Abstract
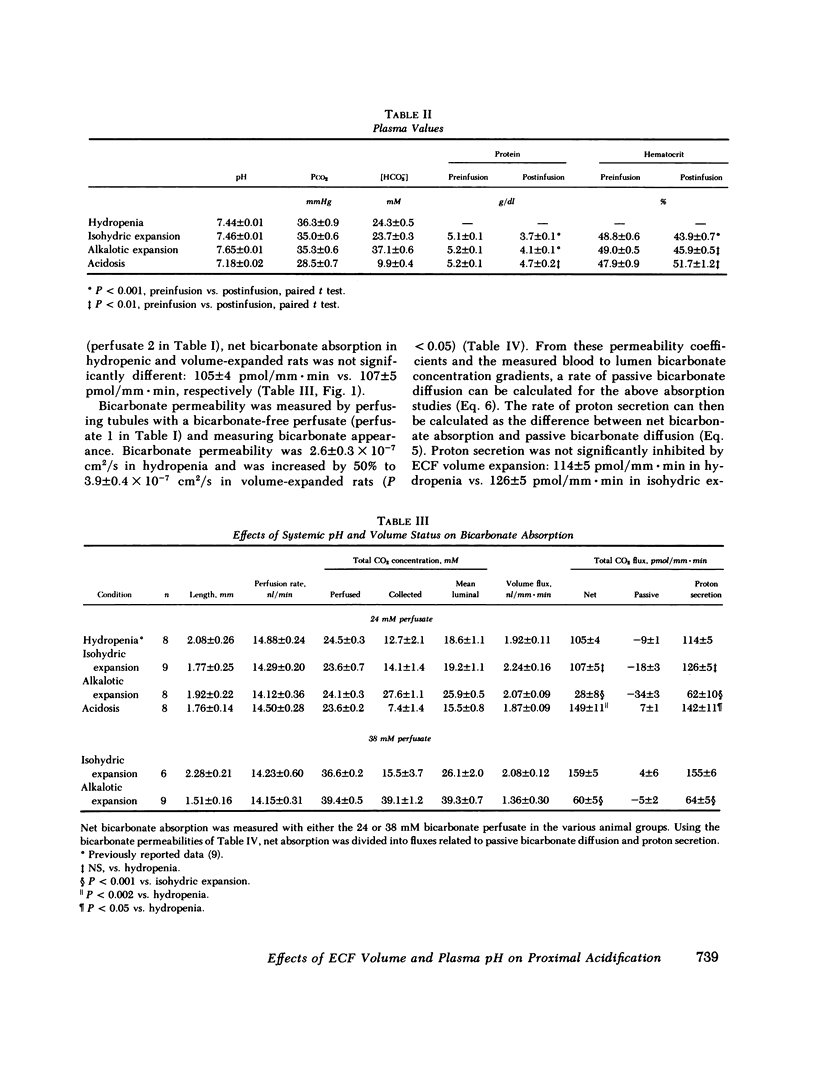
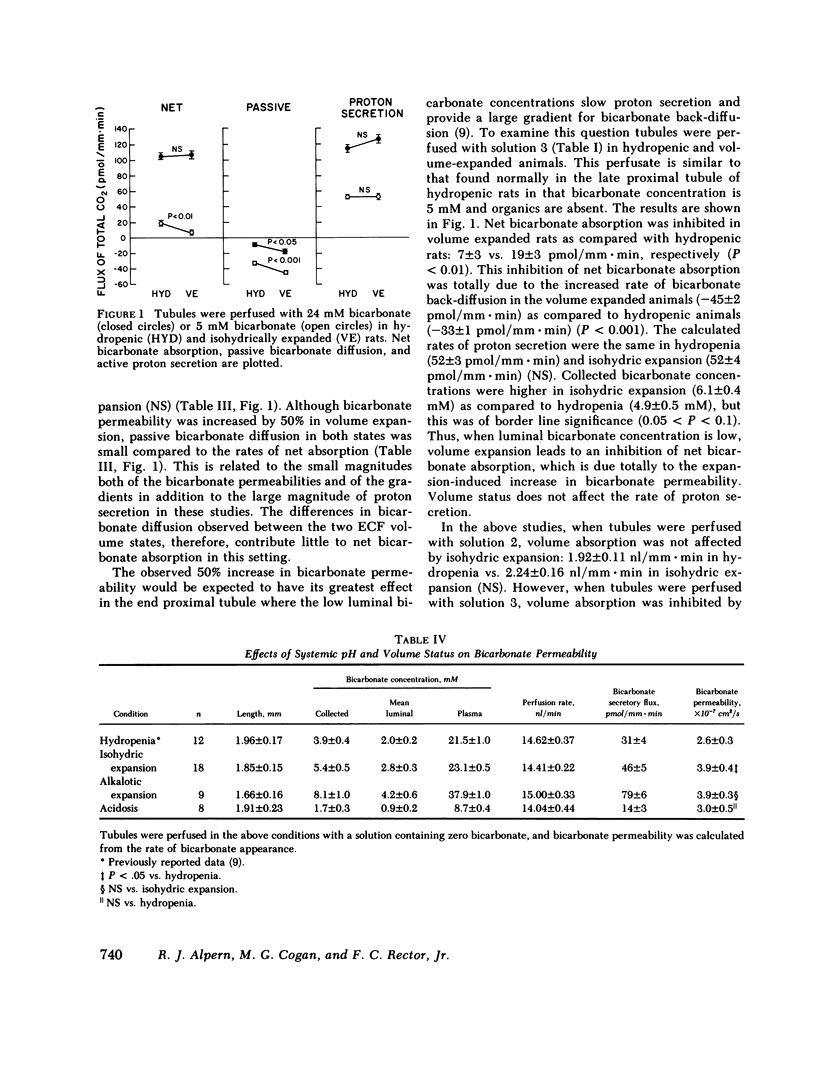
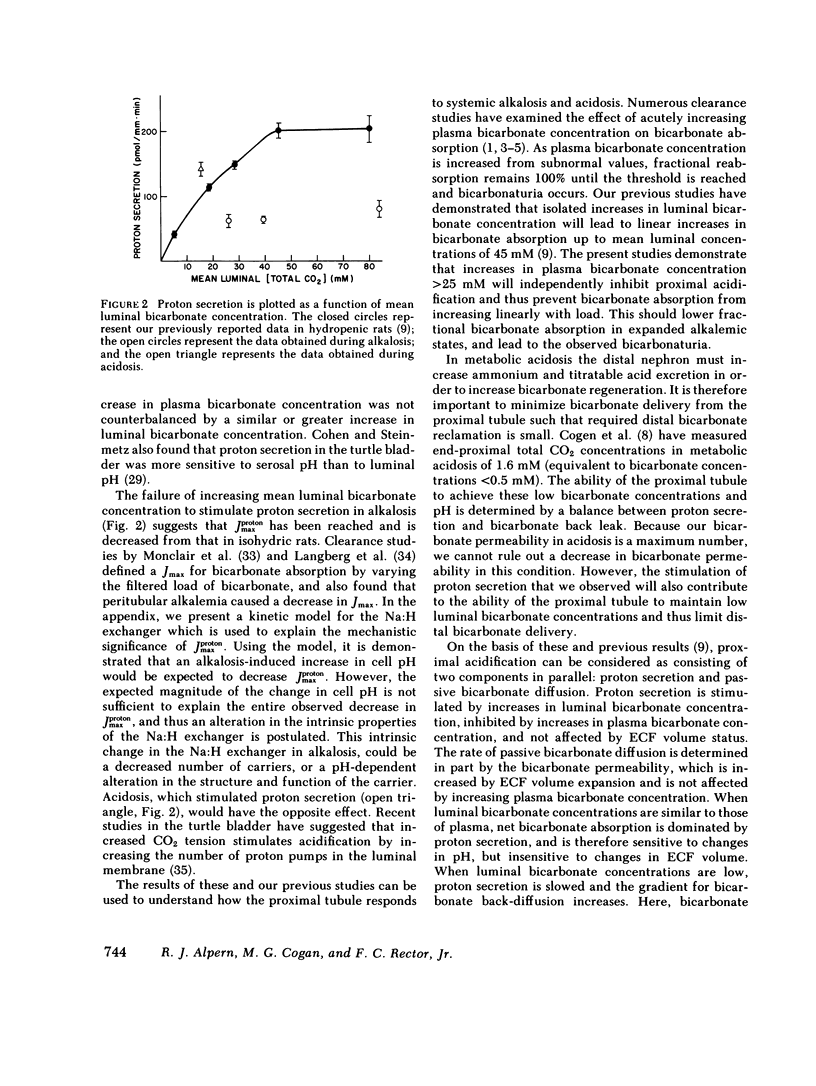
The effects of systemic bicarbonate concentration and extracellular fluid volume status on proximal tubular bicarbonate absorption, independent of changes in luminal composition and flow rate, were examined with in vivo luminal microperfusion of rat superficial proximal convoluted tubules. Net bicarbonate absorption and bicarbonate permeability were measured using microcalorimetry. From these data, net bicarbonate absorption was divided into two parallel components: proton secretion and passive bicarbonate diffusion. The rate of net bicarbonate absorption was similar in hydropenic and volume-expanded rats when tubules were perfused with 24 mM bicarbonate, but was inhibited in volume-expanded rats when tubules were perfused with 5 mM bicarbonate. Volume expansion caused a 50% increase in bicarbonate permeability, which totally accounted for the above inhibition. The rate of proton secretion was unaffected by volume expansion in both studies. The rate of net bicarbonate absorption was markedly inhibited in alkalotic expansion as compared with isohydric expansion. Bicarbonate permeabilities were not different in these two conditions, and the calculated rates of proton secretion were decreased by greater than 50% in alkalosis. Net bicarbonate absorption was stimulated in acidotic rats compared to hydropenic rats. This stimulation was attributable to a 25% increase in the rate of proton secretion. We conclude that (a) proton secretion is stimulated in acidosis, inhibited in alkalosis, and is not altered by volume status; (b) bicarbonate permeability is increased by volume expansion but is not altered by increases in plasma bicarbonate concentration; (c) when luminal bicarbonate concentrations are similar to those of plasma, net bicarbonate absorption is dominated by proton secretion and is thus sensitive to peritubular bicarbonate concentrations, and insensitive to extracellular fluid volume; (d) when luminal bicarbonate concentrations are low and proton secretion is slowed, bicarbonate permeability and thus extracellular fluid volume have a greater influence on net bicarbonate absorption.