Abstract
The ability to get and keep an erection is important to men for several reasons and the inability is known as erectile dysfunction (ED). ED has started to be accepted as an early indicator of systemic endothelial dysfunction and subsequently of cardiovascular diseases. The role of NO in endothelial relaxation and erectile function is well accepted. The discovery of NO as a small signalling gasotransmitter led to the investigation of the role of other endogenously derived gases, carbon monoxide (CO) and hydrogen sulphide (H2S) in physiological and pathophysiological conditions. The role of NO and CO in sexual function and dysfunction has been investigated more extensively and, recently, the involvement of H2S in erectile function has also been confirmed. In this review, we focus on the role of these three sister gasotransmitters in the physiology, pharmacology and pathophysiology of sexual function in man, specifically erectile function. We have also reviewed the role of soluble guanylyl cyclase/cGMP pathway as a common target of these gasotransmitters. Several studies have proposed alternative therapies targeting different mechanisms in addition to PDE-5 inhibition for ED treatment, since some patients do not respond to these drugs. This review highlights complementary and possible coordinated roles for these mediators and treatments targeting these gasotransmitters in erectile function/ED.
Linked Articles
This article is part of a themed section on Pharmacology of the Gasotransmitters. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-6
Keywords: gasotransmitters, H2S, CO, NO, sGC/cGMP, erectile function, erectile dysfunction
Introduction
Erectile function
Erectile physiology is the interplay of vascular, neurological and endocrine factors, which leads to an increase in or facilitates the vasodilatation (tumescence) and/or reduces the contraction (detumescence) of the corpus cavernosum smooth muscle (CCSM) cells. Erection is the final outcome of a complex integration of signals. It is essentially a spinal reflex that can be initiated by recruitment of penile afferents, but also by visual, olfactory and imaginary stimuli and all the stimuli contribute to the increase in vasodilatation of penile tissues (for details, see review by Cirino et al., 2006). Neuronal and endothelial NO are considered as the most important factors for relaxation of penile vessels and CCSM cells.
Erectile dysfunction (ED)
ED is defined as the consistent or recurrent inability to attain or maintain a penile erection sufficient for sexual activity in man (2nd International Consultation on Sexual Dysfunction-Paris, 28 June–1 July 2003). It is interesting to note that ED and cardiovascular disease (CVD) share many of the risk factors that contribute to their development and progression such as age, hypercholesterolaemia, obesity, diabetes, smoking and some less-traditional risk factors including inflammation, hypoxia and homocysteinaemia (Brunner et al., 2005). Moreover it is now well accepted that vascular disturbance of the penile endothelium leads to ED and as a consequence the possibility arises that ED may be an early indicator of systemic endothelial dysfunction and subsequently of CVD. In fact, recognizing ED as a disease marker for CVD may help to identify individuals at risk of having a premature cardiovascular event (Shin et al., 2011).
Nitric oxide
The synthesis and physiological significance of NO in erectile function
Constitutive forms of NOS (see Alexander et al., 2013b), the endothelial (eNOS) and neuronal NOS (nNOS) have a role in erectile process. In contrast, inducible form of NOS, iNOS does not have a direct role but is involved in pathological conditions in the penis (Gonzalez-Cadavid and Rajfer, 2005). nNOS is localized in the pelvic plexus, dorsal penile nerve, cavernous nerve and its branches in the cavernous tissue (Sullivan et al., 1999). eNOS is localized in the arterial and cavernous endothelial cells and also in the CCSM (Andersson, 2001). Penile nNOS variant (PnNOS) has been identified in rat and mouse penis nerves, which is considered to be responsible for the synthesis of NO in the terminal nerve of the penis (Gonzalez-Cadavid et al., 2000).
The role of NO in erectile function as the principal mediator is confirmed by several studies where NOS is genetically or pharmacologically inhibited (Burnett, 1995; Burnett et al., 2002; Cashen et al., 2002; Lasker et al., 2010a).
Depolarization of the cavernous nerves by psychogenic and reflex stimuli leads to rapid nNOS-mediated NO release to initiate tumescence (Burnett, 1995; Burnett et al., 2002; Cashen et al., 2002; Lasker et al., 2010a). NO diffuses the CCSM, activates soluble guanylyl cyclase/cGMP (sGC/cGMP) pathway and causes relaxation which increases blood flow to the penis. Following this, blood flow-induced shear stress causes an increase in sustained NO release via PI3K/Akt/eNOS pathway to supply maintenance of tumescence (Hurt et al., 2012; Lasker et al., 2013). It is believed that eNOS is more significant than nNOS in erectile physiology (Bivalacqua et al., 2007d). However, recently, it has been demonstrated that nNOS also contributes to the maintenance of erectile process via sustained release of NO through PKA activation-induced phosphorylation of nNOS at Ser1412 (Hurt et al., 2012). Protein–protein interaction, subcellular localization, phosphorylation and deacetylation (Fleming and Busse, 2003; Mattagajasingh et al., 2007) are the main regulatory mechanisms for eNOS activity. However only a few of eNOS regulatory mechanisms are recognized in penis such as Ca/calmodulin (Ca/CaM), PI3K/Akt-dependent phosphorylation and protein interaction with caveolin or heat shock protein 90 (hsp90; Hurt et al., 2002; Musicki and Burnett, 2006). While hsp90 activates eNOS, caveolin-1 inactivates it by binding to CaM-binding site on eNOS (Musicki et al., 2009). However hsp90 and caveolin-1 are not targets solely for NOS but also for other gaseous molecules and for sGC (see last section of this review). Recently, it has been demonstrated that urotensin II (U-II), an endogenous peptide identified as the natural ligand of a GPCR, physically interacts with eNOS in penis and activates it via phosphorylation (d'Emmanuele di Villa Bianca et al., 2012). Several agonist and stimuli such as shear stress, VEGF, sildenafil, angiopoietin and sphingosine-1-phosphate (S1-P) cause NO production by phosphorylation of eNOS at Ser1177 (d'Emmanuele di Villa Bianca et al., 2006; Musicki et al., 2009). There are six specific sites of phosphorylation in eNOS. However only phosphorylation sites at the Ser1177 and Thr495 residues, activating and inactivating eNOS respectively, were demonstrated in the penis (Hurt et al., 2002; Musicki et al., 2005a).
Pathophysiological significance of NO in ED
Decreased NO bioavailability in vasculogenic ED is caused by decreased NOS activity/synthesis or the inactivation of NO (Musicki et al., 2005a; Jin et al., 2008a; Claudino et al., 2009; Park et al., 2009; Demir et al., 2010; Soner et al., 2010; Saito et al., 2012; Bivalacqua et al., 2013; Dalaklioglu et al., 2013a; Silva et al., 2013; Yang et al., 2013b). Oxidative stress impairing NO bioavailability is a common mechanism for ED. Reactive oxygen species (ROS) result from an imbalance between antioxidant and ROS-generating systems such as NADPH oxidase, myeloperoxidase and even eNOS itself (Zouaoui Boudjeltia et al., 2007; Jin and Burnett, 2008). Oxidation of tetrahydrobiopterin (BH4) or deficiency in cofactor BH4, leads to eNOS uncoupling, in which eNOS becomes two monomers and generates superoxide anion rather than NO (Förstermann and Li, 2011; Johnson et al., 2011). The lack of dimerization is responsible for the pathophysiology of ED in hypercholesterolaemia (Musicki et al., 2010). Moreover, oxidative stress increases iNOS expression, but decreases both expressions of nNOS and eNOS and the erectile response in ischaemic rabbit CC (Azadzoi et al., 2004).
It has been shown that eNOS phosphorylation is altered in vasculogenic ED induced by aging, diabetes mellitus and hypercholesterolaemia (Musicki et al., 2009) and has an important role in the prolongation of erection. Thus, inhibiting phosphorylation of eNOS (p-eNOS Thr495) and dephosphorylation of eNOS (p-eNOS Ser1177) appear as new drug targets for the treatment of ED.
Myristoylation, palmitoylation and acetylation are necessary for caveolar localization of the enzyme, which inactivates eNOS; however, the first two mechanisms have not been investigated in the penis yet. Sirtuin-1 (SIRT-1) leads to activation of eNOS through its deacetylation (Arunachalam et al., 2010). Although a direct role of SIRT-1 has not been investigated in the penis, decreased expressions of SIRT-1 expression in CC in androgen depletion (Tomada et al., 2013) or diabetes (Yu et al., 2013)-induced ED has been shown.
S-nitrosylation negatively regulates NOS by inhibition of sGC, eNOS itself and eNOS-regulating proteins including hsp90 and Akt (PKB). Palmer and co-workers have shown that S-nitrosoglutathione reductase (GSNOR), which catalyses the reduction of S-nitrosothiols (Lima et al., 2010), is co-localized with eNOS in the endothelium of CC. Moreover, S-nitrosylated eNOS levels are increased during detumescence in wild-type mice compared with GSNOR-/- mice (Palmer et al., 2012). The role of S-nitrosylation/denitrosylation of NOS is well documented in erectile physiology but has not been associated with the pathophysiology of erectile function yet.
Beside NO bioavailability, downstream mechanisms of NO, such as increased PDE-5 enzyme and reduced PKG-1 activation by cGMP have also been reported in ED. The pathophysiological significance of eNOS/NO pathway in ED is presented in Table 1.
Table 1.
eNOS/NO regulation in ED
| Pathology | Molecular mechanisms | References |
|---|---|---|
| Hyperlipidaemia/atherosclerosis | ↓p-VASP | Musicki et al., 2010 |
| ↓cGMP | Musicki et al., 2008 | |
| ↑NADPH oxidase, ROS, TBARS production | Musicki et al., 2008; 2010; Fraga-Silva et al., 2013 | |
↓eNOS and nNOS,  eNOS eNOS |
Musicki et al., 2010; Fraga-Silva et al., 2013 | |
 p-eNOS S1177, uncoupled eNOS p-eNOS S1177, uncoupled eNOS |
Musicki et al., 2008; 2010 | |
| ↑ADMA | Park et al., 2009 | |
↑eNOS binding to Cav-1,  Cav-1 Cav-1 |
Musicki et al., 2008 | |
| ↑Rho A expression | Dalaklioglu et al., 2013b | |
| Aging | ↓p-eNOS S1177 | Dalaklioglu et al., 2013b; Silva et al., 2013 |
| ↑p-eNOS T495, ↓ p-Akt | Musicki et al., 2005a | |
| ↑Arginase activity | Sakai et al., 2004; Numao et al., 2007 | |
| ↓cGMP | Silva et al., 2013 | |
| ↑ROS | Johnson et al., 2011 | |
| ↓L-arginine in CC | Sakai et al., 2004 | |
↓ eNOS and nNOS expression,  total NOS activity total NOS activity |
Numao et al., 2007; Dalaklioglu et al., 2013b | |
| ↑p-AMPK | Labazi et al., 2013 | |
↓p-eNOS S1177,  p-eNOS S1177 p-eNOS S1177 |
Saito et al., 2012; Labazi et al., 2013 | |
| Hypertension | ↓cGMP | Gur et al., 2010; Saito et al., 2012 |
| ↑ROCK mRNA expression, ↑Cav-1 | Yono et al., 2009 | |
| ↑ROS | Jin et al., 2008a | |
↓nNOS and eNOS, ↑iNOS expression,  nNOS nNOS |
Gur et al., 2010; Saito et al., 2012; Labazi et al., 2013 | |
| ↑ERK1/2 Phosphorylation | Labazi et al., 2013 | |
| ↓eNOS, nNOS | Li et al., 2012; Dalaklioglu et al., 2013a; Qiu et al., 2013 | |
| ↓p-eNOS S1177 | Musicki et al., 2005b; Dalaklioglu et al., 2013a; Yang et al., 2013a | |
| ↑RhoA expression | Dalaklioglu et al., 2013a | |
| Diabetes | ↑NADPH oxidase and ROS | Li et al., 2012; Dalaklioglu et al., 2013a; Yang et al., 2013a |
| ↓p-Akt | Musicki et al., 2005b | |
 p-eNOS T495 p-eNOS T495 |
Musicki et al., 2005b | |
| ↓cGMP | Fukuhara et al., 2011; Yang et al., 2013a | |
| ↑Arginase II | Bivalacqua et al., 2001 | |
| ↑Cav-1 | Elçioğlu et al., 2010 | |
| ↑ROS, uncoupled eNOS, ↑cGMP | Bivalacqua et al., 2013 | |
↓p-eNOS S1177, ↓ eNOS- HSP90 interaction, ↓ p-AKT,  p -eNOS T495, p -eNOS T495,  eNOS, eNOS,  Hsp90, Hsp90,  Cav-1 Cav-1 |
Musicki et al., 2011 |
↑, increase; ↓, decrease,  , unchanged; p-VASP, vasodilator stimulated phosphoprotein; Cav-1, caveolin-1; p-AMPK phosphorylated 5′ AMP-activated PK; TBARS, thiobarbituric acid-reactive substances; ADMA, asymmetric dimethylarginine; p-eNOS T495, eNOS phosphorylated on threonine 495; p-eNOS S1177, eNOS phosphorylated on serine 1177; uncoupled eNOS, monomer form of eNOS that is generating ROS rather than NO; ROCK, Rho-kinase.
, unchanged; p-VASP, vasodilator stimulated phosphoprotein; Cav-1, caveolin-1; p-AMPK phosphorylated 5′ AMP-activated PK; TBARS, thiobarbituric acid-reactive substances; ADMA, asymmetric dimethylarginine; p-eNOS T495, eNOS phosphorylated on threonine 495; p-eNOS S1177, eNOS phosphorylated on serine 1177; uncoupled eNOS, monomer form of eNOS that is generating ROS rather than NO; ROCK, Rho-kinase.
