Abstract
Infertility is a global problem that is on the rise, especially during the last decade. Currently, infertility affects approximately 10–15% of the population worldwide. The frequency and origin of different forms of infertility varies. It has been shown that reactive oxygen and nitrogen species (ROS and RNS) are involved in the aetiology of infertility, especially male infertility. Various strategies have been designed to remove or decrease the production of ROS and RNS in spermatozoa, in particular during in vitro fertilization. However, in recent years it has been shown that spermatozoa naturally produce a variety of ROS/RNS, including superoxide anion radical (O2⋅−), hydrogen peroxide and NO. These reactive species, in particular NO, are essential in regulating sperm capacitation and the acrosome reaction, two processes that need to be acquired by sperm in order to achieve fertilization potential. In addition, it has recently been shown that mitochondrial function is positively correlated with human sperm fertilization potential and quality and that NO and NO precursors increase sperm motility by increasing energy production in mitochondria. We will review the new link between sperm NO-driven redox regulation and infertility herein. A special emphasis will be placed on the potential implementation of new redox-active substances that modulate the content of NO in spermatozoa to increase fertility and promote conception.
Linked Articles
This article is part of a themed section on Pharmacology of the Gasotransmitters. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-6
Keywords: nitric oxide, infertility, spermatozoa, in vitro fertilization, mitochondria, redox regulation, capacitation, acrosome reaction, motility
Introduction
Infertility is one of the most serious medical problems worldwide. The prevalence of infertility is on the rise, especially during the last decade. Globally, one of six to seven couples worldwide currently has some difficulty with conception (Sharma et al., 2013). Infertility is usually defined as the inability to conceive after 1 year of regular unprotected intercourse, but in couples in which the female partner is >35 years of age, infertility is diagnosed after an inability to conceive for 6 months (Cooper et al., 2010). Although the frequency and origin of infertility varies, it has been established that nearly 40% of the issues involved with infertility are attributable to a male factor, another 40% due to a female factor, and 20% result from combined male and female factors (Sharlip et al., 2002).
Over the past decade, significant advances have occurred in the diagnosis and treatment of reproductive disorders. However, some of causative factors underlying infertility can only be successfully overcome with the use of assisted reproductive technologies. Various techniques, such as in vitro fertilization (IVF), intracytoplasmic sperm injection, intrauterine insemination using a partner or donor's semen, frozen embryo replacement and egg donation, have been developed to treat infertility. Since 1978, when the first IVF baby was born, the techniques used in IVF have advanced dramatically; indeed, the current procedure success rate based on clinical pregnancies is 25–50%, clearly showing that advances in general and reproductive medicine occur simultaneously. However, modern lifestyle patterns, such as social and environmental factors, account for part of the increase in the prevalence of infertility. For these reasons, one of the most important biomedical directions today is the development of new therapeutic approaches for improvement in fertilization, both under natural and assisted reproduction methods.
Extensive research in infertility has confirmed that excessive production of reactive oxygen and nitrogen species (ROS and RNS), that is, oxidative stress, is involved in the aetiology of infertility, especially male infertility (MacLeod, 1943; Aitken and Clarkson, 1987; Iwasaki and Gagnon, 1992; Sukcharoen et al., 1996; Agarwal and Allamaneni, 2004). All of these reports have associated ROS/RNS, generated both exogenously and endogenously, with several aspects of male infertility, such as decreased sperm motility, abnormal morphology and decreased sperm–egg penetration. The mechanism of action of ROS and RNS involves lipid peroxidation of the sperm plasma membrane, which is highly susceptible to oxidative damage due to large amounts of polyunsaturated fatty acids, by impairing membrane fluidity and mobility (Alvarez and Storey, 1995; Baker et al., 1996). In addition, ROS and RNS can damage sperm axoneme, and impair mitochondrial function, DNA, RNA and proteins (de Lamirande and Gagnon, 1992; Agarwal and Prabakaran, 2005).
Thus, for many years, ROS and RNS have been considered as harmful and various strategies were designed to remove or decrease their production, especially during IVF, when sperm is exposed to higher concentrations of oxygen than in vivo. Some of the strategies used include supplementation of culture media with antioxidants (vitamin E and disulphide-reducing agents), removal of ROS-producing cells (mostly leukocytes and damaged spermatozoa) by density gradient for sperm preparation, and incubation under low O2 tension and illumination (de Lamirande and Gagnon, 1992; Aitken et al., 1996; Irvine, 1996; Parinaud et al., 1997). Nevertheless, in contrast to the involvement of ROS and RNS in male infertility, supplemental intake of antioxidants in humans has been shown to improve reproductive functions only in select cases (Dawson et al., 1992; Kessopoulou et al., 1995). Moreover, some of the studies have reported no changes in semen parameters or male fertility after prolonged oral administration of antioxidants (Hughes et al., 1998; Rolf et al., 1999). These data have given rise to a concern and debate regarding the effectiveness of oral administration of antioxidants as therapy for male infertility.
The basis for a ‘failure’ of antioxidant therapy in the treatment of male infertility is that ROS and RNS in small, controlled, physiological concentrations are needed for normal sperm functioning. Thus, ROS/RNS must be maintained at appropriate levels to ensure a physiological function. Specifically, it has been shown that as a product of normal metabolism in germ cells – spermatozoa and oocytes – small amounts of ROS and RNS were produced, and these reactive species, when produced in controlled, physiological concentrations, function as secondary messengers that regulate crucial reproductive processes, especially in sperm cells (de Lamirande et al., 1997; Leclerc et al., 1997). Specifically, it has been shown that NO and superoxide anion radical (O2⋅−) are essential in regulating sperm capacitation and acrosome reaction (AR), two processes required for sperm to acquire fertilization potential (de Lamirande et al., 1997; Leclerc et al., 1997). It has also been shown that ROS and RNS stimulate sperm-hyperactivated motility (Suzuki et al., 1997) and binding to the zona pellucida (ZP) (Aitken et al., 1989), emphasizing that essential sperm fertilization events are redox sensitive.
