. Donaldson NE. Nurse Staffing and Patient Care Quality and Safety. In: Hughes RG, editor; Patient Safety & Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Health Care Research & Quality; 2008. p. 1. 133. AHRQ Publication No. 07-00151. [PubMed] [Google Scholar]
- Coleman S, Gorecki A, Nelson ES, Closs SJ, Defloor T, Farrain A, Brown J, Schoonhoven L. Nixon J. Patient Risk Factors for Pressure Ulcer Development: Systematic Review. International Journal Nursing Studies. 2013;50(7):974–1003. doi: 10.1016/j.ijnurstu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Cook A, Gaynor M, Stephens M., Jr Taylor L. The Effect of a Hospital Nurse Staffing Mandate on Patient Health Outcomes: Evidence from California's Minimum Staffing Regulation. Journal of Health Economics. 2012;31(2):340–8. doi: 10.1016/j.jhealeco.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Donaldson NE. Shapiro SE. Impact of California Mandated Acute Care Hospital Nurse Staffing Ratios: A Literature Synthesis. Policy, Politics and Nursing Practice. 2010;11(3):184–201. doi: 10.1177/1527154410392240. [DOI] [PubMed] [Google Scholar]
- Donaldson N, Aydin C. Fridman M. Predictors of Unit-Level Medication Administration Accuracy: Microsystem Impacts on Medication Safety. Journal of Nursing Administration. 2014;44(6):353–61. doi: 10.1097/NNA.0000000000000081. [DOI] [PubMed] [Google Scholar]
- Donaldson N, Burnes Bolton L, Aydin C, Brown D, Elashoff JD. Sandhu M. Impact of California/s Licensed Nurse-Patient Ratios on Unit Level Nurse Staffing and Patient Outcomes. Policy, Politics and Nursing Practice. 2005;6(3):198–210. doi: 10.1177/1527154405280107. [DOI] [PubMed] [Google Scholar]
- Duffield C, Diers D, Aisbett C. Roche M. Churn: Patient Turnover and Case Mix. Nursing Economics. 2009;27(3):185–91. [PubMed] [Google Scholar]
- Duffield C, Diers D, O'Brien-Pallas L, Aisbett C, Riche M, King M. Aisbett K. Nursing Staffing, Nursing Workload, the Work Environment and Patient Outcomes. Applied Nursing Research. 2011;24:244–55. doi: 10.1016/j.apnr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Estabrooks CA, Midodzi WK, Cummings GG, Ricker KL. Giovannetti P. The Impact of Hospital Nursing Characteristics on 30-Day Mortality. Nursing Research. 2005;54(2):74–84. doi: 10.1097/00006199-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Jenkins ML. O'Neal E. Pressure Ulcer Prevalence and Incidence in Acute Care. Advances in Skin and Wound Care. 2010;23(12):556–9. doi: 10.1097/01.ASW.0000391184.43845.c1. [DOI] [PubMed] [Google Scholar]
- The Joint Commission. Implementation Guide for the NQF Endorsed Nursing-Sensitive Care Measure Set, Version 2.0. Oakbrook Terrace, IL: The Joint Commission; 2009. [accessed on February 13, 2012]. Available at http://www.jointcommission.org/national_quality_forum_nqf_endorsed_nursing-sensitive_care_performance_measures/ [Google Scholar]
- Kovner C. Gergen PJ. Nurse Staffing Levels and Adverse Events Following Surgery in U.S. Hospitals. Image – The Journal of Nursing Scholarship. 1998;30(4):315–21. [PubMed] [Google Scholar]
- Lake ET. Cheung RB. Are Patient Falls and Pressure Ulcers Sensitive to Nurse Staffing? Western Journal of Nursing Research. 2006;28(6):654–77. doi: 10.1177/0193945906290323. [DOI] [PubMed] [Google Scholar]
- Lyder CH, Wang Y, Metersky M, Curry M, Kliman R, Verzier NR. Hunt DR. Hospital-Acquired Pressure Ulcers: Results from the National Medicare Patient Safety Monitoring System Study. Journal of the American Geriatric Society. 2012;60(9):1603–8. doi: 10.1111/j.1532-5415.2012.04106.x. [DOI] [PubMed] [Google Scholar]