Treatments targeting NO
Known NO-based therapies include NO precursors, NO donors, NO-based gene therapy, pharmacological NOS activators such as resveratrol. See reviews by Decaluwe and co-workers for details (Bryan, 2011; Decaluwe et al., 2013). Although L-arginine substrate of NOS has been found to increase relaxation in human or animal CC (Table 2), clinical studies do not always support the beneficial effects of L-arginine alone. However, it seems successful in combination therapies (Table 3). L-arginine is also a substrate for arginase and its inhibition increases substrate availability of NOS. Arginase inhibitors have been found to increase neuronal and endothelial-dependent relaxation of CC, improve erectile function especially in diabetic ED as well as aging-induced ED. Recently, a protective effect of resveratrol, NOS activator, has been shown in diabetes and hypercholesterolaemia-induced ED (see Table 2). Even though there are many animal studies that indicate the success of NADPH oxidase inhibitors and gene therapies in ED (see Table 2), no clinical trial has been performed with these therapies yet. The importance of the NO/cGMP pathway as drug targets became clear with the discovery of the PDE-5 inhibitors (PDE-5i) and their great success in treating ED. However, there are significant numbers of ED patients with diabetes mellitus and severe vascular disease who do not respond to PDE-5i, suggesting that maintaining physiological levels of NO may not be sufficient in mild or severe ED associated with vascular risk factors. Therefore, drugs targeting the bioavailability or downstream mechanisms of NO or combination therapies have started to be investigated. Clinical and preclinical pharmacological treatments and gene therapies targeting the NOS/cGMP pathway are summarized in Tables 2 and 3 respectively.
Table 2.
Preclinical studies in ED targeting NOS/NO pathway
| Drugs targeting NOS/NO pathway | Effects in ED models | References |
|---|---|---|
| NOS substrate L-arginine and L-citrulline | ↑ICP/MAP in acute arteriogenic rats and relaxation in healthy human | Gur et al., 2007; Shiota et al., 2013 |
| NOS cofactor | ↑ICP/MAP in aged mice and neurogenic relaxation in obese rat CC | Johnson et al., 2011; Sanchez et al., 2012 |
| BH4 | ||
| Arginase inhibitors | ↑Neurogenic and ICP/MAP in aged rats/mice and endothelial relaxation in aged/diabetic mice CC | Bivalacqua et al., 2001; 2007a; Toque et al., 2011; Segal et al., 2012 |
| ABH and BEC | ||
| NADPH oxidase inhibitor apocynin | ↑ICP/MAP in hypertensive/diabetic rats/hypercholesterolaemic mice and endothelium-dependent relaxation in aged rat CC. | Jin et al., 2008a; Musicki et al., 2010; Li et al., 2012; Silva et al., 2013 |
| Pharmacological NOS activator resveratrol | ↑ICP/MAP in diabetic rats and endothelial relaxation in hypercholesterolaemic rabbit and healthy rat CC. | Shin et al., 2008; Soner et al., 2010; Fukuhara et al., 2011; Yu et al., 2013 |
| NO-releasing agents NaNO2 | ↑ICP/MAP in healthy and diabetic rats and endothelial relaxation in hypercholesterolaemic rabbit CC. | Shukla et al., 2005; Lasker et al., 2010b; Soni et al., 2013 |
| Gene therapies eNOS/PnNOS/EC-SOD/iNOS/PKG1α/VEGF gene and angiopoietin-1 | ↑ICP/MAP in aged/ diabetic rat and in healthy /diabetic rat CC. | Bivalacqua et al., 2000; 2003; 2004c; 2005; 2007b,c; Magee et al., 2002; Chancellor et al., 2003; Ryu et al., 2006; Wang et al., 2013 |
| Inhibition of genes PIN/arginase/RhoA (T19NRhoA) | ↑ICP/MAP in healthy/diabetic rat, aged mice and healthy/aged rat CC. | Chitaley et al., 2002b; Bivalacqua et al., 2004b; 2007a; Jin et al., 2006; Magee et al., 2007 |
MAP, mean arterial pressure; BEC, S- (2-boronoethyl)-L-cysteine; ABH, 2(S)-amino-6-boronohexanoic acid; EC-SOD, endothelial cell superoxide dismutase; PIN, protein inhibitor of NOS.
Table 3.
Clinical studies targeting NOS/NO in ED
| Therapy | Drug dose | Patients | Sexual function | References |
|---|---|---|---|---|
| L-arginine supplementation | L-arginine 3 × 500 mg·day−1 | 32 patients with mixed-type impotence | No difference | Klotz et al., 1999 |
| L-arginine (5 g·day−1) | 50 organic ED patients. A double-blind, randomized, placebo-controlled study. | Significant improvement | Chen et al., 1999 | |
| BH4 supplementation | Single oral doses of BH4 200 mg or 500 mg | 18 moderate ED patients in a randomized, placebo-controlled, double-blind crossover study. | Increase duration of penile rigidity. | Sommer et al., 2006 |
| Combined therapies with L-arginine | L-arginine aspartate 8 g + adenosine monophosphate 200 mg | Mild-to-moderate ED whose erectile function domain score between 14 and 22 | Effective | Neuzillet et al., 2013 |
| L-arginine + L-carnitine + nicotinic acid + vardanafil | Insulin-dependent diabetic patients | Better than PDE-5 inhibitor alone | Gentile et al., 2009. | |
| L-arginine 6 g + yohimbine 6 g during 2 weeks or L-arginine 6 g | 42 patients mild to moderate ED. Double-blind, placebo-controlled, three-way crossover, randomized clinical trial. | L-arginine do not improve alone but combined therapy improve erectile function | Lebret et al., 2002 | |
| L-arginine aspartate 1 g during 3 months + pycnogenol 3 × 40 mg during 2 months | 40 ED patients | Improve sexual function. | Stanislavov and Nikolova, 2003 |
Carbon monoxide
CO was known as the ‘silent killer’ until the 21st century because it is odourless and colourless and it can threaten life by hypoxic intoxication without giving an obvious symptoms or indications. Unlike NO and H2S, CO elimination is through exhalation by the lungs and does not involve a biochemical modification (Kreck et al., 2001; Motterlini and Otterbein, 2010). CO might exert its effects during longer periods of time and distances compared with NO or H2S, as it is the most biologically stable gasotransmitter with a half-life of around 3 h (Motterlini and Otterbein, 2010) since it does not have unpaired electrons, and does not chemically dissociate in an aqueous solution to form different chemical species.
The synthesis of CO
The majority of CO is produced by enzymatic haem metabolism (Wu and Wang, 2005) and the remaining fraction of CO arises from other sources that may include lipid peroxidation and xenobiotic metabolism.
Haem oxygenase enzymes (HO) exist in constitutive (HO-2 and HO-3) and inducible (HO-1) isoforms (see Alexander et al., 2013b). HO-1, a 32 kDa mammalian stress protein is induced by several stimuli including hypoxia, stress, ROS, inflammatory cytokines and a variety of transition metals and heavy metals (see review by Ryter for a detailed list of HO inducers; Ryter et al., 2006). HO-1 induction leads to the release of iron and the formation of biliverdin and CO, which are able to regulate ROS level and inflammatory processes to a certain extent. HO expression regulates the level of ROS by increasing antioxidant, such as glutathione and bilirubin. HO-1 is expressed less in nerve fibres but is clearly identifiable in the endothelium lining of penile arteries and the sinusoidal walls of the CC and spongiosum (Hedlund et al., 2000b). However, upon stimulation, HO-1 expression in the testes overpowers the expression of HO-2. HO-2 is a constitutively expressed ‘haem sensor’ in the endothelium and CCSM, engaged in fine-tuning the activity of transcriptional factors and genes that are haem-responsive, including HO-1. HO-2 gene expression has been shown not to be changed by either HO inducers or inhibitors (Abdel Aziz et al., 2005). HO-2 expression is more condensed in the pelvic ganglion and nerve fibres innervating bulbospongiosus muscles in rat and human urogenital system (Burnett et al., 1998; Hedlund et al., 2000b; Ushiyama et al., 2004).
HO-3 has only been found in rat tissues, including brain, liver, kidney and spleen. HO-3 is related to HO-2 and represents pseudo genes originating from HO-2 transcripts (Hayashi et al., 2004).
Physiological significance of CO in erectile function
In isolated vessel preparations, both CO and haem-L-lysinate increase the vasodilatation (Kozma et al., 1997) of which only the latter can be reversed by inhibitors such as HO chromium mesoporphyrin (Kozma et al., 1997). The role of HO/CO pathway in erectile function was first demonstrated by showing HO expression and CO induced relaxation in human CC (Hedlund et al., 2000b). Further, it was confirmed that exogenous CO relaxes the CC dose dependently in rat (Ushiyama et al., 2004). NOS or HO inducers can equally up-regulate expression of both genes and increase the tissue levels of cGMP in CC. Aziz and colleagues suggest that HO/CO system is supervising and dominating NO as a signalling molecule in erectile function (Abdel Aziz et al., 2005). Thus, induction of HO may have therapeutic implications for the management of ED (Decaluwe et al., 2013).
Electrical field stimulation (EFS)-induced relaxations are inhibited by HO inhibitors; tin-protoporphyrin (SnPP) and zinc-protoporphyrin (ZnPP; Ushiyama et al., 2004) and increased by exogenous CO in rat CC (Kim et al., 2010). The suppression of EFS-induced relaxation by SnPP has been found to be specific to HO inhibition and not related to NOS inhibition, as is the case in the hippocampus (Meffert et al., 1994), since the relaxation that remained in HO inhibitor treated group was further inhibited by L-NNA. On the contrary, neurogenic relaxation by EFS is not inhibited in rabbit CC by ZnPP (Kim et al., 1994) or in HO-2 knockout mice (Burnett et al., 1998). Nevertheless, more rigorous investigations need to be performed before suggesting that the role of CO in neurogenic erection may be different in rats compared with other species, since the expression and the biological status of HO-1 are not clear in this HO-2-deficient mice model and HO-1 may also cause neurogenic erection.
In addition to the involvement in penile erection control, CO also plays an important role in regulating ejaculation (Burnett et al., 1998). HO-2 knockout mice have less reflex activity of the bulbospongiosus muscle, where the HO-2 localization is condensed, and substantially reduced ejaculation, without a significant change in erectile function. In the same year, another study reported that prenatal exposure to CO (150 ppm) leads to increased mount/intromission latency, decreased mount/intromission frequency, and a significant decrease in ejaculation frequency, which are associated with changes in mesolimbic dopaminergic function in male rats (Cagiano et al., 1998). The authors speculated that prenatal exposure to CO might influence the development or function of neurons releasing CO locally in the penis and decreases HO-2 expression/activity parallel to the findings in HO-2 −/− animals.
Pathophysiological significance and treatments targeting CO in ED
Drugs targeting activation of HO/CO pathway
A number of studies have suggested that impaired CO-mediated vasodilatation is implicated in ED (Abdel Aziz et al., 2009c; Shamloul, 2009). Ushiyama and colleagues clearly showed that NO- and CO-dependent relaxation of the CC in response to EFS is diminished in spontaneously hypertensive rats (SHR) and suggested that this may be due to decreased activity of HO-2, since the HO-2 gene expression was unchanged (Ushiyama et al., 2004). This study for the first time showed that the impairment of neurogenic relaxation induced by HO/CO systems may, to a certain degree, be involved in the diminished erectile responses in SHR (Ushiyama et al., 2004). Two studies suggest that HO inducers may ameliorate the erectile function in SHR by showing that; (i) a potent HO-1 inducer, haemin, increased both intracavernous pressure (ICP) and HO-1 level, but not HO-2, as well as HO-1 downstream molecule sGC expression in SHR (Shamloul and Wang, 2006); and (ii) the improved erectile function by the antioxidant α-tocopherol in SHR could be blocked by an HO inhibitor (Ushiyama et al., 2008).
An HO-1 inducer reversed the decreased erectile function, gene expression and enzymatic activity of HO-1 in CC of diabetic rats (Abdel Aziz et al., 2009a). This study suggests that the decline in erectile function in diabetic rats may be attributed to a down-regulation of the HO/CO pathway and indicates that stimulating this pathway could be an effective treatment for ED in diabetic patients. In addition, HO-1 induction also restores decreased eNOS expression and vascular responses as well as reversing the increased iNOS expression in diabetic rat aorta (Ahmad et al., 2005). It has been suggested that the antioxidant effects of HO might also contribute to its endothelial protective effect in diabetes (Kruger et al., 2006).
In addition, chronic administration of the HO-1 inducers in hypertensive and diabetic rats and an in vivo gene therapy using HO-1 cDNA-liposome complex transfer have been found to be beneficial for ED induced by aging (Abdel Aziz et al., 2009b). HO-1 inducers, which have been reported to augment HO-1 expressions and/or cGMP concentrations together with subsequent relaxation in CC, are listed in Table 4. Several studies show that HO-1 induction by losartan and/or CoPP (Abdel Aziz et al., 2009a), hemin (Abdel Aziz et al., 2008), curcumin (Abdel Aziz et al., 2010) restores ED through the up-regulation of the local tissue levels of cGMP. The erectile function induced by HO-1 induction was found to be as effective as up-regulating NOS by L-Arg. (Abdel Aziz et al., 2005), complementary to and even dominating NO in mediating erectile function (Abdel Aziz et al., 2009a).
Table 4.
Targeting HO/CO in erectile function
| HO-1 inducing drug | Model | References |
|---|---|---|
| HO-1 cDNA-liposome complex transfer | Aged rats | Abdel Aziz et al., 2009b |
| Hemine | SHR | Shamloul and Wang, 2006 |
| Losartan | Diabetic rats | Abdel Aziz et al., 2009a |
| Hemin | Healthy rat | Abdel Aziz et al., 2008 |
| Curcumin | Healthy rat | Abdel Aziz et al., 2010 |
| α-tocopherol | SHR | Ushiyama et al., 2008 |
| PDE-5 inhibitors; sildenafil, tedalafil, verdanafil | Healthy rat | Abdel Aziz et al., 2007a,b,c; Liu et al., 2012 |
Approaches increasing HO-1 induction and subsequently erectile functions are listed.
Interestingly, NO itself has been shown to induce HO-1 to produce CO (Durante et al., 1997). Thus if the HO/CO pathway is involved in the mechanism of the NO targeting, drug-induced beneficial effects in relaxation should be investigated. Moreover, the effect of sildenafil on ED has been attributed to interactions between CO and NO (Abdel Aziz et al., 2007a). α-tocopherol has been shown to enhance erectile function in both a NOS- and HO-dependent manner in ED in SHR (Ushiyama et al., 2008). Some of the cardiovascular drugs targeting HO/CO pathway are listed in Table 5 as well as losartan and sildenafil, which are listed in Table 4. Among those drugs, losartan, α-tocopherol and PDE-5i are shown to induce HO/CO in CC but HO-related effects of statins have not been investigated in the penis yet. However, there is increasing knowledge concerning the significance of the HO/CO pathway in the pathophysiology, which has led to the development of CO-releasing molecules, known as CORMs, a safe therapeutic strategy, releasing CO with controllable kinetics (Motterlini et al., 2002). Tayem and colleagues indicated that CORMs can induce HO-1 and thus have a dual action, releasing CO and increasing HO-1 (Tayem et al., 2006). This is not surprising since the ability of CO to induce HO activity has already been shown (Kim et al., 2007).