It is important to note that the major production site and target for ROS and RNS are the mitochondria. Consequently, by production of ROS/RNS and subsequent activation of various redox-dependent signalling pathways, mitochondria are involved in the regulation of sperm functions. In addition, it has been shown that through ATP production, mitochondria regulate spermatogenesis, differentiation, the AR, oocyte fusion and fertilization (Agarwal et al., 2008). Furthermore, through Ca2+ signalling, mitochondria regulate sperm hyperactivation, flagellar activity during events leading up to fertilization and energy metabolism (Publicover et al., 2008). The importance of mitochondria in sperm functioning was strengthened with recent evidence showing that mitochondrial function is positively correlated with human sperm fertilization potential and quality (Kasai et al., 2002; Marchetti et al., 2004; Gallon et al., 2006; Otasevic et al., 2013). In contrast, mitochondria are the main site for ROS and RNS targeting. In agreement with this observation, multiple regulatory effects of NO on mitochondria functioning have become evident during the last decade (Nisoli et al., 2003; Petrović et al., 2005; 2008; 2010a,b,,,; Vucetic et al., 2011). In our recent work, we have shown that NO achieves beneficial effects on sperm function through improvement of functional status of sperm mitochondria (Otasevic et al., 2013). For these reasons, involvement of NO on sperm mitochondria and sperm function warrants more consideration, and is discussed in more detail in this review.
The current review has addressed infertility from the aspect of redox regulation. We focused on male (in)fertility and NO-mediated redox regulation of crucial sperm-fertilizing events. A special emphasis was placed on the potential implementation of new redox-active substances that through modulation of the content of NO in spermatozoa could increase male fertility and promote conception.
NO as a signalling molecule: a historical overview
NO represents a simple diatomic gas that functions as a cell signalling molecule in mammalian cells, controlling fundamental functions in the cardiovascular system, brain, host defence, immunity, reproductive system, gastrointestinal system and secretory tissues (Alderton et al., 2001; Govers and Oess, 2004). The first description of NO was linked to the discovery of nitroglycerine (NG) in 1847 by the Italian scientist Sobrero (Marsh and Marsh, 2000). A few years later, NG was used to treat headaches, while since 1870 it has been used in the treatment of angina pectoris. A century after, it was shown that NG activates soluble GC (sGC), as well as increasing the production of cGMP. The largest contribution to the discovery of the effects of NO was the discovery of endothelial-derived relaxing factor (EDRF) by Furchgott and Zawadzki (1980). Seven years later, Ignarro et al. (1987a,b,) showed that EDRF is actually NO, and a year later Moncada's group (Palmer et al., 1988) reported that mammalian cells endogenously synthesize NO from l-arginine. The importance of this discovery was demonstrated by the naming of NO as the ‘molecule of the year’ by the editor of Science in 1992. In 1998, the scientists who were involved in the discovery of NO and defined the role of NO in the cardiovascular system were awarded the Nobel Prize in Medicine.
NO synthesis
NO is synthesized in virtually all mammalian cells via l-arginine oxidation by a family of NOS isoforms (Moncada et al., 1989). Three distinct NOS isoforms have been identified thus far (see Alexander et al., 2013), as follows: neuronal (nNOS), which was originally described in neuronal tissue; endothelial (eNOS), which was originally described in endothelial cells; and inducible NOS (iNOS), which was originally identified in macrophages (Moncada et al., 1991; Alderton et al., 2001). All mammalian NOS isoforms characterized are haem-containing proteins that are dimeric in native conditions with a monomeric molecular mass of 126–160 kDa. NOS isoforms display different affinities for calmodulin, whereas nNOS and eNOS are Ca2+-calmodulin dependent, and iNOS is nearly Ca2+-calmodulin independent. Another dissimilarity between the NOS isoforms is related to the level of NO production because iNOS has been shown to produce higher NO levels (μM-mM) and to remain active for a longer period of time compared with nNOS and eNOS (NO concentrations from nM-μM) (Moncada et al., 1991; Alderton et al., 2001; Buzadzic et al., 2006; Otasevic et al., 2011). All NOS isoforms may be found attached to or within the mitochondria, in which case NO synthase is referred to as the fourth isoform mitochondrial NOS (mtNOS) (Kobzik et al., 1995; Bates et al., 1996; Ghafourifar and Richter, 1999). However, the existence of mtNOS is controversial and the full characterization of mtNOS is still in progress.
Mechanisms by which NO acts
In general, NO has been shown to exhibit its effects through two different signalling pathways as follows: cGMP-dependent signalling and cGMP-independent signalling (classical and non-classical signalling respectively) (Martínez-Ruiz et al., 2011; Otasevic et al., 2011). Classical signalling involves activation of sGC, generation of cGMP, and the subsequent activation of specific cGMP-dependent enzymes (protein kinases, channels and phosphodiesterases). The sensitivity of sGC to NO is within the nanomolar range (Martínez-Ruiz et al., 2011). Non-classical signalling occurs through covalent post-translational modification of target proteins, that is, S-nitrosylation, S-glutathionylation and tyrosine nitration (Mieyal et al., 2008; Souza et al., 2008; Lima et al., 2010). This non-classical signalling is governed by NO concentrations higher than needed for activation of sGC. S-nitrosylation of the proteins and thiols is mediated not only by NO, but also by other NO species, metal-NO complexes, peroxynitrite or nitrite (Otasevic et al., 2011). Almost all classes of proteins, including receptors, enzymes and transcriptional factors, are targets of S-nitrosylation. In contrast, protein modifications by tyrosine nitration, which are mainly mediated by peroxynitrite, potentially result in alteration, loss or gain of function, but reversibility has not been demonstrated, and hence a clear assignment to redox regulation cannot be made.