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Cogan M. G., Rector F. C., Jr Effect of luminal bicarbonate concentration on proximal acidification in the rat. Am J Physiol. 1982 Jul;243(1):F53–F59. doi: 10.1152/ajprenal.1982.243.1.F53. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Cogan M. G. Influence of peritubular protein on solute absorption in the rabbit proximal tubule. A specific effect on NaCl transport. J Clin Invest. 1981 Aug;68(2):506–516. doi: 10.1172/JCI110282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A. Lack of effect of peritubular protein on passive NaCl transport in the rabbit proximal tubule. J Clin Invest. 1983 Feb;71(2):268–281. doi: 10.1172/JCI110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Burg M., Green N. Bicarbonate transport by isolated perfused rabbit proximal convoluted tubules. Am J Physiol. 1977 Oct;233(4):F307–F314. doi: 10.1152/ajprenal.1977.233.4.F307. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Giebisch G. Relationship between sodium and bicarbonate transport in the rat proximal convoluted tubule. Am J Physiol. 1981 Mar;240(3):F222–F230. doi: 10.1152/ajprenal.1981.240.3.F222. [DOI] [PubMed] [Google Scholar]
- Chantrelle B., Cogan M. G., Rector F. C., Jr Evidence for coupled sodium/hydrogen exchange in the rat superficial proximal convoluted tubule. Pflugers Arch. 1982 Nov 11;395(3):186–189. doi: 10.1007/BF00584807. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Maddox D. A., Lucci M. S., Rector F. C., Jr Control of proximal bicarbonate reabsorption in normal and acidotic rats. J Clin Invest. 1979 Nov;64(5):1168–1180. doi: 10.1172/JCI109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Selective Cl retention in repair of metabolic alkalosis without increasing filtered load. Am J Physiol. 1970 Jan;218(1):165–170. doi: 10.1152/ajplegacy.1970.218.1.165. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Steinmetz P. R. Control of active proton transport in turtle urinary bladder by cell pH. J Gen Physiol. 1980 Sep;76(3):381–393. doi: 10.1085/jgp.76.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Rumrich G., Ullrich K. J. Phenomenologic description of Na+, Cl- and HCO-3 absorption from proximal tubules of rat kidney. Pflugers Arch. 1973 Oct 22;343(3):189–220. doi: 10.1007/BF00586045. [DOI] [PubMed] [Google Scholar]