Table 5.
Cardiovascular treatments targeting HO/CO in the vascular system
| Cardiovascular drug | Explanation | References |
|---|---|---|
| Atorvastatin | Activates HO-1 to get compensatory anti-inflammatory and vasorelaxant effect in hypercholesterolaemic rabbit aorta | Muchova et al., 2007; Fujita et al., 2010; Ong et al., 2011 |
| Atorvastatin-clinical study | Decreases inflammation and oxidant stress via mechanisms associated with HO-1 induction and CO, but not bilirubin | Ong et al., 2011 |
| Angiotensin II | Regulates HO-1 in rat vascular smooth muscle cells | Ishizaka and Griendling, 1997 |
| Resveratrol | Induces HO-1 in human aortic smooth muscle cells in a concentration-dependent manner | Juan et al., 2005 |
| NO donors (SpermineNONOate, SNAP) | Inhibits HO activity in vascular smooth muscle cell | Durante et al., 1997; Hartsfield et al., 1997 |
In vivo delivery of CORM-3 increases blood flow in penile arterioles and sinuses (Abdel Aziz et al., 2008). CORM-2 also induces relaxation in mice CC but differently from CO, CORM-2-induced corporal relaxation was not affected by sGC inhibition. (Decaluwe et al., 2012b) Readers interested in CO targeting molecules as therapeutics in specific pathological conditions are referred to a recent review by Motterlini and co-workers (Motterlini and Foresti, 2014).
Drugs leading to inhibition of HO/CO pathway
Priapism represents a ‘medical emergency’ with a persistent, usually painful erection that lasts for more than 4 h and occurs without sexual stimulation. It may lead to permanent ED and penile necrosis if left untreated and occurs in approximately 40% of patients with sickle cell disease (SCD; Kato and Gladwin, 2008). HO-1 expression is increased in SCD patients (Nath et al., 2001; Jison et al., 2004) in transgenic sickle mice (Belcher et al., 2006) and in artificially- induced veno-occlusive, low-flow priapism (Jin et al., 2008b).
The question arising is this; should we try to prevent HO-1 activation before it appears? Or inhibit the HO-1 activity in late priapism? Prompt treatment for priapism is usually needed to prevent tissue damage that could result in ED. The evidence to recommend medical prophylaxis is sparse but based on a consensus of experts and small phase 2 or 3 clinical trials (Olujohungbe and Burnett, 2013). It has been shown that HO inhibition by ZnPP reversed the apoptosis induced by ischaemic priapism in rats and seems promising for preserving erectile function in late priapism (Karakeci et al., 2013).
Hydrogen sulphide
This molecule, now considered to be the third gaseous transmitter, shares many characteristics with the other gaseous transmitters: NO and CO (Wang, 2002). The role of H2S in the homeostatic control of our body is now consistently supported by the literature (Wang, 2012).
H2S presence in mammalian tissues was known even in the eighties but it was considered a metabolic waste product, and its potential physiological activity was ignored. Kruszyna and co-workers in 1985 described an influence by cyanide and sulphide compounds in the relaxation induced by nitrogenous compounds (Kruszyna et al., 1985). The first evidence indicating this gas as an endogenous mediator was in 1996 by Abe and Kimura (1996) and it was in the brain.
Solid evidence demonstrated that H2S acts as a potential neurotransmitter (Gadalla and Snyder, 2010) and exerts many activities in mammalian cardiovascular and respiratory systems (Hosoki et al., 1997; Zhao et al., 2001). Regarding the physiological significance of H2S, a turning point has been achieved by the development of the knockout (KO) strain for both cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) enzymes. CBS is essential for life since in homozygous KO of CBS mice the lifespan would only be (about) 4 weeks (Watanabe et al., 1995) and CSE-KO mice develop hypertension (Yang et al., 2008).
Synthesis of H2S
H2S is generated within the mammalian cells via both enzymatic and non-enzymatic pathways, although the major contribution comes from the enzymatic one. CBS and CSE use L-cysteine (L-Cys) as the substrate to produce H2S, while CBS can also use homocysteine to produce cystathionine that is metabolized by CSE to H2S. Both CBS and CSE use pyridoxal 5′-phosphate, as a cofactor. The main H2S-producing enzyme in the CNS is CBS while in the cardiovascular system, it is CSE (Zhao et al., 2001; Eto et al., 2002). Moreover, it has been suggested that H2S could exert a negative feedback effect on the enzyme activity to regulate its synthesis and release (Kredich et al., 1973). Other enzymes mainly localized in endothelial cell have been proposed to synthesize this gas, the 3-mercaptopyruvate sulphurtransferase and the cysteine aminotransferase. For more details, see review by Wang (2012).
Physiological significance of H2S in erectile function
In 2006, it was shown that intracavernosal injection of sodium hydrogen sulphide (NaHS) resulted in a significant increase in penile length and cavernous pressure in primates. Administration of DL-propargylglycine (PAG, CSE inhibitor) to rats resulted in a significant reduction in cavernous nerve stimulation-evoked perfusion pressure. On the basis of these results, a possible role for endogenous H2S in erectile function has been suggested (Srilatha et al., 2006).
In 2009, d'Emmanuele and co-workers clearly demonstrated that the L-Cys/H2S pathway is present in human CC tissues. In particular, it was shown that both CBS and CSE are present and are active in human CC since tissue homogenates efficiently convert L-Cys to H2S. CBS and CSE are localized within muscular trabeculae and the smooth-muscle component of the penile artery. Conversely, CSE but not CBS is also expressed in peripheral nerves. Moreover, both H2S and L-Cys cause a concentration-dependent relaxation of human CC strips. This relaxation effect was inhibited by the CBS inhibitor, aminoxyacetic acid (AOAA), glibenclamide, a KATP (Kir6.1-6.2) channel (see Alexander et al., 2013a) inhibitor, and was only slightly reduced by L-NG-nitroarginine methyl ester (L-NAME), a NOS inhibitor. EFS of human penile tissue, under resting conditions, caused an increase in tension that was significantly potentiated by either PAG or AOAA. The role of this pathway in erectile function was also confirmed in vivo, in fact, NaHS and L-Cys increased the ICP in rat, and the response to L-Cys was blocked by PAG (d'Emmanuele di Villa Bianca et al., 2009).
Pathophysiological significance and treatments targeting H2S in ED
The altered expression of CSE and H2S levels are involved in some acute inflammatory processes (Zanardo et al., 2006; d'Emmanuele di Villa Bianca et al., 2010) in atherosclerosis (Wang, 2009b; Wang et al., 2009), diabetes (Wu et al., 2009), hypertension (Yang et al., 2008), hyper-homocysteinaemia (d'Emmanuele di Villa Bianca et al., 2013) and obesity (Elshorbagy et al., 2012), which are pathological conditions associated with ED. A link between male sexual hormones and H2S has been suggested by Bucci and co-authors, who demonstrated that testosterone (T) causes an increase in the H2S concentration acting on KATP channels. Thus, H2S contributes to the vasodilator effect of testosterone (Bucci et al., 2009). Testosterone induces relaxation by activating smooth muscle KATP channels in human CC strips (Yildiz et al., 2009) and in horse penile resistance arteries (Ruiz Rubio et al., 2004). It has been demonstrated that aging significantly reduces NO and H2S levels both in plasma and CC and a reduction of the ICP was countered by NaHS or sildenafil treatment for 10 weeks. To confirm that there is a link between T and H2S, Syrilatha and co-authors have shown a marked increase in T or oestradiol after NaHS supplementation. These data support the idea that ED related to aging may be also linked to a derangement in the H2S pathway accompanied by low T levels (Srilatha et al., 2012).
If T can modulate H2S production, the decline in T level with aging or hypogonadism may also affect H2S biosynthesis. All these data suggest the involvement of the L-cys/H2S pathway in penile erection mechanisms of T (for details, see review by d'Emmanuele di Villa Bianca et al., 2011). This very interesting issue needs to be addressed more accurately to translate this preclinical data to humans.
The efficacy of PDE-5i, the mainstay in the treatment of ED, seems to be tightly associated with the integrity of nerves and endothelium in CC and in several pathologies such as CVD, diabetes, obesity and post-prostatectomy state, this integrity is severely compromised leading to lack of the NO/cGMP pathway. Thus, there is a pressing need to discover new therapies for targeting other pathways not totally dependent on endothelium integrity. In this regard, the H2S pathway could offer one opportunity since CBS and CSE are mainly localized within muscular trabeculae and in human penile tissues and the H2S-induced relaxation is only partially reduced by L-NAME treatment. A tentative move towards developing a drug working on H2S and cGMP pathways (i.e. not totally dependent on endothelium integrity) was performed by Shukla and co-workers who synthesized and characterized an H2S-donating derivative of sildenafil (ACS6; Shukla et al., 2009). Surprisingly, ACS6 had a similar efficacy to sildenafil and this result can be explained by the fact that H2S and PDE-5i share the same target (e.g. PDE-5). Most probably, the development of drugs that either deliver H2S directly or stimulate the enzyme activity responsible for its synthesis might be more efficacious.
While the inorganic forms of H2S-releasing molecules, NaHS or Na2S, are basic tools used to understand the H2S role in the body, they are not eligible for treatments due to the rapid H2S donation because of high solubility. For instance, we need the H2S long-term releasing molecule. The best way to obtain a controlled gas release is to induce its synthesis endogenously by using L-Cys and/or N-acetylcysteine, but this approach could not work in a condition where a down-regulation of the enzyme CBS and CSE occurs. Until now, no studies have addressed the potential effect of L-Cys on human ED.
Concerning natural plant-derived compounds, the S-allyl cysteine, a bioactive component derived from garlic, can restore erectile function in diabetic rats by preventing ROS formation through modulation of NADPH oxidase subunit expression (Yang et al., 2013b). However, whether it plays a role as a H2S precursor or a modulator of H2S-related enzymes is controversial (Jacob et al., 2008). Other garlic-derived molecules, generally considered as precursors of H2S metabolized in blood, have been studied for their potential anti-inflammatory and anti-cancer effects such as diallyl trisulphide, diallyl sulphide, diallyl disulphide and diallyl tetrasulphide but no data concerning their efficacy on CC are available.
In contrast, the synthetic H2S donor that is attracting most interest is GYY4137. It inhibits lipid accumulation exhibiting anti-atherosclerotic activity both in vitro and in vivo (Yang et al., 2013b). However, there is no data available on the effect of GYY4137 in ED.
Recently, it has been shown that H2S can elicit vasoprotection by both scavenging O2− and by reducing vascular NADPH oxidase-derived O2− production in vascular tissues (Vacek et al., 2010; Hamar et al., 2012; Al-Magableh et al., 2014), Since ROS is the common cause of the ED and when eNOS is uncoupled it can produce ROS, the beneficial effects of drugs targeting H2S in ED is not surprising.
The three gases – is there a convergence point?
The three gasotransmitters share similarities as modulators of physiological processes (Wang, 2002). CO, NO and H2S are all able to induce SM relaxation contributing to penile erection. The common mechanism of these gases to cause erectile function is ‘increasing the cGMP level’ (Figure 1). The enzyme sGC is accepted as the most important target for NO to increase cGMP, which contributes to penile erection. Besides NO, CO can also bind to the enzyme sGC for its activation. However, sGC is not always associated as the target molecule for the three of them. H2S increases the cGMP level without stimulating sGC. In this section, we will discuss the relationship between he gasotransmitters (NO, CO and H2S) and their molecular mechanism in erectile function.
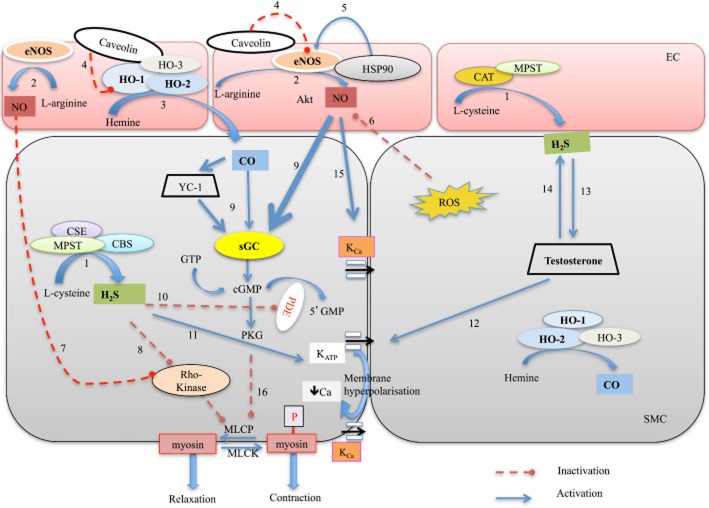
Figure 1.
Synthesis and mechanisms of gaseous neurotransmitters in the relaxation of penile or other vascular tissues. Unbold fonts indicate evidence obtained in other vascular tissues rather than the penis. Dashed red lines indicate inhibition, whereas straight lines indicate activation. Endothelial cells (EC) are shown as pink boxes and smooth muscle cells (SMC) are shown as grey boxes. 1: CBS, CSE and MPST synthesize H2S from L-cysteine. (CBS, CSE and MPST are expressed in smooth muscle cells in the penis. MPST can be also expressed in the endothelium of some vascular tissues). 2: eNOS synthesizes NO from L-arginine. 3: CO is synthesized from hemine by constitutive (HO-2 and HO-3) and inducible (HO-1) haem oxygenases. 4: Caveolin interacts and inactivates both eNOS and HO-1. 5: Hsp90 (HSP90) activates eNOS. 6: ROS decreases the availability of NO to act on sGC. 7: NO induces relaxation via inhibition of Rho-kinase (ROCK) signalling in the penile tissue. 8: H2S-induced relaxations are increased in CC precontracted with endothelin, indicating a possible involvement of the RhoA/ROCK pathway in H2S-induced erectile function. 9: Both NO and CO activate sGC to produce cGMP. CO-induced activation of sGC is lower than NO-induced activation of sGC. However, CO favours YC-1-induced haem-independent activation of sGC. 10: H2S inhibits cGMP breakdown by PDE-5. 11: H2S activates KATP and leads to membrane hyperpolarization, which decreases intracellular calcium level via KCa channels and consequently causes relaxation. 12: Testosterone induces relaxation by activating smooth muscle KATP channels in human CC strips. 13: NaHS treatment increases testosterone level in aging rats. 14: Testosterone causes an increase in H2S level. 15: NO activates large conductance KCa (KCa1.1 also known as BKCa) in horse penile resistance arteries. 16: PKG can cause relaxation through activation of MLCP and reduce Ca2+ sensitivity in the penis.
sGC
sGC is a heterodimer and it is similar to other nucleotide-converting enzymes. Two different subunits with two isoforms of each have been identified: α1, α2, β1 and β2. The most abundant form of the heterodimer sGC is α1/β1 in CC (Behrends et al., 1995). Both show sensitivity towards NO-releasing substances and to sGC activators. CC from sGCα1-/- mice showed significantly less or no relaxation in response to bradykinin (BK) and ACh, respectively, emphasizing the requirement of sGCα1 subunit for the erectile function of endothelium-derived NO (Nimmegeers et al., 2008). The absence of EFS-induced relaxation in these mice indicates sGCα1β1 as the predominant target for neuronal NO. The minor contribution of sGCα2β1 isoform in erectile function has been suggested in this study since some responsiveness to exogenous NO (SNP and NO-gas) and sGC stimulator (BAY 41-2272) remains in the sGCα1-/- mice CC (Nimmegeers et al., 2008).