It is important to highlight specifically NO acting in a cGMP-independent manner, in which NO interacts with other haemoproteins, such as cytochrome c oxidase (Cleeter et al., 1994). This cGMP-independent pathway is a less classic but equally important pathway for NO, which might have important implications for cell respiration and metabolism (Brookes et al., 2002; Erusalimsky and Moncada, 2007; Vucetic et al., 2011).
NOS isoforms in spermatozoa
Human spermatozoa express all three isoforms of NOS, which are located in the head and/or flagellum regions of the sperm (Herrero et al., 2003; de Lamirande and Lamothe, 2009; de Lamirande et al., 2009). It has been shown that under capacitating conditions, the activity of NOSs increases in a time-dependent manner (de Lamirande and Lamothe, 2009; de Lamirande et al., 2009). Furthermore, the presence and activity of sperm NOSs has been recently shown to depend on the maturity of male germ cells (Roessner et al., 2010). The correlation between NOS activity and sperm maturity clearly underlies the physiological role played by NO in spermatozoa. It is often very difficult to define a function regulated exclusively by one specific NOS isoform. However, Table 1 summarizes the reported specific function, so far, that each NOS isoform plays in the regulation of events important for sperm functionality.
Table 1.
Specific function of NOS isoforms in the regulation of events important for sperm functionality
| Isoform | Localization (Lee and Cheng, 2008) | Function |
|---|---|---|
| eNOS | Germ cells, Sertoli cells, Leydig cells, myofibroblasts, endothelial cells in blood vessels, head and midpiece of the spermatozoon | Control of tight junction in the testis, germ cell apoptosis, sperm motility and follicular fluid-induced acrosome reaction, spermatogenesis, regulating penile erection |
| (Burnett et al., 1996; Zini et al., 1996; 1998; 1999; O'Bryan et al., 1998; Revelli et al., 1999; Lee and Cheng, 2008) | ||
| nNOS | Sertoli cells, acrosome, Leydig cells, myofibroblasts, endothelial cells in blood vessels, head and midpiece of the spermatozoon | Sperm motility, regulating penile erection |
| (de Lamirande et al., 1984; Burnett et al., 1996; Schaad et al., 1996; Donnelly et al., 1997) | ||
| iNOS | Leydig cells, Sertoli cells, germ cells, head and midpiece of the spermatozoon | Control of tight junction in the testis, germ cell apoptosis, fertilization and different aspects of sperm–egg interaction during fertilization, germ cell apoptosis and sperm number |
| (Lue et al., 2003; Yang et al., 2005; Vera et al., 2006; Lee and Cheng, 2008) | ||
| TnNOS | Leydig cells | Steroidogenesis |
| (Wang et al., 2002) |
TnNOS, testis-specific nNOS.
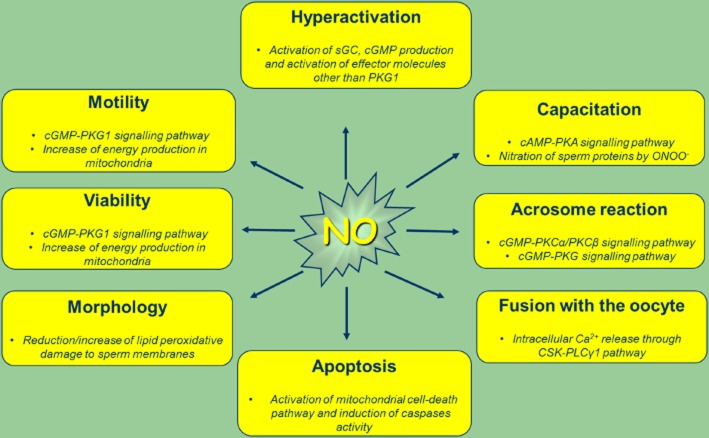
Specifically, NO is known to be involved in the regulation of sperm motility, viability, hyperactivation, capacitation, AR and fusion with the oocyte ( Figure 1) (Herrero et al., 1999; 2003; de Lamirande and Lamothe, 2009; de Lamirande et al., 2009; Kothari et al., 2010; Doshi et al., 2012).
Figure 1.
NO regulates sperm functions. At physiological levels, NO and RNS regulate essential sperm-fertilizing events. At supra-physiological levels NO achieves detrimental effects on above shown sperm function. PLCγ1, phosphoinositide-specific phospholipase γ1.
Effects of NO on sperm-fertilizing effects
Sperm motility, morphology and viability
Sperm motility is a crucial parameter of semen quality (Wang et al., 2012). Accordingly, a primary cause of male infertility is decreased sperm motility, and men with poorly motile or immotile sperm are usually sterile (Turner, 2006). The very first report that showed a regulatory role of NO on sperm motility came from Hellstrom et al. (1994), who determined the effects of NO-releasing compounds on sperm motility and viability. They showed (Hellstrom et al., 1994) that the NO releaser sodium nitroprusside (SNP) in low concentrations (25–100 nM) is beneficial to the maintenance of post-thaw human sperm motion and viability. In contrast, several reports have shown a decrease in progressive sperm motility in the presence of high concentrations of pure NO (25–125 μM) and SNP (0.25–2.5 mM), or S-nitroso-N-acetylpenicillamine (12–600 μM) and 3-morpholinosydnonimine (100–125 μM) (Herrero et al., 1994; Rosselli et al., 1995; Weinberg et al., 1995; Nobunaga et al., 1996). It is clear that, as in other systems, NO exerts a bimodal action on sperm motility; specifically, low concentrations of NO increase sperm motility, while high concentrations of NO reduce the motility of spermatozoa (Weinberg et al., 1995; Herrero and Gagnon, 2001; Miraglia et al., 2011; Doshi et al., 2012). Consistent with this finding, high NO levels have been reported in the semen of infertile men with decreased sperm motility (Rosselli et al., 1995; Perera et al., 1996; Balercia et al., 2004). Moreover, it has been shown that production of nitrite by asthenozoospermic men is lower than that in normozoospermic men, indicating that a decrease in endogenous NO influences sperm motility, and consequently sperm-fertilizing potential (Lewis et al., 1996; Bolanos et al., 2008). Accordingly, the addition of NG-nitro-l-arginine methyl ester (l-NAME) to the sperm-incubating medium significantly reduces human sperm motility (Donnelly et al., 1997; Yeoman et al., 1998).