- Gennari F. J., Caflisch C. R., Johns C., Maddox D. A., Cohen J. J. PCO2 measurements in surface proximal tubules and peritubular capillaries of the rat kidney. Am J Physiol. 1982 Jan;242(1):F78–F85. doi: 10.1152/ajprenal.1982.242.1.F78. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., De Mello G. B., De Mello Aires M. Kinetics of luminal acidification in cortical tubules of the rat kidney. J Physiol. 1977 Jun;267(3):571–599. doi: 10.1113/jphysiol.1977.sp011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp A., Boulpaep E. L. Pressure control of sodium reabsorption and intercellular backflux across proximal kidney tubule. J Clin Invest. 1974 Jul;54(1):69–82. doi: 10.1172/JCI107751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman J. G., Brown W. W., Ware R. A., Schwartz J. H. Cell pH and acid transport in renal cortical tissue. Am J Physiol. 1980 Nov;239(5):F440–F444. doi: 10.1152/ajprenal.1980.239.5.F440. [DOI] [PubMed] [Google Scholar]
- Kurtzman N. A. Regulation of renal bicarbonate reabsorption by extracellular volume. J Clin Invest. 1970 Mar;49(3):586–595. doi: 10.1172/JCI106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H., Mathisen O., Holdaas H., Kiil F. Filtered bicarbonate and plasma pH as determinants of renal bicarbonate reabsorption. Kidney Int. 1981 Dec;20(6):780–788. doi: 10.1038/ki.1981.211. [DOI] [PubMed] [Google Scholar]
- Levine D. Z., Nash L. A., Chan T., Dubrovskis A. H. Proximal bicarbonate reabsorption during Ringer and albumin infusions in the rat. J Clin Invest. 1976 Jun;57(6):1490–1497. doi: 10.1172/JCI108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Mello Aires M., Malnic G. Peritubular pH and PCO'2 in renal tubular acidification. Am J Physiol. 1975 Jun;228(6):1766–1774. doi: 10.1152/ajplegacy.1975.228.6.1766. [DOI] [PubMed] [Google Scholar]