Activation of sGC by NO involves binding to the enzyme's prosthetic haem group since its removal abolishes NO-induced activation (Stone and Marletta, 1995). After binding to the sGC haem, NO increases sGC activity by several hundred-fold (Derbyshire and Marletta, 2009) promoting the conversion of GTP to cGMP. In contrast, CO causes only a few fold increases in sGC activity, whereas this enzyme is unlikely to be activated by H2S (Zhao and Wang, 2002). Despite the lower ability of CO to activate sGC compared with NO, it was reported that the vasodilator and erectile effects of CO are mediated by sGC activation (Friebe et al., 1996; Nakane et al., 2002; Decaluwe et al., 2012a).
Besides the well-established NO/haem-mediated stimulation, other mechanisms for sGC activation have been identified. The activation induced by 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) and 5-cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-4-ylamine (BAY 41-2272) involves binding to a site different from the haem group (Stone and Marletta, 1995; Friebe et al., 1998). In vascular SM cells, YC-1 sensitizes sGC to NO and CO (Liu et al., 2009). These compounds together with the amino dicarboxylic acid substance, BAY 58-2667 can evoke erectile responses, enhance cGMP formation and/or CC relaxation synergistically with NO (Mizusawa et al., 2002; Nakane et al., 2002; Stasch et al., 2002; Baracat et al., 2003; Hsieh et al., 2003; Teixeira et al., 2007; Frey et al., 2012).
Although CO stimulates purified sGC very poorly, only 3–4-fold (Schmidt et al., 2001), in the presence of sGC activators such as YC-1, sGC activation by CO is drastically enhanced, near to that stimulated by NO (Friebe et al., 1996; Lee et al., 2000; Ma et al., 2007). CO relaxes CC through activation of sGC, indicated by the inhibiting effect of (1H-[1,2,4] oxadiazolo[4,3,-a]quinoxalin-1-one) and potentiating effect of YC-1 on the CO-induced responses in mice CC (Decaluwe et al., 2012a).
The findings that (i) CO activates sGC in a similar way to NO, and (ii) it can activate sGC in nNOS-deficient mice (Zakhary et al., 1997), suggest that endogenous CO might serve as a backup system when constitutive enzymes for NO are not functional or available.
Hsp90
We have previously observed that the relaxation mediated by sGC is regulated by the molecular chaperone 90-kDa heat shock protein hsp90 (Yetik-Anacak et al., 2006). Inherent ATPase activity of hsp90 helps to protect cells against stressors through the control of maturation, trafficking, stability and activity of client proteins, such as the enzymes NOS and sGC (Garcia-Cardena et al., 1998; Venema et al., 2003; Yetik-Anacak et al., 2006). Hsp90 is important to drive haem insertion and maturation of sGC (Ghosh and Stuehr, 2012). We demonstrated hsp90 and eNOS interaction and functional significance in mice CC (Yetik-Anacak et al., 2013). Musicki et al. also showed the decreased complex formation of hsp90-eNOS in sickle cell anaemia-induced ED (Musicki et al., 2011). There is evidence that it also regulates CO and H2S activities in myocardial cells and astrocytes (Choi et al., 2010; Yang et al., 2011). Whether hsp90 interacts with CO and H2S in the CC and contributes to penile erection remain to be elucidated.
cGMP
The product formed following sGC activation from GTP is the second messenger cGMP, that modulates the activity of several effector proteins leading to vasorelaxation (Schmidt et al., 1993). NO and CO induce an increase in cGMP levels in CC (Priviero and Webb, 2010; Decaluwe et al., 2012b). H2S is also able to induce an increase in cGMP levels; however, it does not seem to directly activate sGC as mentioned above (Coletta et al., 2012). As sildenafil, H2S has been implicated as an inhibitor of PDE-5 delaying cGMP degradation (Bucci et al., 2010; Coletta et al., 2012). Furthermore, although it has been suggested that cGMP or cAMP analogues cause an increase in H2S production in human bladder (Fusco et al., 2012), this issue has to be confirmed in penile tissue.
PKG
Once formed, the principal intracellular mediator of the cGMP is the PK dependent on cGMP, PKG, which is a key step in the signal cascade leading to penile erection (Hedlund et al., 2000a). PKG plays a role in mediating NO-, CO- and H2S-dependent signalling in vascular tissue and BP control (Lohmann et al., 1997; Lincoln et al., 2001; Schlossmann et al., 2003; Leffler et al., 2005; Bucci et al., 2012; Burgoyne et al., 2012).
PKG can cause vascular relaxation through activation of myosin light chain phosphatase (MLCP) and reduce Ca2+ sensitivity in the penis (Mills et al., 2002). Additionally, it has been shown that PKG phosphorylates and inhibits RhoA in the aorta (Sauzeau et al., 2000).
RhoA/Rho-kinase pathway
Rho-kinase (ROCK) phosphorylates and inhibits MLCP thus promoting the binding of actin and myosin for contraction of CC (Chitaley et al., 2002a; Wang et al., 2002; Jin and Burnett, 2006) Chitaley and colleagues (2001) were the first to demonstrate the involvement of RhoA/Rho-kinase signalling in erectile response (Chitaley et al., 2001). This signalling pathway is increased in the CCSM of several models of ED in rats, such as those associated with hypertension, diabetes and aging (Bakircioglu et al., 2001; Chitaley et al., 2001; Bivalacqua et al., 2004c). There is evidence that NO induces relaxation via inhibition of ROCK signalling in the penis (Mills et al., 2002) and CO in aorta (Awede et al., 2010). Furthermore, co-localization of eNOS and Rho-kinase was found in sinusoidal endothelium of CC (Mills et al., 2002; Bivalacqua et al., 2004a) CO inhibits the production of the potent vasoconstrictor, endothelin-1, which has been shown to activate RhoA (Morita and Kourembanas, 1995). H2S may also interfere with the contractile mechanism mediated by the RhoA/ROCK pathway. In fact, in human CC strips pre-contracted with either U46619 or h-ET1, there was a marked increase in the H2S vasorelaxant effect compared with that observed in strips pre-contracted with 1-agonist (d'Emmanuele di Villa Bianca et al., 2009).
Caveolin-1
The enzyme sGC that was believed to be present only at the cytosol has been also detected in association with the plasma membrane (Zabel et al., 2002; Venema et al., 2003). sGC translocates to caveolar domain to be sensitized by NO (Zabel et al., 2002; Venema et al., 2003). In the CC of caveolin-1 knockout mice, the relaxation induced by EFS and by the NO donor is impaired compared with wild-type mice (Shakirova et al., 2009), supporting a role for caveolae and caveolin-1 in erection. Previously, we observed that the relaxation induced by the sGC activator YC-1 is impaired in both the endothelium-intact aortic rings and CC after treatment with methyl-β-cyclodextrin, a compound that depletes plasma membrane cholesterol and disassembles caveolae (Linder et al., 2005; 2006). In the aortic and sinusoidal endothelium, we observed colocalization of sGC and the major coat protein of caveolae, caveolin-1 (Linder et al., 2005; 2006). These findings establish the association of sGC to caveolae in the endothelium introducing a potential therapeutic strategy for CVDs related to endothelial dysfunction, such as ED.
The well-established association of the enzyme eNOS with the plasma membrane protein, caveolin-1, maintains the enzyme in an inactive state (Feron et al., 1996) and an increase in intracellular calcium concentration in the endothelial cell is a key step for the dissociation of these proteins and, consequently eNOS activation (Gratton et al., 2000).
Similar to eNOS, HO-1 also appears in caveolae and physically interacts with caveolin-1 (Jung et al., 2003; Kim et al., 2004). HO enzyme activity increases in the absence of caveolin-1. In contrast, caveolin-1 causes inhibition of HO induction (Taira et al., 2011). The negative regulation of both eNOS and HO-1 activity by caveolin-1 give rise to the hypothesis that caveolin-1 may serve as a molecular brake on signalling mechanisms involving small gaseous second messengers. H2S-producing enzymes are also expressed in endothelium (Chertok and Kotsyuba, 2012; Baragatti et al., 2013). Recent studies showed that H2S is produced in adipose tissue, which is enriched by caveolin-1, but it is not known yet if H2S-producing enzymes are located at caveolae and if H2S interacts with caveolin-1. The only study addressing H2S-caveolin relation demonstrated the lack of effect of H2S donor (NaHS) on caveolin-1 expression in the CC (Meng et al., 2013) but it remains to be investigated whether caveolin-1 regulates H2S producing enzymes or H2S-induced relaxations in penile tissue.
Alterations in caveolin-1 expression were reported in different animal models such as decreased caveolin-1 expression in diabetic, aged and nerve injured rats penis (Becher et al., 2009) or increased caveolin-1 mRNA expression in SHR and protein expression in hypercholesterolaemic rat penis (Bakircioglu et al., 2000; Yono et al., 2009). Investigating the role of caveolar domains in erectile function of these gasotransmitters may bring new targets for ED treatment.
ATP-sensitive potassium channels: KATP channels
Activation of KATP channels leads to subsequent membrane hyperpolarization, which causes closure of voltage-dependent calcium channels resulting in smooth muscle relaxation. With respect to the physiology of erection, K channels in corporeal smooth muscle cells are accepted to represent a critical modulator of the flow of blood to and from the penis and, thus, an important determinant of erectile capacity (Spektor et al., 2002). NO activates KATP channels via a cGMP-dependent mechanism in vascular smooth muscle cells (Kubo et al., 1994) but not in horse penile resistance arteries (Simonsen et al., 1995) or horse corpus cavernosum (Recio et al., 1998). Glibenclamide inhibits CO-induced relaxation in vascular tissue (Foresti et al., 2004) but not in mice CC suggesting that CO-induced erectile function does not involve KATP channels (Friebe et al., 1996; Nakane et al., 2002; Decaluwe et al., 2012a). It has been proposed that H2S causes opening of KATP channels by a protein S-sulphydration (Mustafa et al., 2009; Jiang et al., 2010). The role of these channels in H2S-induced relaxation of human CC has also been confirmed (d'Emmanuele di Villa Bianca et al., 2009). These studies show that both NO- and CO-induced relaxation mechanisms in the penis are different from those in other vascular tissues.
Calcium-activated potassium channels KCa
The endothelium-dependent vasodilatation evoked by ACh is resistant to blockade of NOS in penile small arteries (Prieto, 2008). The relaxant effect of NO is due in part to activation of large-conductance KCa (KCa1.1 also known as BKCa, see Alexander et al., 2013a) in horse penile resistance arteries, (Simonsen et al., 1995) but not in horse CC (Recio et al., 1998) suggesting the diameter of the vessel may determine the involvement of KCa in the relaxation. ACh is the most common agonist that causes relaxation mediated by endothelial-derived hyperpolarizing factor (EDHF). Muscarinic cholinergic receptor activation causes CSE activation and in turn H2S production and there are data supporting H2S as an EDHF (Wang, 2003; 2009a). The exact nature of EDHF is still unknown but many hypotheses have been proposed. (Feletou and Vanhoutte, 2009). It is believed that KCa channels are the main mediator for vasodilator effects of the EDHF. The combination of KCa blockers, charybdotoxin and apamine significantly reduces the H2S-induced endothelial-dependent relaxation, underlining that KCa channels are targets for H2S and as it is well known, these channels are also the targets of EDHF (d'Emmanuele di Villa Bianca et al., 2011; Mustafa et al., 2011).
CO also leads to stimulation of KCa channels in several vascular tissues (Dubuis et al., 2005; Decaluwe et al., 2012a); however, CO-induced relaxation in mice CC does not involve KCa channels (Decaluwe et al., 2012b).
The interactions among the three sister gases
The interactions among these gases are mostly shown in other vascular tissues rather than the penis. The traffic between these gasotransmitters and downstream molecules and their implication in erectile function/dysfunction represent a very complicated but intriguing issue. There is evidence that the effects induced by CO and H2S are partially mediated by NO/cGMP (Wegiel et al., 2010; Coletta et al., 2012; Fusco et al., 2012; Meng et al., 2013). In other words, H2S and CO potentiate the stimulating action of endogenously synthesized NO. Additionally, Meng and colleagues have shown that H2S enhances NOS expression in endothelial cells of CC leading to NO production (Meng et al., 2013). The crosstalk among the gases was summarized in a representative figure (Figure 2).
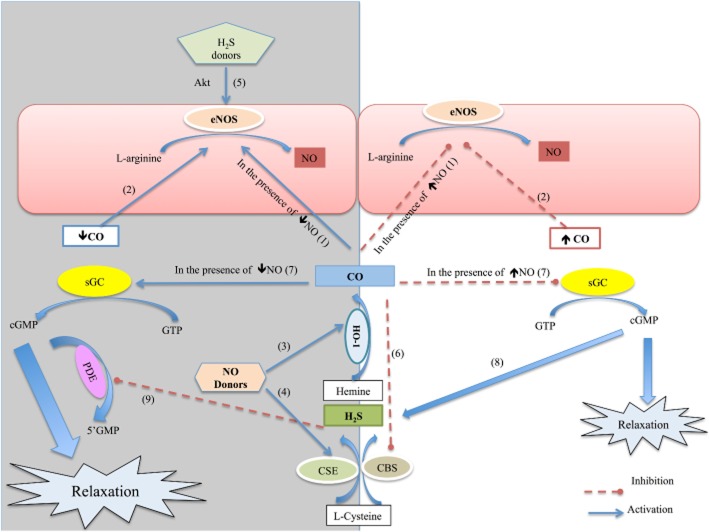
Figure 2.