Miraglia et al. (2011) reported that NO influences sperm motility via the activation of sGC, the subsequent synthesis of cGMP and the activation of cGMP-dependent protein kinases. In addition, our recent data suggest that NO increases the motility of spermatozoa by increasing energy production originating in the mitochondrial compartment (Otasevic et al., 2013). Accordingly, high levels of NO in spermatozoa detected in infertile men are linked to a decrease in sperm metabolism (Doshi et al., 2012).
Moreover, NO contributes to normal sperm morphology, which has been shown to facilitate the accurate prediction of fertility status and pregnancy outcomes during IVF (Revelli et al., 2001). Nevertheless, similar to sperm motility, it has been shown that in high concentrations NO induces atypical sperm morphology (Wu et al., 2004). Sperm viability is also affected by NO. Specifically, researchers have reported that viability of sperm in the presence of 10−4 M SNP is significantly lower than sperm without this treatment (Doshi et al., 2012).
Sperm capacitation
Motile and morphologically mature human spermatozoa are not fertile immediately after ejaculation, but rather achieve fertilizing ability during transition in the female genital tract. Sperm first undergoes a complex and timely series of biochemical and physiological changes, which is termed capacitation (Chang, 1951; Austin, 1952). In fact, capacitation is the final maturation process that ensures that only fertile spermatozoa are able to reach, bind to and penetrate the oocyte (Baker and Aitken, 2004). Membrane and metabolic changes that occur during capacitation confer upon the spermatozoa an ability to gain hyperactive motility, interact with the oocyte ZP, undergo an AR and initiate oocyte plasma membrane fusion (Baker and Aitken, 2004).
The involvement of NO in capacitation was first demonstrated in human spermatozoa incubated with low concentrations of NO-releasing compounds (Zini et al., 1995). Specifically, it has been shown that NO donors (with different potencies) accelerate capacitation, while NOS inhibitors significantly decrease this process (Herrero et al., 1999). These data clearly demonstrate that endogenous NO is necessary for spermatozoa to display full fertilizing ability. Furthermore, it was subsequently shown that this NO-dependent redox control appears crucial for capacitation triggered by different agents, such as albumin, progesterone and fetal cord serum ultrafiltrate, which modulate the same actions independently of the inducer (O'Flaherty et al., 2006; de Lamirande and O'Flaherty, 2008).
At the molecular level, capacitation involves a cascade of events (an efflux of cholesterol, fluxes of bicarbonate, and a rise in cAMP, Ca2+ and intracellular pH) that is initiated by ROS and RNS produced by spermatozoa (de Lamirande et al., 1997; 2009; Herrero et al., 1999; 2003; O'Flaherty et al., 2006; de Lamirande and O'Flaherty, 2008; de Lamirande and Lamothe, 2009). Specifically, it has been shown that NO and O2⋅− are generated in spermatozoa from the beginning of capacitation and control the increase in cAMP and most of the known serine/threonine phosphorylation of PKA substrates, tyrosine phosphorylation of fibrous sheath proteins as well as tyrosine nitration of proteins. Moreover, it has been shown that NO is needed over the course of capacitation and more than one NOS is probably involved in this process (de Lamirande and Lamothe, 2009).
Hyperactivation
Hyperactivation coincides with the onset of capacitation and represents a subcategory of capacitation. In non-hyperactive spermatozoa, low-amplitude flagellar movements are associated with slow, linear movements of the cell. In contrast, hyperactive spermatozoa exhibit high amplitude asymmetric flagellar movements and non-linear motility. In the female genital tract, hyperactivated spermatozoa exhibit less stagnation in the oviductal epithelium and the surrounding viscous mucus and a propulsive force to penetrate the cells of the oocyte (Miraglia et al., 2007; Kothari et al., 2010). Results supporting the role of NO in the initiation of hyperactivation are well accepted. Similar to the NO effects on ‘regular’ sperm motility, it has been shown that NO concentrations < 1 μM increase sperm hyperactivation, while NO in concentrations > 1 μM decreases sperm-hyperactivated motility (Lewis et al., 1996; Miraglia et al., 2011; Otasevic et al., 2013).
AR
When hyperactivated motility has drowned the capacitated spermatozoa to the ovum, sperm binds to the ZP and begins to bypass this barrier by the exocytotic release of proteolytic enzymes in a process referred to as the AR. The AR encompasses the release of proteolytic enzymes from the acrosome cap of the spermatozoa that create a pore in the extracellular matrix of the ZP and allows spermatozoa to bind appropriately, penetrate the barrier and fuse with the oocyte. At the molecular level, the AR shares a significant overlap with molecular events of capacitation, such as phosphorylation of similar tyrosine residues in proteins, an influx of Ca2+, activities of AC, cAMP and PKA (de Lamirande and O'Flaherty, 2008). Similar to capacitation, reactive species, including NO, stimulate the AR by activating AC that triggers downstream target molecules to initiate the exocytotic event (Zini et al., 1995).