- Manojlovich M, Sidani S, Covell CL. Antonakos CL. Nurse Dose: Linking Staffing Variables to Adverse Patient Outcomes. Nursing Research. 2011;60(4):214–20. doi: 10.1097/NNR.0b013e31822228dc. [DOI] [PubMed] [Google Scholar]
- Mark BA, Harless DW, McCue M. Xu Y. A Longitudinal Examination of Hospital Registered Nurse Staffing and Quality of Care. Health Services Research. 2004;39(2):279–300. doi: 10.1111/j.1475-6773.2004.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington, DC: National Pressure Ulcer Advisory Panel; 2009. [Google Scholar]
- National Quality Forum. National Voluntary Consensus Standards for Nursing-Sensitive Care: An Initial Performance Measure Set. Washington, DC: Author; 2004. [Google Scholar]
- Needleman J, Buerhaus P, Mattke S, Stewart M. Zelevinsky K. Nurse-Staffing Levels and the Quality of Care in Hospitals. New England Journal of Medicine. 2002;346(22):1715–22. doi: 10.1056/NEJMsa012247. [DOI] [PubMed] [Google Scholar]
- Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR. Harris M. Nurse Staffing and Inpatient Hospital Mortality. New England Journal of Medicine. 2011;364(11):1037–45. doi: 10.1056/NEJMsa1001025. [DOI] [PubMed] [Google Scholar]
- Park SH, Blegen MA, Spetz J, Chapman SA. De Groot H. Patient Turnover and the Relationship between Nurse Staffing and Patient Outcomes. Research in Nursing and Health. 2012;35(3):277–88. doi: 10.1002/nur.21474. [DOI] [PubMed] [Google Scholar]
- Preventing Pressure Ulcers in Hospitals. 2011. Agency for Healthcare Research and Quality, Rockville, MD [accessed on May 10, 2011]. Available at http://www.ahrq.gov/professionals/systems/long-term-care/resources/pressure-ulcers/pressureulcertoolkit/index.html.
- Russo CA, Steiner C. Spector W. Hospitalizations Related to Pressure Ulcers among Adults 18 Years and Older, 2006: Statistical Brief #64. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research (US); 2008. 2006 Feb [accessed on February 8, 2009]. Available at http://www.hcup-us.ahrq.gov/reports/statbriefs/sb64.jsp. [PubMed] [Google Scholar]
- Stotts NA, Brown DS, Donaldson NE, Aydin C. Fridman M. Eliminating Hospital-Acquired Pressure Ulcers: Within Our Reach. Advances in Skin and Wound Care. 2013;26(1):13–8. doi: 10.1097/01.ASW.0000425935.94874.41. [DOI] [PubMed] [Google Scholar]
- UHC. 2010. UHC Membership List [accessed March 25, 2010]. Available at https://www.uhc.edu/docs/003675405_UHCMembershipList.pdf.
- Unruh L. Licensed Nurse Staffing and Adverse Events in Hospitals. Medical Care. 2003;41(1):142–52. doi: 10.1097/00005650-200301000-00016. [DOI] [PubMed] [Google Scholar]
- Unruh LY. Zhang NJ. Nurse Staffing and Patient Safety in Hospitals: New Variable and Longitudinal Approaches. Nursing Research. 2012;61(1):3–12. doi: 10.1097/NNR.0b013e3182358968. [DOI] [PubMed] [Google Scholar]
- Wachter RM, Foster NE. Dudley RA. Medicare's Decision to Withhold Payment for Hospital Errors: The Devil is in the Details. Joint Commission Journal on Quality and Patient Safety. 2008;34(2):116–23. doi: 10.1016/s1553-7250(08)34014-8. [DOI] [PubMed] [Google Scholar]
- Zulkowski K, Ayello EA. Wexler S. Certification and Education: Do They Affect Pressure Ulcer Knowledge in Nursing? Advances in Skin and Wound Care. 2007;20(1):34–8. doi: 10.1097/00129334-200701000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