Crosstalk among NO/CO/H2S/sGC pathways in vascular tissues including the penis. Bold fonts indicate the evidence obtainded in the penis. 1: CO inhibits eNOS in the presence of higher amounts of NO. However, CO activates eNOS when there is a low amount of NO (renal arteries; Botros and Navar, 2006). 2: High levels of CO inhibit NOS activity and NO generation, lower concentrations of CO induce release of NO (Thorup et al., 1999). 3: NO donors activate HO-1 (Foresti and Motterlini, 1999). 4: NO donors up-regulate the expression and activity of CSE in vascular tissues and cultured aortic smooth muscle cells (Leffler et al., 2005 and Zhao et al., 2001). 5: H2S cause eNOS activation in aorta through Akt. Coletta et al., 2012, and directly increase the expression of eNOS in CC (Meng et al., 2013). 6: CO inhibits CBS sensor (Taoka and Banerjee, 2001). 7: CO modulates NO-stimulated sGC activation dependent on NO concentration. In that, in the presence of low concentrations of NO, CO stimulates, otherwise CO inhibit sGC activation (Kajimura et al., 2003). 8: cGMP causes an increase in H2S production in vasculature (Bucci et al., 2012). 9: H2S acts as an endogenous inhibitor of PDE activity (Bucci et al., 2010).
H2S–CO interaction
Recently, the data showing inhibition of H2S producing enzyme CBS by constitutive CO suggests an H2S-HO-2/CO interaction to coordinate cerebral vasodilatation (Morikawa et al., 2012). Whereas, it has been shown that H2S up-regulates HO-1 expression in HUVEC (Pan et al., 2011). However, the interactions between these gasotransmitters have not been studied in the penis yet.
NO–CO interaction
The NO–CO crosstalk seems dependent on the concentration of gasotransmitters; such that low concentrations of CO induce release of NO and, therefore, may mimic the vascular effects of NO. (Thorup et al., 1999). In contrast, supra-physiological high levels of CO or HO-1 gene over-expression inhibit NOS activity and NO generation (Abdel Aziz et al., 2009c). Supporting this, it has been found that elevated levels of endogenous CO contribute to arteriolar NO dysfunction in Dahl salt-sensitive rats (Johnson et al., 2003). This CO-induced preconditioning conforms with a defence mechanism to inhibit iNOS-induced higher concentration of NO in pathological conditions.
In the same way, CO inhibits the NO-cGMP pathway under high NO concentrations, but compensates for NO to prevent excess vasoconstriction when insufficient NO is available (Botros and Navar, 2006). This study also suggests that the effect of CO on modulating sGC activity is also not static but dynamic. Supporting this low tissue availability of NO makes CO a stimulating modulator of sGC, while high tissue availability of NO causes the opposite (Kajimura et al., 2003). Thus, it is believed that CO regulates NOS and sGC activity in a way that the HO/CO pathway is compensatory for NOS.
In contrast, NO donors cause HO-1 induction (Durante et al., 1997; Foresti and Motterlini, 1999). Since CO inhibits NOS, under high concentrations of NO as in the case of exogenous NO administration, NO-induced HO-1 induction controls itself later by inhibiting NOS, representing a negative feedback mechanism. For further information on how the two systems are interrelated, readers are referred to the review by Foresti (Foresti and Motterlini, 1999).
H2S–NO interaction
In 1997, a physiological role for H2S in the vasculature and a link between NO and H2S (Hosoki et al., 1997) were suggested. Studies showing that H2S enhances cGMP levels in isolated aortic rings, and inhibits both cGMP and cAMP breakdown in a cell-free system provide direct evidence that H2S acts as an endogenous inhibitor of PDE activity (Bucci et al., 2010). In line with this evidence, it has been demonstrated that exposure of endothelial cells to H2S increases intracellular cGMP in a NO-dependent manner; H2S activates PI3K/Akt and increases eNOS phosphorylation, demonstrating the requirement of NO in vascular H2S signalling. NO and H2S are mutually required for the physiological control of smooth muscle tone and function in the aorta (Coletta et al., 2012). A contribution of NO/cGMP pathway in NaHS-induced human CC relaxation has also been addressed (d'Emmanuele di Villa Bianca et al., 2009). NO donors up-regulate the expression and activity of CSE in vascular tissues and cultured aortic smooth muscle cells (Zhao et al., 2001; Leffler et al., 2005). Recently, it has been shown that H2S promotes NO production in CC by enhancing he expression of eNOS (Meng et al., 2013). However, NO–H2S interactions have not been investigated in-depth in penile tissue.
Future directions
The evidence showing beneficial effects of CO-producing approaches in diabetes, hypertension or aging-induced ED as well as H2S donors in aging-induced ED are encouraging the development of drugs that target H2S or CO pathways and clinical studies. In addition, NO donors have been shown to increase both the H2S level and HO-1 activity in vascular tissues, thus drugs acting on the NO pathway may also be further beneficial in ED treatment because of their pleiotropic effects on other gasotransmitters. As a consequence when the endothelium is disrupted, a compound that supplies NO and increases both HO/CO and the H2S pathways could be beneficial in ED. Interestingly, PDE-5i have been shown to increase the activity of the HO/CO pathway in penile tissues (Abdel Aziz et al., 2007a) and H2S production in human bladder (Fusco et al., 2012) as well as limiting myocardial infarction through H2S signalling (Salloum et al., 2009). Moreover, our preliminary study suggests that H2S signalling may represent a new mechanism involved in the effect of sildenafil on erectile function (Dikmen et al., 2013). Thus, a specific study needs to be performed to clarify the H2S-related mechanisms of PDE-5 inhibitors in CC as well. Furthermore, an in-depth investigation into the close relationship among the testosterone, H2S and cGMP pathways will help urologists to decide the best therapeutic approach to counteract or prevent ED. More importantly, the trafficking among these gasotransmitters and downstream molecules and their implication in erectile function/dysfunction represent a very complicated but intriguing issue.
Conclusion
Although the role of the NOS/NO pathway in erectile function and dysfunction is fundamental, the clinical studies targeting the NOS pathway in ED have not been successful to reach full erectile response recovery. Besides NO, the role of both CO and H2S in erectile function has been well established in preclinical studies. The finding that CO can activate sGC in nNOS-deficient mice (Zakhary et al., 1997), and can compensate for NO to relax the vessel, when the NO level is low, may be important from bench to bedside translation to find a compensatory alternative therapy for ED. On the contrary, since H2S is mainly produced by smooth muscle in human CC, this pathway may complement NO signalling in ED especially in conditions associated with endothelial dysfunction. Moreover, since ROS is the main cause of ED and when eNOS is uncoupled it can be converted to ROS-producing enzyme, the antioxidant effects of H2S and the HO/CO pathway, as well as haem-independent activation of sGC by CO or endothelium-independent erectile effects of H2S, may have additional benefits in ED when NO-dependent cGMP formation is impaired because of either decreased synthesis/bioavailability, ROS-induced disruption of NO or the inability of haem-dependent activation of sGC in vasculogenic ED. Thus, targeting the other sister gases, H2S and CO, may represent new therapeutic potentials in ED.
Acknowledgments
The authors would like to thank the financial supports by Turkish Academia Young investigator award programme; TUBA-Gebip (to G. Y. A.), Turkish Scientific Research Council TÜBİTAK for the grant #109S453 and # 109S432 (to G. Y. A.), EBİLTEM (to G. Y. A.), Conselho Nacional de Desenvolvimento Científico e Tecnológico; CNPq (to A. E. L.) and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina; FAPESC (to A. E. L.) and the COST action BM1005 (ENOG: European Network on Gasotransmitters).
Glossary
- CC
corpus cavernosum
- ED
erectile dysfunction
Conflict of interest
None.
References
- Abdel Aziz MT, El-Asmar MF, Mostafa T, Atta H, Wassef MAA, Fouad HH, et al. Effects of nitric oxide synthase and heme oxygenase inducers and inhibitors on molecular signaling of erectile function. J Clin Biochem Nutr. 2005;37:103–111. [Google Scholar]
- Abdel Aziz MT, Al-Asmar MF, Mostafa T, Atta H, Rashed L, Sabry D, et al. Assessment of heme oxygenase-1 (HO-1) activity in the cavernous tissues of sildenafil citrate-treated rats. Asian J Androl. 2007a;9:377–381. doi: 10.1111/j.1745-7262.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, El-Asmer MF, Mostafa T, Mostafa S, Atta H, Aziz Wassef MA, et al. Heme oxygenase versus nitric oxide synthase in signaling mediating sildenafil citrate action. J Sex Med. 2007b;4(4):1098–1107. doi: 10.1111/j.1743-6109.2007.00533.x. Pt 2): [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, Mostafa T, Atta H, Rashed L, Marzouk SA, Obaia EM, et al. Oral phosphodiesterase-5 inhibitors: effect of heme oxygenase inhibition on cGMP signalling in rat cavernous tissue. Andrologia. 2007c;39:66–70. doi: 10.1111/j.1439-0272.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, El-Asmar MF, Mostafa T, Atta H, Fouad HH, Roshdy NK, et al. Effect of hemin and carbon monoxide releasing molecule (CORM-3) on cGMP in rat penile tissue. J Sex Med. 2008;5:336–343. doi: 10.1111/j.1743-6109.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, El Asmer MF, Mostafa T, Atta H, Mahfouz S, Fouad H, et al. Effects of losartan, HO-1 inducers or HO-1 inhibitors on erectile signaling in diabetic rats. J Sex Med. 2009a;6:3254–3264. doi: 10.1111/j.1743-6109.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, Mostafa T, Atta H, Mahfouz S, Wassef M, Fouad H, et al. Effect of HO-1 cDNA-liposome complex transfer on erectile signalling of aged rats. Andrologia. 2009b;41:176–183. doi: 10.1111/j.1439-0272.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, Mostafa T, Atta H, Wassef MA, Fouad HH, Rashed LA, et al. Putative role of carbon monoxide signaling pathway in penile erectile function. J Sex Med. 2009c;6:49–60. doi: 10.1111/j.1743-6109.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, El Asmer MF, Rezq A, Kumosani TA, Mostafa S, Mostafa T, et al. Novel water-soluble curcumin derivative mediating erectile signaling. J Sex Med. 2010;7:2714–2722. doi: 10.1111/j.1743-6109.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Turkseven S, Mingone CJ, Gupte SA, Wolin MS, Abraham NG. Heme oxygenase-1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol (Noisy-Le-Grand) 2005;51:371–376. [PubMed] [Google Scholar]
- Al-Magableh MR, Kemp-Harper BK, Ng HH, Miller AA, Hart JL. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:67–74. doi: 10.1007/s00210-013-0920-x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013a;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K-E. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awede B, Lemaire MC, Hyvelin JM, Halimi JM, Bonnet P, Eder V. Hemin, a carbon monoxide donor, improves systemic vascular compliance by inhibiting the RhoA-Rhokinase pathway in spontaneous hypertensive rats. Eur J Pharmacol. 2010;626:256–261. doi: 10.1016/j.ejphar.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Azadzoi KM, Master TA, Siroky MB. Effect of chronic ischemia on constitutive and inducible nitric oxide synthase expression in erectile tissue. J Androl. 2004;25:382–388. doi: 10.1002/j.1939-4640.2004.tb02804.x. [DOI] [PubMed] [Google Scholar]
- Bakircioglu ME, Hsu K, El-Sakka A, Sievert K-D, Lin CS, Lue TF. Effect of a Chinese herbal medicine mixture on a rat model of hypercholesterolemic erectile dysfunction. J Urol. 2000;164:1798–1801. [PubMed] [Google Scholar]
- Bakircioglu ME, Sievert K-D, Nunes L, Lau A, Lin C-S, Lue TF. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–738. [PubMed] [Google Scholar]
- Baracat JS, Teixeira CE, Okuyama CE, Priviero FB, Faro R, Antunes E, et al. Relaxing effects induced by the soluble guanylyl cyclase stimulator BAY 41-2272 in human and rabbit corpus cavernosum. Eur J Pharmacol. 2003;477:163–169. doi: 10.1016/j.ejphar.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Baragatti B, Ciofini E, Sodini D, Luin S, Scebba F, Coceani F. Hydrogen sulfide in the mouse ductus arteriosus: a naturally occurring relaxant with potential EDHF function. Am J Physiol Heart Circ Physiol. 2013;304:H927–H934. doi: 10.1152/ajpheart.00718.2012. [DOI] [PubMed] [Google Scholar]