The fact that NO participates in ZP binding, as well as in the AR has been well characterized (Herrero and Gagnon, 2001). Treatment of human spermatozoa with low concentrations of SNP in the capacitation medium increases the number of spermatozoa bound to hemizona (Sengoku et al., 1998), and increases sperm affinity for the ZP. In contrast, it has been demonstrated that addition of various concentrations of l-NAME to capacitated spermatozoa do not affect ZP binding, but significantly inhibit the fusion effect (Francavilla et al., 2000). Similarly, NO-releasing compounds induce the AR in human spermatozoa in a concentration-dependent manner, whereas haemoglobin, as a NO scavenger, prevents the AR in spermatozoa capacitated by human follicular fluid (Revelli et al., 1999).
NO and sperm mitochondria
Mitochondria are essential in the regulation of a variety of cellular activities, including ATP synthesis, intracellular calcium homeostasis and apoptosis (Duchen, 2000). In sperm cells, mitochondria supply sperm with energy for several purposes (Figure 2), and thus play important roles in spermatogenesis, differentiation, motility, AR, oocyte fusion and fertilization (Agarwal et al., 2008). In addition to the generation of ATP to supply the above sperm fertilization processes, other functions of mitochondria in sperm, such as Ca2+ signalling and protein synthesis, have been documented (Gur and Breitbart, 2006; Publicover et al., 2008) (Figure 2). Several studies have confirmed that mitochondrial functionality positively correlates with human sperm fertilizing potential and quality (Kasai et al., 2002; Marchetti et al., 2004; Gallon et al., 2006; Otasevic et al., 2013). The major determinants of sperm mitochondrial functionality are the activity and expression of components of the electron transport chain (ETC) (Ruiz-Pesini et al., 1998; Hüttemann et al., 2003) as well as mitochondrial membrane potential (MMP) (Marchetti et al., 2004; Gallon et al., 2006; Otasevic et al., 2013) Accordingly, any disruption of mitochondrial ETC components or MMP is associated with decreased sperm motility, reduced sperm fertilization potential and male sterility (Marchetti et al., 2002; 2004; Rossato, 2008; Otasevic et al., 2013).
Figure 2.
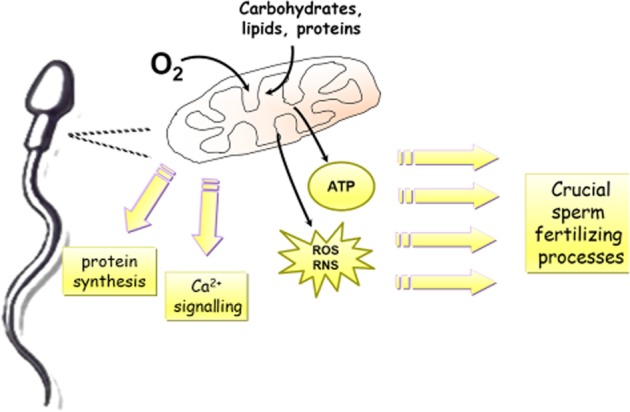
Importance of mitochondria in regulation of sperm functions. Mitochondria supply spermatozoa with energy (ATP) for vital sperm-fertilizing processes. In addition, through production of ROS and RNS and subsequent activation of various redox-dependent signalling pathways, mitochondria are involved in the regulation of sperm functions. Other roles of mitochondria in spermatozoa, such as Ca2+ signalling and protein synthesis, have also been presented.
NO has been shown to affect the mitochondria on several levels, as follows: regulates mitochondrial respiration; biogenesis, remodelling and increasing O2 and substrate supply to the mitochondria (Nisoli et al., 2003; Petrović et al., 2005; 2008; 2010a,b; Vasilijevic et al., 2010; Vucetic et al., 2011). Current data show that NO can adversely influence the mitochondrial function of sperm cells depending on the NO level. A growing amount of data have shown that higher levels of NO (μM) inhibit respiration in sperm mitochondria (Rosselli et al., 1995; Weinberg et al., 1995), while iNOS-derived high (mM) NO levels have been shown to induce mitochondrial hyperpolarization, cytochrome c release and sperm cell death (Mishra and Shaha, 2005). Low concentrations of NO (nM) increase sperm motility and this has been shown to be strongly associated with energy production in mitochondria (Hellstrom et al., 1994). Our very recent work revealed that NO improved the functional status of sperm mitochondria and sperm quality (Otasevic et al., 2013). The functional state of sperm mitochondria, as indicated by the percentage of positive MitoTracker®Green FM (Molecular Probes, Inc., Eugene, OR, USA) sperm cells and the expression of components of ETC, was significantly higher in sperm samples with higher NO concentration. These data were positively correlated with sperm motility, a major indicator of semen quality, and clearly showed that NO improved the fertilizing potential of spermatozoa.
Therapeutic applications
To date, the majority of trials oriented to NO-dependent improvement of sperm function have been performed with a precursor of NO synthesis, l-arginine (Scibona et al., 1994; Aydin et al., 1995; Patel et al., 1998; Stanislavov et al., 2009). In addition to l-arginine per se, the products of its metabolism play significant roles in the regulation of fundamental cellular processes and physiological functions (Vasilijevic et al., 2007; Stancic et al., 2012). However, many of the positive effects of l-arginine in male fertility disorders (improvement in the sperm count and motility in oligospermic and asthenospermic patients) have been proven to be mediated through increased biosynthesis of NO (Scibona et al., 1994; Aydin et al., 1995; Srivastava et al., 2000). Stanislavov et al. (2009) reported that supplementation of subfertile men with a combination of pycnogenol and l-arginine aspartate in a double-blind, placebo-controlled, crossover study significantly increased sperm volume, concentration, motility and % normal morphology through the antioxidative effect of pycnogenol, induction of eNOS and consequent endogenous production of NO in spermatozoa.