- Monclair T., Mathisen O., Kiil F. Renal bicarbonate reabsorption during bicarbonate loading. Kidney Int. 1980 May;17(5):577–585. doi: 10.1038/ki.1980.68. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. A study by continuous microperfusion of water and electrolyte movements in the loop of Henle and distal tubule of the rat. Nephron. 1969;6(3):388–405. doi: 10.1159/000179741. [DOI] [PubMed] [Google Scholar]
- Purkerson M. L., Lubowitz H., White R. W., Bricker N. S. On the influence of extracellular fluid volume expansion on bicarbonate reabsorption in the rat. J Clin Invest. 1969 Sep;48(9):1754–1760. doi: 10.1172/JCI106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Berry C. A., Rector F. C., Jr Effect of luminal and peritubular HCO3(-) concentrations and PCO2 on HCO3(-) reabsorption in rabbit proximal convoluted tubules perfused in vitro. J Clin Invest. 1982 Sep;70(3):639–649. doi: 10.1172/JCI110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J. Na+-dependent H+ efflux from proximal tubule: evidence for reversible Na+-H+ exchange. Am J Physiol. 1981 Oct;241(4):F380–F385. doi: 10.1152/ajprenal.1981.241.4.F380. [DOI] [PubMed] [Google Scholar]
- Seely J. F. Effects of peritubular oncotic pressure on rat proximal tubule electrical resistance. Kidney Int. 1973 Jul;4(1):28–35. doi: 10.1038/ki.1973.77. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Hoffsten P., Purkerson M., Bricker N. S. On the influence of extracellular fluid volume expansion and of uremia on bicarbonate reabsorption in man. J Clin Invest. 1970 May;49(5):988–998. doi: 10.1172/JCI106318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R. Acid-base relations in epithelium of turtle bladder: site of active step in acidification and role of metabolic CO2. J Clin Invest. 1969 Jul;48(7):1258–1265. doi: 10.1172/JCI106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Baumann K. Renal proximal tubular buffer-(glycodiazine) transport. Inhomogeneity of local transport rate, dependence on sodium, effect of inhibitors and chronic adaptation. Pflugers Arch. 1975 Jun 26;357(3-4):149–163. doi: 10.1007/BF00585971. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]