- Becher EF, Toblli JE, Castronuovo C, Nolazco C, Rosenfeld C, Grosman H, et al. Expression of caveolin-1 in penile cavernosal tissue in a denervated animal model after treatment with sildenafil citrate. J Sex Med. 2009;6:1587–1593. doi: 10.1111/j.1743-6109.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- Behrends S, Harteneck C, Schultz G, Koesling D. A variant of the alpha 2 subunit of soluble guanylyl cyclase contains an insert homologous to a region within adenylyl cyclases and functions as a dominant negative protein. J Biol Chem. 1995;270:21109–21113. doi: 10.1074/jbc.270.36.21109. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Mehta YS, Abdel-Mageed AB, Sikka SC, Ignarro LJ, et al. Adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to the penis improves age-related erectile dysfunction in the rat. Int J Impot Res. 2000;12(Suppl. 3):S8–S17. doi: 10.1038/sj.ijir.3900556. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Hellstrom WJG, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Usta MF, Champion HC, Adams D, Namara DB, Abdel-Mageed AB, et al. Gene transfer of endothelial nitric oxide synthase partially restores nitric oxide synthesis and erectile function in streptozotocin diabetic rats. J Urol. 2003;169:1911–1917. doi: 10.1097/01.ju.0000051881.14239.4a. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004a;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004b;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Usta MF, Champion HC, Leungwattanakij S, Dabisch PA, McNamara DB, et al. Effect of combination endothelial nitric oxide synthase gene therapy and sildenafil on erectile function in diabetic rats. Int J Impot Res. 2004c;16:21–29. doi: 10.1038/sj.ijir.3901054. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, et al. Original research – basic science: superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–197. doi: 10.1111/j.1743-6109.2005.20228_1.x. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Burnett AL, Hellstrom WJG, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007a;292:H1340–H1351. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007b;292:H1278–H1290. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Kendirci M, Champion HC, Hellstrom WJG, Andersson K-E, Hedlund P. Dysregulation of cGMP-dependent protein kinase 1 (PKG-1) impairs erectile function in diabetic rats: influence of in vivo gene therapy of PKG1α. BJU Int. 2007c;99:1488–1494. doi: 10.1111/j.1464-410X.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007d;51:1732–1740. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Musicki B, Hsu LL, Berkowitz DE, Champion HC, Burnett AL. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS ONE. 2013;8:e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botros FT, Navar LG. Interaction between endogenously produced carbon monoxide and nitric oxide in regulation of renal afferent arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2772–H2778. doi: 10.1152/ajpheart.00528.2006. [DOI] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the working group on endothelins and endothelial factors of the european society of hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Bryan NS. Application of nitric oxide in drug discovery and development. Expert Opinion on Drug Discovery. 2011;6:1139–1154. doi: 10.1517/17460441.2011.613933. [DOI] [PubMed] [Google Scholar]
- Bucci M, Mirone V, Di Lorenzo A, Vellecco V, Roviezzo F, Brancaleone V, et al. Hydrogen sulphide is involved in testosterone vascular effect. Eur Urol. 2009;56:378–383. doi: 10.1016/j.eururo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, et al. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS ONE. 2012;7:e53319. doi: 10.1371/journal.pone.0053319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension. 2012;60:1301–1308. doi: 10.1161/HYPERTENSIONAHA.112.198754. [DOI] [PubMed] [Google Scholar]
- Burnett AL. Role of nitric oxide in the physiology of erection. Biol Reprod. 1995;52:485–489. doi: 10.1095/biolreprod52.3.485. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Johns DG, Kriegsfeld LJ, Klein SL, Calvin DC, Demas GE, et al. Ejaculatory abnormalities in mice with targeted disruption of the gene for heme oxygenase-2. Nat Med. 1998;4:84–87. doi: 10.1038/nm0198-084. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Chang AG, Crone JK, Huang PL, Sezen SF. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002;23:92–97. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Cagiano R, Ancona D, Cassano T, Tattoli M, Trabace L, Cuomo V. Effects of prenatal exposure to low concentrations of carbon monoxide on sexual behaviour and mesolimbic dopaminergic function in rat offspring. Br J Pharmacol. 1998;125:909–915. doi: 10.1038/sj.bjp.0702143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashen DE, MacIntyre DE, Martin WJ. Effects of sildenafil on erectile activity in mice lacking neuronal or endothelial nitric oxide synthase. Br J Pharmacol. 2002;136:693–700. doi: 10.1038/sj.bjp.0704772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor MB, Tirney S, Mattes CE, Tzeng E, Birder LA, Kanai AJ, et al. Nitric oxide synthase gene transfer for erectile dysfunction in a rat model. BJU Int. 2003;91:691–696. doi: 10.1046/j.1464-410x.2003.04219.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Wollman Y, Chernichovsky T, Iaina A, Sofer M, Matzkin H. Effect of oral administration of high-dose nitric oxide donor l-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study. BJU Int. 1999;83:269–273. doi: 10.1046/j.1464-410x.1999.00906.x. [DOI] [PubMed] [Google Scholar]
- Chertok VM, Kotsyuba AE. Distribution of H2S synthesis enzymes in the walls of cerebral arteries in rats. Bull Exp Biol Med. 2012;154:104–107. doi: 10.1007/s10517-012-1886-2. [DOI] [PubMed] [Google Scholar]
- Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- Chitaley K, Bivalacqua TJ, Champion HC, Usta MF, Hellstrom WJ, Mills TM, et al. Adeno-associated viral gene transfer of dominant negative RhoA enhances erectile function in rats. Biochem Biophys Res Commun. 2002a;298:427–432. doi: 10.1016/s0006-291x(02)02458-0. [DOI] [PubMed] [Google Scholar]
- Chitaley K, Bivalacqua TJ, Champion HC, Usta MF, Hellstrom WJG, Mills TM, et al. Adeno-associated viral gene transfer of dominant negative RhoA enhances erectile function in rats. Biochem Biophys Res Commun. 2002b;298:427–432. doi: 10.1016/s0006-291x(02)02458-0. [DOI] [PubMed] [Google Scholar]
- Choi YK, Kim CK, Lee H, Jeoung D, Ha KS, Kwon YG, et al. Carbon monoxide promotes VEGF expression by increasing HIF-1alpha protein level via two distinct mechanisms, translational activation and stabilization of HIF-1alpha protein. J Biol Chem. 2010;285:32116–32125. doi: 10.1074/jbc.M110.131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino G, Fusco F, Imbimbo C, Mirone V. Pharmacology of erectile dysfunction in man. Pharmacol Ther. 2006;111:400–423. doi: 10.1016/j.pharmthera.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Claudino MA, Franco-Penteado CF, Corat MAF, Gimenes AP, Passos LAC, Antunes E, et al. Increased cavernosal relaxations in sickle cell mice priapism are associated with alterations in the NO-cGMP signaling pathway. J Sex Med. 2009;6:2187–2196. doi: 10.1111/j.1743-6109.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Emmanuele di Villa Bianca R, Sorrentino R, Imbimbo C, Palmieri A, Fusco F, Maggi M, et al. Sphingosine 1-phosphate induces endothelial nitric-oxide synthase activation through phosphorylation in human corpus cavernosum. J Pharmacol Exp Ther. 2006;316:703–708. doi: 10.1124/jpet.105.093419. [DOI] [PubMed] [Google Scholar]
- d'Emmanuele di Villa Bianca R, Sorrentino R, Maffia P, Mirone V, Imbimbo C, Fusco F, et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci U S A. 2009;106:4513–4518. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Emmanuele di Villa Bianca R, Coletta C, Mitidieri E, De Dominicis G, Rossi A, Sautebin L, et al. Hydrogen sulphide induces mouse paw oedema through activation of phospholipase A2. Br J Pharmacol. 2010;161:1835–1842. doi: 10.1111/j.1476-5381.2010.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Emmanuele di Villa Bianca R, Sorrentino R, Mirone V, Cirino G. Hydrogen sulfide and erectile function: a novel therapeutic target. Nat Rev Urol. 2011;8:286–289. doi: 10.1038/nrurol.2011.45. [DOI] [PubMed] [Google Scholar]
- d'Emmanuele di Villa Bianca R, Mitidieri E, Fusco F, D'Aiuto E, Grieco P, Novellino E, et al. Endogenous urotensin II selectively modulates erectile function through eNOS. PLoS ONE. 2012;7:e31019. doi: 10.1371/journal.pone.0031019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- d'Emmanuele di Villa Bianca R, Mitidieri E, Di Minno MN, Kirkby NS, Warner TD, Di Minno G, et al. Hydrogen sulphide pathway contributes to the enhanced human platelet aggregation in hyperhomocysteinemia. Proc Natl Acad Sci U S A. 2013;110:15812–15817. doi: 10.1073/pnas.1309049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalaklioglu S, Kuscu N, Celik-Ozenci C, Bayram Z, Nacitarhan C, Ozdem SS. Chronic treatment with taurine ameliorates diabetes-induced dysfunction of nitric oxide-mediated neurogenic and endothelium-dependent corpus cavernosum relaxation in rats. Fundam Clin Pharmacol. 2013a doi: 10.1111/fcp.12041. doi: 10.1111/fcp.12041. [DOI] [PubMed] [Google Scholar]
- Dalaklioglu S, Sahin P, Tasatargil A, Celik-Ozenci C. Pravastatin improves the impaired nitric oxide-mediated neurogenic and endothelium-dependent relaxation of corpus cavernosum in aged rats. Aging Male. 2013b doi: 10.3109/13685538.2013.832194. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Decaluwe K, Pauwels B, Boydens C, Van de Voorde J. Divergent molecular mechanisms underlay CO- and CORM-2-induced relaxation of corpora cavernosa. J Sex Med. 2012a;9:2284–2292. doi: 10.1111/j.1743-6109.2012.02831.x. [DOI] [PubMed] [Google Scholar]
- Decaluwe K, Pauwels B, Verpoest S, Van de Voorde J. Divergent mechanisms involved in CO and CORM-2 induced vasorelaxation. Eur J Pharmacol. 2012b;674:370–377. doi: 10.1016/j.ejphar.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Decaluwe K, Pauwels B, Boydens C, Van de Voorde J. Treatment of erectile dysfunction: new targets and strategies from recent research. Pharmacol Biochem Behav. 2013 doi: 10.1016/j.pbb.2013.11.024. doi: 10.1016/j.pbb.2013.11.024 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Demir O, Murat N, Soner BC, Demir T, Bal E, Can E, et al. Acute effects of hypercholesterolemic diet on erectile responses in rats. Urol Int. 2010;85:112–117. doi: 10.1159/000296286. [DOI] [PubMed] [Google Scholar]
- Derbyshire ER, Marletta MA. Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol. 2009;191:17–31. doi: 10.1007/978-3-540-68964-5_2. [DOI] [PubMed] [Google Scholar]
- Dikmen A, d'Emmanuele di Villa Bianca R, Mitidieri E, Donnarumma E, Sevin G, Cirino G. New mechanism for the beneficial effect of sildenafil on erectile function: H2S. In: Neil Hogg, et al., editors. Second European Conference on the Biology of Hydrogen Sulfide. Vol. 31. Exeter: Nİtric oxide; 2013. p. 38. In: (ed.). , Vol. [Google Scholar]
- Dubuis E, Potier M, Wang R, Vandier C. Continuous inhalation of carbon monoxide attenuates hypoxic pulmonary hypertension development presumably through activation of BKCa channels. Cardiovasc Res. 2005;65:751–761. doi: 10.1016/j.cardiores.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- Elçioğlu KH, Kabasakal L, Çetinel Ş, Conturk G, Sezen SF, Ayanoğlu-Dülger G. Changes in caveolin-1 expression and vasoreactivity in the aorta and corpus cavernosum of fructose and streptozotocin-induced diabetic rats. Eur J Pharmacol. 2010;642:113–120. doi: 10.1016/j.ejphar.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Elshorbagy AK, Kozich V, Smith AD, Refsum H. Cysteine and obesity: consistency of the evidence across epidemiologic, animal and cellular studies. Curr Opin Clin Nutr Metab Care. 2012;15:49–57. doi: 10.1097/MCO.0b013e32834d199f. [DOI] [PubMed] [Google Scholar]
- Eto K, Ogasawara M, Umemura K, Nagai Y, Kimura H. Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci. 2002;22:3386–3391. doi: 10.1523/JNEUROSCI.22-09-03386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic Res. 1999;31:459–475. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, et al. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga-Silva RA, Costa-Fraga FP, Savergnini SQ, De Sousa FB, Montecucco F, da Silva D, et al. An oral formulation of angiotensin-(1–7) reverses corpus cavernosum damages induced by hypercholesterolemia. J Sex Med. 2013;10:2430–2442. doi: 10.1111/jsm.12262. [DOI] [PubMed] [Google Scholar]
- Frey R, Scheerans C, Blunck M, Muck W, Gnoth MJ, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase activator cinaciguat in individuals with hepatic impairment. J Clin Pharmacol. 2012;52:1714–1724. doi: 10.1177/0091270011426143. [DOI] [PubMed] [Google Scholar]
- Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Schultz G, Koesling D. Stimulation of soluble guanylate cyclase by superoxide dismutase is mediated by NO. Biochem J. 1998;335:527–531. doi: 10.1042/bj3350527. (Pt 3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Shimizu A, Masuda Y, Kuwahara N, Arai T, Nagasaka S, et al. Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages. Am J Pathol. 2010;177:1143–1154. doi: 10.2353/ajpath.2010.090608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Tsujimura A, Okuda H, Yamamoto K, Takao T, Miyagawa Y, et al. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin-induced diabetic rat: preliminary findings. J Sex Med. 2011;8:1061–1071. doi: 10.1111/j.1743-6109.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- Fusco F, di Villa Bianca R, Mitidieri E, Cirino G, Sorrentino R, Mirone V. Sildenafil effect on the human bladder involves the L-cysteine/hydrogen sulfide pathway: a novel mechanism of action of phosphodiesterase type 5 inhibitors. Eur Urol. 2012;62:1174–1180. doi: 10.1016/j.eururo.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- Gentile V, Antonini G, Antonella Bertozzi M, Dinelli N, Rizzo C, Ashraf Virmani M, et al. Effect of propionyl-l-carnitine, l-arginine and nicotinic acid on the efficacy of vardenafil in the treatment of erectile dysfunction in diabetes. Curr Med Res Opin. 2009;25:2223–2228. doi: 10.1185/03007990903138416. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Stuehr DJ. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc Natl Acad Sci U S A. 2012;109:12998–13003. doi: 10.1073/pnas.1205854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cadavid NF, Rajfer J. The pleiotropic effects of inducible nitric oxide synthase (iNOS) on the physiology and pathology of penile erection. Curr Pharm Des. 2005;11:4041–4046. doi: 10.2174/138161205774913372. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cadavid NF, Burnett AL, Magee TR, Zeller CB, Vernet D, Smith N, et al. Expression of penile neuronal nitric oxide synthase variants in the rat and mouse penile nerves. Biol Reprod. 2000;63:704–714. doi: 10.1095/biolreprod63.3.704. [DOI] [PubMed] [Google Scholar]
- Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- Gur S, Kadowitz PJ, Trost L, Hellstrom WJG. Optimizing nitric oxide production by time dependent L-arginine administration in isolated human corpus cavernosum. J Urol. 2007;178:1543–1548. doi: 10.1016/j.juro.2007.05.121. [DOI] [PubMed] [Google Scholar]
- Gur S, Kadowitz PJ, Gurkan L, Chandra S, DeWitt SY, Harbin A, et al. Chronic inhibition of nitric-oxide synthase induces hypertension and erectile dysfunction in the rat that is not reversed by sildenafil. BJU Int. 2010;106:78–83. doi: 10.1111/j.1464-410X.2009.09104.x. [DOI] [PubMed] [Google Scholar]