However, important progress in l-arginine research suggests that this amino acid and its metabolites play a more complex role in the regulation of whole body energy homeostasis, and thus dietary supplementation of l-arginine must be taken with vigilance. When supplemented in appropriate physiological doses, the duration and intake frequency of l-arginine shows multiple beneficial effects; however, with supplementation in high, supra-physiological doses (Luiking et al., 1998; Grimble, 2007) and over a long-term period (Park, 1993; Tome et al., 1999), l-arginine can exert some side effects (nausea, gastrointestinal discomfort, diarrhoea, and changes in electrolytes in the blood, including potassium). In addition, there are a few pathophysiological states in which l-arginine must be applied with caution, such as myocardial infarction (Schulman et al., 2006), asthma (Takano et al., 1998), viral infections (Tankersley, 1964), cancer (Yeatman et al., 1991; Grossie, 1996), and renal and liver diseases (Tome et al., 1999; Appleton, 2002). Bearing in mind all of these findings and the fact that infertility currently affects one in seven couples worldwide (NB, many experts in the field predict this could double over the next decade), it is clear that novel strategies to treat sterility must be developed (Table 2).
Table 2.
List of redox-active substances in clinical trials or commercially available that ameliorate sperm-fertilizing capacity by (at least partially) modulating the NO-signalling pathway
| Compound | Effect | |
|---|---|---|
| l-arginine | Increases sperm volume, concentration, motility and morphology by increasing eNOS activity | Scibona et al., 1994; Aydin et al., 1995; Srivastava et al., 2000 |
| Superoxide dismutase mimic, M40403 | Improves the functional status of sperm mitochondria, and thus the fertilizing potential of spermatozoa, by increasing NO bioavailability | Otasevic et al., 2013 |
| Sildenafil (Viagra™) | Improves erectile function through inhibition of cGMP-dependent phosphodiesterase | Schwartz and Kloner, 2010 |
| Increase velocity, capacitation and amplitude of lethal head displacement in spermatozoa (mechanism unclear) | Lefièvre et al., 2000 | |
| Tadalafil (Cialis™) | Improves erectile function through inhibition of cGMP-dependent phosphodiesterase | Schwartz and Kloner, 2010 |
| Vardenafil (Levitra™) | Improves erectile function through inhibition of cGMP-dependent phosphodiesterase | Schwartz and Kloner, 2010 |
| Ginsenoside Re | Improves sperm motility through induction of NOS activity (increase of NO production) | Zhang et al., 2006 |
Accordingly, our recent data shed light on the development of new pharmacological strategies to treat male infertility using novel, safe and effective redox-active substances (Otasevic et al., 2013). Due to the important implications of the redox status in male factor infertility, we examined how changes in the sperm redox milieu affect sperm functionality. To modulate the sperm cell redox state, sperm incubation medium was supplemented with the superoxide dismutase (SOD) mimic, M40403, which is representative of the Mn II pentaazamacrocyclic class of complexes (Figure 3). This is a potent low molecular mass SOD mimic with marked protective effects in inflammation, stroke, atherosclerosis and hypertension (Salvemini et al., 2002; Vallance and Leiper, 2002). The main advantage of M40403 is high selectivity for O2⋅–, lack of peroxidase/catalase activity and lack of reactivity with NO. Our results have shown that M40403 restores the population of sperm with functionally active mitochondria, thus stimulating sperm mitochondrial activity and subsequent sperm quality. The SOD mimic positively affected another component of the functional status of sperm mitochondria, that is, increased mRNA expression of the nucleus encoded subunits of both complexes I and IV of the ETC, while subunits encoded by the mitochondrial genome were restored to the control level. These data undoubtedly showed improvement of functional status of sperm mitochondria, and thus the fertilizing potential of spermatozoa by M40403, while definitive proof of improvements in sperm-fertilizing potential was shown by the finding that the mimic increased sperm motility, a crucial parameter of semen quality.
Figure 3.
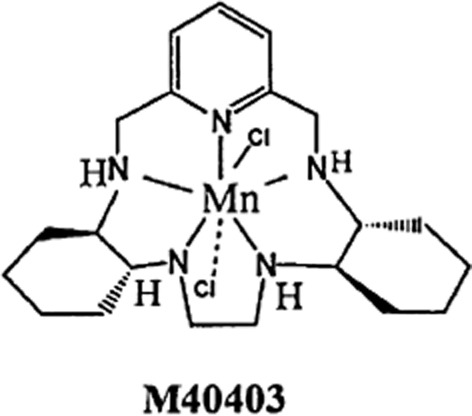
Structure of M40403, a SOD mimic.
This is the very first time that M40403 has been shown to improve the molecular basis of sperm mitochondrial function, as well as increase sperm motility, and thus has a positive effect on the functionality of spermatozoa. Although the precise mechanism of this M40403-acting remains to be elucidated, the increase in NO content in spermatozoa after treatment with M40403 implies the involvement of NO in the observed beneficial effects of this drug. This seems quite plausible considering that NO affects mitochondria on several levels and that NO and its precursors increase sperm motility by increasing energy production in the mitochondrial compartment (Hellstrom et al., 1994). In addition, M40403 increased eNOS gene expression, which strongly indicates that an increase in the NO level after M40403 may depend, at least partly, on drug-elicited induction of eNOS and an increase in endogenous NO production. Thus, in future studies designed to define the precise mechanism underlying the M40403-driven increase in the population of sperm with active mitochondria and motile spermatozoa, the involvement of NO warrants further consideration (Figure 4).
Figure 4.
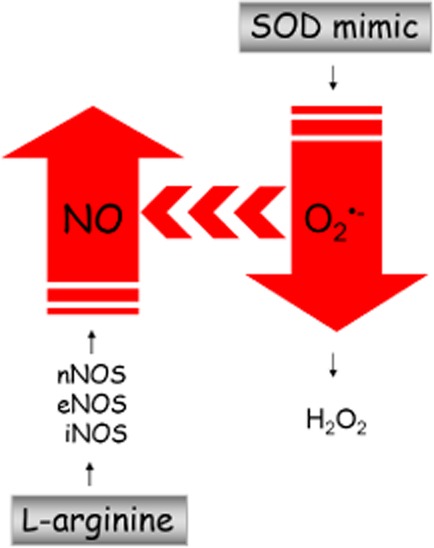
Strategies to modify NO concentration in spermatozoa. As a substrate for NOSs, l-arginine increases NO production. In contrast, the SOD mimic removes O2⋅−, and by decreasing the bioavailability of O2− for interaction with NO, the amount of NO consequently increases.
Nevertheless, what is certain and clearly shown by this study is that the redox modulator, M40403, is a promising pharmacological tool for the improvement of sperm function during assisted fertilization and for the treatment of infertile states accompanied by mitochondrial impairments and/or a disturbed sperm redox state.
In addition, another potentially important fact that could be significant for determination of NO bioavailability and roles that NO plays in male (in)fertility is NOS uncoupling (Alderton et al., 2001). That is, it is known that in a number of pathological conditions NOS enzymatic activity becomes uncoupled, resulting in the production of O2⋅– instead of NO, and leading to a decrease in NO bioavailability. A number of potential mechanisms are responsible for uncoupling of NOSs, although the most consistent evidence suggests it is due to tetrahydrobiopterin deficiency (Kietadisorn et al., 2012). NOS uncoupling also occurs when there is lack of other NOS cofactors, substrate – l-arginine, when they are dephosphorylated or redistributed to the cytosolic fraction. Thus, this could be potentially very important from the aspect of male infertility because, theoretically, modulation of NOS uncoupling (by providing their substrate, cofactors, or stabilizing their activity) could be an attractive therapeutic approach to increase NO synthesis and consequently improve sperm functions.
In line with this, it has been shown that when eNOS activity is uncoupled, treatments with redox-active substances, that is, redox changes in endothelium, can substantially ameliorate NO production and thus achieve beneficial effects in cardiovascular system (Visioli and Hagen, 2011). Specifically, it was shown that vitamin C facilitates eNOS-derived NO synthesis by maintaining/increasing intracellular tetrahydrobiopterin in a highly reduced state, necessary for NOS activity, thereby promoting or maintaining eNOS coupling in endothelium (Heller et al., 1999; Carr et al., 2000; Huang et al., 2000). In addition, GSH and stimulators of GSH synthesis, such as (R)-α-lipoic acid, markedly increase eNOS activity by promoting/preserving eNOS phosphorylation, especially at the S1176 residue (Visioli et al., 2002; Suh et al., 2004), whereas polyphenols increase endothelial NO synthesis markedly, but the underlying mechanisms are still elusive. Based on these data obtained in vascular dysfunction, there is compelling rationale to use these substances to increase NO production in spermatozoa, at least in conditions that could lead to uncoupled activity of NOSs. In addition, a recently reported link between vascular dysfunction and various forms of infertility (Visioli and Hagen, 2011) further strengthens the rationale for the use of these molecules to increase bioavailability of NO in spermatozoa.
Conclusions and perspectives
Although infertility is a common global problem, treatment is sometimes ineffective, and the number of couples seeking infertility services has increased dramatically over the last decade. Such an increase in infertility rates clearly indicates the need for the development of new strategies to treat sterility.
It has become evident that redox regulation in reproductive biology, that is, regulation driven by ROS and RNS, represents an inevitable new tool for studying fundamental fertilization processes. Accordingly, the present article dealt with the problem of sterility from the perspective of redox regulation. We aimed here not only to review the known facts of the multiple roles that NO plays in spermatozoa and integrating different aspects of the physiological act, but also to open new fields for future research of its complex action in male infertility, emphasizing it as a potentially useful strategy for prevention and management of infertility. The ubiquitous involvement of NO in the regulation of crucial sperm-fertilizing events may have huge clinical implications in developing new therapeutic strategies to treat fertilization disorders in men.
In this regard, use of new potent substances that modulate sperm redox state by selective production/removal of certain ROS/RNS could be a new power tool to increase male fertility and promote conception.
Acknowledgments
This work was supported by the COST BM1005 action and by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant Nos. 173054 and 173055. We would like to acknowledge that the drug/molecular target nomenclature used in this work conforms to BJP's Concise Guide to Pharmacology (Alexander et al., 2013).