- Hamar J, Solymar M, Tanai E, Cseplo P, Springo Z, Berta G, et al. Bioassay-comparison of the antioxidant efficacy of hydrogen sulfide and superoxide dismutase in isolated arteries and veins. Acta Physiol Hung. 2012;99:411–419. doi: 10.1556/APhysiol.99.2012.4.5. [DOI] [PubMed] [Google Scholar]
- Hartsfield CL, Alam J, Cook JL, Choi AM. Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am J Physiol. 1997;273(5):L980–L988. doi: 10.1152/ajplung.1997.273.5.L980. Pt 1): [DOI] [PubMed] [Google Scholar]
- Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, et al. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci U S A. 2000a;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P, Ny L, Alm P, Andersson KE. Cholinergic nerves in human corpus cavernosum and spongiosum contain nitric oxide synthase and heme oxygenase. J Urol. 2000b;164(3):868–875. doi: 10.1097/00005392-200009010-00064. Pt 1): [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, O'Neill AB, Moreland RB, Sullivan JP, Brioni JD. YC-1 potentiates the nitric oxide/cyclic GMP pathway in corpus cavernosum and facilitates penile erection in rats. Eur J Pharmacol. 2003;458:183–189. doi: 10.1016/s0014-2999(02)02730-9. [DOI] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. PNAS. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt KJ, Sezen SF, Lagoda GF, Musicki B, Rameau GA, Snyder SH, et al. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. PNAS. 2012;109:16624–16629. doi: 10.1073/pnas.1213790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka N, Griendling KK. Heme oxygenase-1 is regulated by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1997;29:790–795. doi: 10.1161/01.hyp.29.3.790. [DOI] [PubMed] [Google Scholar]
- Jacob C, Anwar A, Burkholz T. Perspective on recent developments on sulfur-containing agents and hydrogen sulfide signaling. Planta Med. 2008;74:1580–1592. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- Jiang B, Tang G, Cao K, Wu L, Wang R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid Redox Signal. 2010;12:1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci (Lond) 2006;110:153–165. doi: 10.1042/CS20050255. [DOI] [PubMed] [Google Scholar]
- Jin L, Burnett AL. NADPH oxidase: recent evidence for its role in erectile dysfunction. Asian J Androl. 2008;10:6–13. doi: 10.1111/j.1745-7262.2008.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006;20:536–538. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- Jin L, Lagoda G, Leite R, Webb RC, Burnett AL. NADPH oxidase activation: a mechanism of hypertension-associated erectile dysfunction. J Sex Med. 2008a;5:544–551. doi: 10.1111/j.1743-6109.2007.00733.x. [DOI] [PubMed] [Google Scholar]
- Jin YC, Gam SC, Jung JH, Hyun JS, Chang KC. Expression and activity of heme oxygenase-1 in artificially induced low-flow priapism in rat penile tissues. J Sex Med. 2008b;5:1876–1882. doi: 10.1111/j.1743-6109.2008.00886.x. [DOI] [PubMed] [Google Scholar]
- Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FK, Durante W, Peyton KJ, Johnson RA. Heme oxygenase inhibitor restores arteriolar nitric oxide function in dahl rats. Hypertension. 2003;41:149–155. doi: 10.1161/01.hyp.0000046923.52222.58. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Bivalacqua TJ, Lagoda GA, Burnett AL, Musicki B. eNOS-uncoupling in age-related erectile dysfunction. Int J Impot Res. 2011;23:43–48. doi: 10.1038/ijir.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol. 2005;69:41–48. doi: 10.1016/j.bcp.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Jung NH, Kim HP, Kim BR, Cha SH, Kim GA, Ha H, et al. Evidence for heme oxygenase-1 association with caveolin-1 and −2 in mouse mesangial cells. IUBMB Life. 2003;55:525–532. doi: 10.1080/15216540310001620968. [DOI] [PubMed] [Google Scholar]
- Kajimura M, Shimoyama M, Tsuyama S, Suzuki T, Kozaki S, Takenaka S, et al. Visualization of gaseous monoxide reception by soluble guanylate cyclase in the rat retina. FASEB J. 2003;17:506–508. doi: 10.1096/fj.02-0359fje. [DOI] [PubMed] [Google Scholar]
- Karakeci A, Firdolas F, Ozan T, Unus I, Ogras MS, Orhan I. Second pathways in the pathophysiology of ischemic priapism and treatment alternatives. Urology. 2013;82:625–629. doi: 10.1016/j.urology.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT. Evolution of novel small-molecule therapeutics targeting sickle cell vasculopathy. JAMA. 2008;300:2638–2646. doi: 10.1001/jama.2008.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Zhao C, Kim MK, Park JK. Direct effect of carbon monoxide on relaxation induced by electrical field stimulation in rat corpus cavernosum. Korean J Urol. 2010;51:572–578. doi: 10.4111/kju.2010.51.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- Kim KM, Pae H-O, Zheng M, Park R, Kim Y-M, Chung H-T. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007;101:919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- Kim YC, Davies MG, Marson L, Hagen PO, Carson CC., 3rd Lack of effect of carbon monoxide inhibitor on relaxation induced by electrical field stimulation in corpus cavernosum. Urol Res. 1994;22:291–293. doi: 10.1007/BF00297197. [DOI] [PubMed] [Google Scholar]
- Klotz T, Mathers MJ, Braun M, Bloch W, Engelmann U. Effectiveness of oral L-arginine in first-line treatment of erectile dysfunction in a controlled crossover study. Urol Int. 1999;63:220–223. doi: 10.1159/000030454. [DOI] [PubMed] [Google Scholar]
- Kozma F, Johnson RA, Nasjletti A. Role of carbon monoxide in heme-induced vasodilation. Eur J Pharmacol. 1997;323:R1–R2. doi: 10.1016/s0014-2999(97)00145-3. [DOI] [PubMed] [Google Scholar]
- Kreck TC, Shade ED, Lamm WJ, McKinney SE, Hlastala MP. Isocapnic hyperventilation increases carbon monoxide elimination and oxygen delivery. Am J Respir Crit Care Med. 2001;163:458–462. doi: 10.1164/ajrccm.163.2.2003039. [DOI] [PubMed] [Google Scholar]
- Kredich NM, Foote LJ, Keenan BS. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem. 1973;248:6187–6196. [PubMed] [Google Scholar]
- Kruger AL, Peterson SJ, Schwartzman ML, Fusco H, McClung JA, Weiss M, et al. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- Kruszyna H, Kruszyna R, Smith RP. Cyanide and sulfide interact with nitrogenous compounds to influence the relaxation of various smooth muscles. Proc Soc Exp Biol Med. 1985;179:44–49. doi: 10.3181/00379727-179-42062. [DOI] [PubMed] [Google Scholar]
- Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994;74:471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- Labazi H, Wynne BM, Tostes R, Webb RC. Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J Sex Med. 2013;10:2154–2164. doi: 10.1111/jsm.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker GF, Maley JH, Kadowitz PJ. A review of the pathophysiology and novel treatments for erectile dysfunction. Adv Pharmacol Sci. 2010a;2010:730861. doi: 10.1155/2010/730861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker GF, Matt CJ, Badejo JAM, Casey DB, Dhaliwal JS, Murthy SN, et al. Intracavernosal administration of sodium nitrite as an erectile pharmacotherapy. Can J Physiol Pharmacol. 2010b;88:770–776. doi: 10.1139/y10-032. [DOI] [PubMed] [Google Scholar]
- Lasker GF, Pankey EA, Kadowitz PJ. Modulation of soluble guanylate cyclase for the treatment of erectile dysfunction. Physiology. 2013;28:262–269. doi: 10.1152/physiol.00001.2013. [DOI] [PubMed] [Google Scholar]
- Lebret T, Hervé J-M, Gorny P, Worcel M, Botto H. Efficacy and safety of a novel combination of L-arginine glutamate and yohimbine hydrochloride: a new oral therapy for erectile dysfunction. Eur Urol. 2002;41:608–613. doi: 10.1016/s0302-2838(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Lee YC, Martin E, Murad F. Human recombinant soluble guanylyl cyclase: expression, purification, and regulation. Proc Natl Acad Sci U S A. 2000;97:10763–10768. doi: 10.1073/pnas.190333697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Fedinec AL, Parfenova H, Jaggar JH. Permissive contributions of NO and prostacyclin in CO-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol. 2005;289:H432–H438. doi: 10.1152/ajpheart.01195.2004. [DOI] [PubMed] [Google Scholar]
- Li M, Zhuan L, Wang T, Rao K, Yang J, Quan W, et al. Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression. J Sex Med. 2012;9:3041–3050. doi: 10.1111/j.1743-6109.2012.02960.x. [DOI] [PubMed] [Google Scholar]
- Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- Linder AE, McCluskey LP, Cole KR, 3rd, Lanning KM, Webb RC. Dynamic association of nitric oxide downstream signaling molecules with endothelial caveolin-1 in rat aorta. J Pharmacol Exp Ther. 2005;314:9–15. doi: 10.1124/jpet.105.083634. [DOI] [PubMed] [Google Scholar]
- Linder AE, Leite R, Lauria K, Mills TM, Webb RC. Penile erection requires association of soluble guanylyl cyclase with endothelial caveolin-1 in rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1302–R1308. doi: 10.1152/ajpregu.00601.2005. [DOI] [PubMed] [Google Scholar]
- Liu XM, Peyton KJ, Mendelev NN, Wang H, Tulis DA, Durante W. YC-1 stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells. Mol Pharmacol. 2009;75:208–217. doi: 10.1124/mol.108.048314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Peyton KJ, Wang X, Durante W. Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling pathways. Biochem Pharmacol. 2012;84:1045–1054. doi: 10.1016/j.bcp.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- Ma X, Sayed N, Beuve A, van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. EMBO J. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee TR, Ferrini M, Garban HJ, Vernet D, Mitani K, Rajfer J, et al. Gene therapy of erectile dysfunction in the rat with penile neuronal nitric oxide synthase. Biol Reprod. 2002;67:20–28. doi: 10.1093/biolreprod/67.1.20. [DOI] [PubMed] [Google Scholar]
- Magee TR, Kovanecz I, Davila HH, Ferrini MG, Cantini L, Vernet D, et al. Antisense and short hairpin rna (shRNA) constructs targeting PIN (protein inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J Sex Med. 2007;4:633–643. doi: 10.1111/j.1743-6109.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim C-S, Naqvi A, Yamamori T, Hoffman TA, Jung S-B, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. PNAS. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert MK, Haley JE, Schuman EM, Schulman H, Madison DV. Inhibition of hippocampal heme oxygenase, nitric oxide synthase, and long-term potentiation by metalloporphyrins. Neuron. 1994;13:1225–1233. doi: 10.1016/0896-6273(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Meng J, Ganesan Adaikan P, Srilatha B. Hydrogen sulfide promotes nitric oxide production in corpus cavernosum by enhancing expression of endothelial nitric oxide synthase. Int J Impot Res. 2013;25:86–90. doi: 10.1038/ijir.2012.39. [DOI] [PubMed] [Google Scholar]
- Mills TM, Chitaley K, Lewis RW, Webb RC. Nitric oxide inhibits RhoA/Rho-kinase signaling to cause penile erection. Eur J Pharmacol. 2002;439:173–174. doi: 10.1016/s0014-2999(02)01408-5. [DOI] [PubMed] [Google Scholar]
- Mizusawa H, Hedlund P, Brioni JD, Sullivan JP, Andersson KE. Nitric oxide independent activation of guanylate cyclase by YC-1 causes erectile responses in the rat. J Urol. 2002;167:2276–2281. [PubMed] [Google Scholar]
- Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A. 2012;109:1293–1298. doi: 10.1073/pnas.1119658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- Muchova L, Wong RJ, Hsu M, Morioka I, Vitek L, Zelenka J, et al. Statin treatment increases formation of carbon monoxide and bilirubin in mice: a novel mechanism of in vivo antioxidant protection. Can J Physiol Pharmacol. 2007;85:800–810. doi: 10.1139/y07-077. [DOI] [PubMed] [Google Scholar]
- Musicki B, Burnett AL. eNOS function and dysfunction in the penis. Exp Biol Med. 2006;231:154–165. doi: 10.1177/153537020623100205. [DOI] [PubMed] [Google Scholar]
- Musicki B, Kramer MF, Becker RE, Burnett AL. Age-related changes in phosphorylation of endothelial nitric oxide synthase in the rat penis. J Sex Med. 2005a;2:347–357. doi: 10.1111/j.1743-6109.2005.20349.x. [DOI] [PubMed] [Google Scholar]
- Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005b;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Liu T, Strong T, Jin L, Laughlin MH, Turk JR, et al. Low-fat diet and exercise preserve eNOS regulation and endothelial function in the penis of early atherosclerotic pigs: a molecular analysis. J Sex Med. 2008;5:552–561. doi: 10.1111/j.1743-6109.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Ross AE, Champion HC, Burnett AL, Bivalacqua TJ. Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl. 2009;30:352–362. doi: 10.2164/jandrol.108.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, et al. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010;7:3023–3032. doi: 10.1111/j.1743-6109.2010.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Champion HC, Hsu LL, Bivalacqua TJ, Burnett AL. Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis. J Sex Med. 2011;8:419–426. doi: 10.1111/j.1743-6109.2010.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane M, Hsieh G, Miller LN, Chang R, Terranova MA, Moreland RB, et al. Activation of soluble guanylate cyclase causes relaxation of corpus cavernosum tissue: synergism of nitric oxide and YC-1. Int J Impot Res. 2002;14:121–127. doi: 10.1038/sj.ijir.3900843. [DOI] [PubMed] [Google Scholar]
- Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, et al. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzillet Y, Hupertan V, Cour F, Botto H, Lebret T. A randomized, double-blind, crossover, placebo-controlled comparative clinical trial of arginine aspartate plus adenosine monophosphate for the intermittent treatment of male erectile dysfunction. Andrology. 2013;1:223–228. doi: 10.1111/j.2047-2927.2012.00046.x. [DOI] [PubMed] [Google Scholar]
- Nimmegeers S, Sips P, Buys E, Decaluwe K, Brouckaert P, Van de Voorde J. Role of the soluble guanylyl cyclase alpha1-subunit in mice corpus cavernosum smooth muscle relaxation. Int J Impot Res. 2008;20:278–284. doi: 10.1038/sj.ijir.3901627. [DOI] [PubMed] [Google Scholar]
- Numao N, Masuda H, Sakai Y, Okada Y, Kihara K, Azuma H. Roles of attenuated neuronal nitric-oxide synthase protein expression and accelerated arginase activity in impairing neurogenic relaxation of corpus cavernosum in aged rabbits. BJU Int. 2007;99:1495–1499. doi: 10.1111/j.1464-410X.2007.06860.x. [DOI] [PubMed] [Google Scholar]
- Olujohungbe A, Burnett AL. How I manage priapism due to sickle cell disease. Br J Haematol. 2013;160:754–765. doi: 10.1111/bjh.12199. [DOI] [PubMed] [Google Scholar]
- Ong KL, Wu BJ, Cheung BM, Barter PJ, Rye KA. Association of lower total bilirubin level with statin usage: the United States National Health and Nutrition Examination Survey 1999–2008. Atherosclerosis. 2011;219:728–733. doi: 10.1016/j.atherosclerosis.2011.07.094. [DOI] [PubMed] [Google Scholar]
- Palmer L, Kavoussi P, Lysiak J. S-Nitrosylation of endothelial nitric oxide synthase alters erectile function. Nitric Oxide. 2012;27(Suppl):S22–S23. [Google Scholar]
- Pan LL, Liu XH, Gong QH, Wu D, Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park K, Lee DG, Kim SW, Paick JS. Dimethylarginine dimethylaminohydrolase in rat penile tissue: reduced enzyme activity is responsible for erectile dysfunction in a rat model of atherosclerosis. Int J Impot Res. 2009;21:228–234. doi: 10.1038/ijir.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- Priviero FB, Webb RC. Heme-dependent and independent soluble guanylate cyclase activators and vasodilation. J Cardiovasc Pharmacol. 2010;56:229–233. doi: 10.1097/FJC.0b013e3181eb4e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio P, Lopez PG, Hernandez M, Prieto D, Contreras J, Garcia-Sacristan A. Nitrergic relaxation of the horse corpus cavernosum. Role of cGMP. Eur J Pharmacol. 1998;351:85–94. doi: 10.1016/s0014-2999(98)00282-9. [DOI] [PubMed] [Google Scholar]
- Ruiz Rubio JL, Hernandez M, Rivera de los Arcos L, Benedito S, Recio P, Garcia P, et al. Role of ATP-sensitive K+ channels in relaxation of penile resistance arteries. Urology. 2004;63:800–805. doi: 10.1016/j.urology.2003.10.071. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Cho CH, Shin HY, Song SU, Oh SM, Lee M, et al. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol Ther. 2006;13:705–715. doi: 10.1016/j.ymthe.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Saito M, Ohmasa F, Dimitriadis F, Tsounapi P, Sejima T, Shimizu S, et al. Hydroxyfasudil ameliorates penile dysfunction in the male spontaneously hypertensive rat. Pharmacol Res. 2012;66:325–331. doi: 10.1016/j.phrs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Masuda H, Kihara K, Kurosaki EMI, Yamauchi Y, Azuma H. Involvement of increased arginase activity in impaired cavernous relaxation with aging in the rabbit. J Urol. 2004;172:369–373. doi: 10.1097/01.ju.0000121691.06417.40. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, et al. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120(11 Suppl):S31–S36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Contreras C, Martinez MP, Climent B, Benedito S, Garcia-Sacristan A, et al. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitrergic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLoS ONE. 2012;7:e36027. doi: 10.1371/journal.pone.0036027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35:21–27. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Schrammel A, Koesling D, Mayer B. Molecular mechanisms involved in the synergistic activation of soluble guanylyl cyclase by YC-1 and nitric oxide in endothelial cells. Mol Pharmacol. 2001;59:220–224. doi: 10.1124/mol.59.2.220. [DOI] [PubMed] [Google Scholar]
- Segal R, Hannan JL, Liu X, Kutlu O, Burnett AL, Champion HC, et al. Chronic oral administration of the arginase inhibitor 2(S)-amino-6-boronohexanoic acid (ABH) improves erectile function in aged rats. J Androl. 2012;33:1169–1175. doi: 10.2164/jandrol.111.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirova Y, Hedlund P, Sward K. Impaired nerve-mediated relaxation of penile tissue from caveolin-1 deficient mice. Eur J Pharmacol. 2009;602:399–405. doi: 10.1016/j.ejphar.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Shamloul R. The potential role of the heme oxygenase/carbon monoxide system in male sexual dysfunctions. J Sex Med. 2009;6:324–333. doi: 10.1111/j.1743-6109.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- Shamloul R, Wang R. Increased intracavernosal pressure response in hypertensive rats after chronic hemin treatment. J Sex Med. 2006;3:619–627. doi: 10.1111/j.1743-6109.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Shin D, Pregenzer G, Jr, Gardin JM. Erectile dysfunction: a disease marker for cardiovascular disease. Cardiol Rev. 2011;19:5–11. doi: 10.1097/CRD.0b013e3181fb7eb8. [DOI] [PubMed] [Google Scholar]
- Shin S, Jeon JH, Park D, Jang MJ, Choi JH, Choi BH, et al. trans-Resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality in vivo. Arch Pharm Res. 2008;31:83–87. doi: 10.1007/s12272-008-1124-7. [DOI] [PubMed] [Google Scholar]
- Shiota A, Hotta Y, Kataoka T, Morita M, Maeda Y, Kimura K. Oral l-citrulline supplementation improves erectile function in rats with acute arteriogenic erectile dysfunction. J Sex Med. 2013;10:2423–2429. doi: 10.1111/jsm.12260. [DOI] [PubMed] [Google Scholar]
- Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol. 2005;517:224–231. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Shukla N, Rossoni G, Hotston M, Sparatore A, Del Soldato P, Tazzari V, et al. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009;103:1522–1529. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FH, Mónica FZ, Báu FR, Brugnerotto AF, Priviero FBM, Toque HA, et al. Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: prevention by antioxidant therapy. J Sex Med. 2013;10:960–971. doi: 10.1111/jsm.12063. [DOI] [PubMed] [Google Scholar]
- Simonsen U, Prieto D, Sanez de Tejada I, Garcia-Sacristan A. Involvement of nitric oxide in the non-adrenergic non-cholinergic neurotransmission of horse deep penile arteries: role of charybdotoxin-sensitive K(+)-channels. Br J Pharmacol. 1995;116:2582–2590. doi: 10.1111/j.1476-5381.1995.tb17211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Klotz T, Steinritz D, Bloch W. Evaluation of tetrahydrobiopterin (BH4) as a potential therapeutic agent to treat erectile dysfunction. Asian J Androl. 2006;8:159–167. doi: 10.1111/j.1745-7262.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- Soner BC, Murat N, Demir O, Guven H, Esen A, Gidener S. Evaluation of vascular smooth muscle and corpus cavernosum on hypercholesterolemia. Is resveratrol promising on erectile dysfunction? Int J Impot Res. 2010;22:227–233. doi: 10.1038/ijir.2010.8. [DOI] [PubMed] [Google Scholar]
- Soni SD, Song W, West JL, Khera M. Nitric oxide-releasing polymeric microspheres improve diabetes-related erectile dysfunction. J Sex Med. 2013;10:1915–1925. doi: 10.1111/jsm.12216. [DOI] [PubMed] [Google Scholar]
- Spektor M, Rodriguez R, Rosenbaum RS, Wang HZ, Melman A, Christ GJ. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J Urol. 2002;167:2628–2635. [PubMed] [Google Scholar]
- Srilatha B, Adaikan PG, Moore PK. Possible role for the novel gasotransmitter hydrogen sulphide in erectile dysfunction – a pilot study. Eur J Pharmacol. 2006;535:280–282. doi: 10.1016/j.ejphar.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Srilatha B, Muthulakshmi P, Adaikan PG, Moore PK. Endogenous hydrogen sulfide insufficiency as a predictor of sexual dysfunction in aging rats. Aging Male. 2012;15:153–158. doi: 10.3109/13685538.2012.668722. [DOI] [PubMed] [Google Scholar]
- Stanislavov R, Nikolova V. Treatment of erectile dysfunction with pycnogenol and L-arginine. J Sex Marital Ther. 2003;29:207–213. doi: 10.1080/00926230390155104. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002;136:773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Heme stoichiometry of heterodimeric soluble guanylate cyclase. Biochemistry. 1995;34:14668–14674. doi: 10.1021/bi00045a007. [DOI] [PubMed] [Google Scholar]
- Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, et al. Nitric oxide and penile erection: Is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43:658–665. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- Taira J, Sugishima M, Kida Y, Oda E, Noguchi M, Higashimoto Y. Caveolin-1 is a competitive inhibitor of heme oxygenase-1 (HO-1) with heme: identification of a minimum sequence in caveolin-1 for binding to HO-1. Biochemistry. 2011;50:6824–6831. doi: 10.1021/bi200601t. [DOI] [PubMed] [Google Scholar]
- Taoka S, Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem. 2001;87:245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol. 2006;290:F789–F794. doi: 10.1152/ajprenal.00363.2005. [DOI] [PubMed] [Google Scholar]
- Teixeira CE, Priviero FB, Webb RC. Effects of 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]pyrimidin-4- ylamine (BAY 41–2272) on smooth muscle tone, soluble guanylyl cyclase activity, and NADPH oxidase activity/expression in corpus cavernosum from wild-type, neuronal, and endothelial nitric-oxide synthase null mice. J Pharmacol Exp Ther. 2007;322:1093–1102. doi: 10.1124/jpet.107.124594. [DOI] [PubMed] [Google Scholar]
- Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277(6):F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. Pt 2): [DOI] [PubMed] [Google Scholar]
- Tomada I, Tomada N, Almeida H, Neves D. Androgen depletion in humans leads to cavernous tissue reorganization and upregulation of Sirt1–eNOS axis. Age (Omaha) 2013;35:35–47. doi: 10.1007/s11357-011-9328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toque HA, Tostes RC, Yao L, Xu Z, Webb RC, Caldwell RB, et al. Arginase II deletion increases corpora cavernosa relaxation in diabetic mice. J Sex Med. 2011;8:722–733. doi: 10.1111/j.1743-6109.2010.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama M, Morita T, Kuramochi T, Yagi S, Katayama S. Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens Res. 2004;27:253–261. doi: 10.1291/hypres.27.253. [DOI] [PubMed] [Google Scholar]
- Ushiyama M, Kuramochi T, Yagi S, Katayama S. Antioxidant treatment with alpha-tocopherol improves erectile function in hypertensive rats. Hypertens Res. 2008;31:1007–1013. doi: 10.1291/hypres.31.1007. [DOI] [PubMed] [Google Scholar]
- Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 2010;39:1161–1169. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]
- Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, et al. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285:H669–H678. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]
- Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2 + sensitization in erectile function. J Biol Chem. 2002;277:30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- Wang R. Hydrogen sulfide: a new EDRF. Kidney Int. 2009a;76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- Wang R. Is H2S a stinky remedy for atherosclerosis? Arterioscler Thromb Vasc Biol. 2009b;29:156–157. doi: 10.1161/ATVBAHA.108.180190. [DOI] [PubMed] [Google Scholar]
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- Wang T, Li M, Yuan H, Zhan Y, Xu H, Wang S, et al. saRNA guided iNOS up-regulation improves erectile function of diabetic rats. J Urol. 2013;190:790–798. doi: 10.1016/j.juro.2013.03.043. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Gallo DJ, Raman KG, Karlsson JM, Ozanich B, Chin BY, et al. Nitric oxide-dependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation. 2010;121:537–548. doi: 10.1161/CIRCULATIONAHA.109.887695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- Wu L, Yang W, Jia X, Yang G, Duridanova D, Cao K, et al. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest. 2009;89:59–67. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wang T, Rao K, Zhan Y, Chen RB, Liu Z, et al. S-allyl cysteine restores erectile function through inhibition of reactive oxygen species generation in diabetic rats. Andrology. 2013a;1:487–494. doi: 10.1111/j.2047-2927.2012.00060.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang T, Rao K, Zhan Y, Chen RB, Liu Z, et al. S-allyl cysteine restores erectile function through inhibition of reactive oxygen species generation in diabetic rats. Andrology. 2013b;1:487–494. doi: 10.1111/j.2047-2927.2012.00060.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang C, Xiao L, Liao X, Lan A, Wang X, et al. Novel insights into the role of HSP90 in cytoprotection of H2S against chemical hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med. 2011;28:397–403. doi: 10.3892/ijmm.2011.682. [DOI] [PubMed] [Google Scholar]
- Yetik-Anacak G, Xia T, Dimitropoulou C, Venema RC, Catravas JD. Effects of hsp90 binding inhibitors on sGC-mediated vascular relaxation. Am J Physiol Heart Circ Physiol. 2006;291:H260–H268. doi: 10.1152/ajpheart.01027.2005. [DOI] [PubMed] [Google Scholar]
- Yetik-Anacak GEE, Ozsarlak-Sozer G, Turkseven S, Koylu S, Gözen O, Sevin G, et al. 2013. Functional significance of hsp90-eNOS interaction in penile tissues. FASEB J 27 (Meeting Abstract Suppl): 920.5. [Google Scholar]
- Yildiz O, Seyrek M, Irkilata HC, Yildirim I, Tahmaz L, Dayanc M. Testosterone might cause relaxation of human corpus cavernosum by potassium channel opening action. Urology. 2009;74:229–232. doi: 10.1016/j.urology.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Yono M, Yoshida M, Yamamoto Y, Imanishi A, Fukagawa A, Latifpour J, et al. Molecular mechanisms regulating urogenital expression of nitric oxide synthase in spontaneously hypertensive rats. Life Sci. 2009;85:334–338. doi: 10.1016/j.lfs.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Yu W, Wan Z, Qiu XF, Chen Y, Dai YT. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15:646–651. doi: 10.1038/aja.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel U, Kleinschnitz C, Oh P, Nedvetsky P, Smolenski A, Muller H, et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol. 2002;4:307–311. doi: 10.1038/ncb775. [DOI] [PubMed] [Google Scholar]
- Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci U S A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouaoui Boudjeltia K, Roumeguere T, Delree P, Moguilevsky N, Ducobu J, Vanhaeverbeek M, et al. Presence of LDL modified by myeloperoxidase in the penis in patients with vascular erectile dysfunction: a preliminary study. Eur Urol. 2007;51:262–268. doi: 10.1016/j.eururo.2006.08.040. discussion 268–269. [DOI] [PubMed] [Google Scholar]