Glossary
- AR
acrosome reaction
- EDRF
endothelial-derived relaxing factor
- eNOS
endothelial NOS
- ETC
electron transport chain
- iNOS
inducible NOS
- IVF
in vitro fertilization
- l-NAME
NG-nitro-l-arginine methyl ester
- MMP
mitochondrial membrane potential
- mtNOS
mitochondrial NOS
- NG
nitroglycerine
- nNOS
neuronal NOS
- O2⋅−
superoxide anion radical
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- sGC
soluble GC
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- ZP
zona pellucida
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9:338–347. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Aitken KJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81:459–469. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Buckingham DW, West K, Brindle J. On the use of paramagnetic beads and ferrofluids to assess and eliminate the leukocytic contribution to oxygen radical generation by human sperm suspensions. Am J Reprod Immunol. 1996;35:541–551. doi: 10.1111/j.1600-0897.1996.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;1:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–346. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- Appleton J. Arginine: clinical potential of a semi-essential amino. Altern Med Rev. 2002;7:512–522. [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Aydin S, Inci O, Alagöl B. The role of arginine, indomethacin and kallikrein in the treatment of oligoasthenospermia. Int Urol Nephrol. 1995;27:199–202. doi: 10.1007/BF02551320. [DOI] [PubMed] [Google Scholar]
- Baker HW, Brindle J, Irvine DS, Aitken RJ. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65:411–419. doi: 10.1016/s0015-0282(16)58109-6. [DOI] [PubMed] [Google Scholar]
- Baker MA, Aitken RJ. The importance of redox regulated pathways in sperm cell biology. Mol Cell Endocrinol. 2004;216:47–54. doi: 10.1016/j.mce.2003.10.068. [DOI] [PubMed] [Google Scholar]
- Balercia G, Moretti S, Vignini A, Magagnini M, Mantero F, Boscaro M, et al. Role of nitric oxide concentrations on human sperm motility. J Androl. 2004;25:245–249. doi: 10.1002/j.1939-4640.2004.tb02784.x. [DOI] [PubMed] [Google Scholar]
- Bates TE, Loesch A, Burnstock G, Clark JB. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochem Biophys Res Commun. 1996;218:40–44. doi: 10.1006/bbrc.1996.0008. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Delgado-Esteban M, Herrero-Mendez A, Fernandez-Fernandez S, Almeida A. Regulation of glycolysis and pentose-phosphate pathway by nitric oxide: impact on neuronal survival. Biochim Biophys Acta. 2008;1777:789–793. doi: 10.1016/j.bbabio.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Nelson RJ, Calvin DC, Liu JX, Demas GE, Klein SL, et al. Nitric oxide-dependent penile erection in mice lacking nitric oxide synthase. Mol Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- Buzadzic B, Korac A, Petrovic V, Korac B. Redox regulation of brown adipocytes: molecular and cellular targets in tissue remodeling. Acta Physiol Pharamacol Serb. 2006;42:141–159. [Google Scholar]
- Carr AC, Zhu BZ, Frei B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and alpha-tocopherol (vitamin E) Circ Res. 2000;87:349–354. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implication for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- Dawson EB, Harris WA, Teter MC, Powell LC. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril. 1992;58:1034–1039. [PubMed] [Google Scholar]
- Donnelly ET, Lewis SE, Thompson W, Chakravarthy U. Sperm nitric oxide and motility: the effects of nitric oxide synthase stimulation and inhibition. Mol Hum Reprod. 1997;3:755–762. doi: 10.1093/molehr/3.9.755. [DOI] [PubMed] [Google Scholar]
- Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012;10:109. doi: 10.1186/1477-7827-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- Francavilla F, Santucci R, Macerola B, Ruvolo G, Romano R. Nitric oxide synthase inhibition in human sperm affects sperm-oocyte fusion but not zona pellucida binding. Biol Reprod. 2000;63:425–429. doi: 10.1095/biolreprod63.2.425. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;27:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gallon F, Marchetti C, Jouy N, Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril. 2006;86:1526–1530. doi: 10.1016/j.fertnstert.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Richter C. Mitochondrial nitric oxide synthase regulates mitochondrial matrix pH. Biol Chem. 1999;380:1025–1028. doi: 10.1515/BC.1999.127. [DOI] [PubMed] [Google Scholar]
- Govers R, Oess S. To NO or not to NO: ‘where?’ is the question. Histol Histopathol. 2004;19:585–605. doi: 10.14670/HH-19.585. [DOI] [PubMed] [Google Scholar]
- Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. J Nutr. 2007;37:1693–1701. doi: 10.1093/jn/137.6.1693S. [DOI] [PubMed] [Google Scholar]
- Grossie VB. Citrulline and arginine increase the growth of the ward colon tumor in parenterally fed rats. Nutr Cancer. 1996;26:91–97. doi: 10.1080/01635589609514466. [DOI] [PubMed] [Google Scholar]
- Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006;20:411–416. doi: 10.1101/gad.367606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Münscher-Paulig F, Gräbner R, Till U. L-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem. 1999;274:8254–8260. doi: 10.1074/jbc.274.12.8254. [DOI] [PubMed] [Google Scholar]
- Hellstrom WJ, Bell M, Wang R, Sikka SC. Effect of sodium nitroprusside on sperm motility, viability, and lipid peroxidation. Fertil Steril. 1994;61:1117–1122. doi: 10.1016/s0015-0282(16)56766-1. [DOI] [PubMed] [Google Scholar]
- Herrero MB, Gagnon C. Nitric oxide: a novel mediator of sperm function. J Androl. 2001;22:349–356. [PubMed] [Google Scholar]
- Herrero MB, Cebral E, Boquet M, Viggiano JM, Vitullo A, Gimeno MA. Effect of nitric oxide on mouse sperm hyperactivation. Acta Physiol Pharmacol Ther Latinoam. 1994;44:65–69. [PubMed] [Google Scholar]
- Herrero MB, de Lamirande E, Gagnon C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol Reprod. 1999;61:575–581. doi: 10.1095/biolreprod61.3.575. [DOI] [PubMed] [Google Scholar]
- Herrero MB, de Lamirande E, Gagnon C. Nitric oxide is a signaling molecule in spermatozoa. Curr Pharm Des. 2003;9:419–425. doi: 10.2174/1381612033391720. [DOI] [PubMed] [Google Scholar]
- Huang A, Vita JA, Venema RC, Keaney JF., Jr Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod. 1998;13:1240–1247. doi: 10.1093/humrep/13.5.1240. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Schmidt TR, Grossman LI. A third isoform of cytochrome c oxidase subunit VIII is present in mammals. Gene. 2003;312:95–102. doi: 10.1016/s0378-1119(03)00604-8. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987a;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987b;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DS. Glutathione as a treatment for male infertility. Rev Reprod. 1996;1:6–12. doi: 10.1530/ror.0.0010006. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992;57:409–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, et al. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J Androl. 2002;4:97–103. [PubMed] [Google Scholar]
- Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–831. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012;302:E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48:425–435. [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl. 1992;16:21–25. doi: 10.1111/j.1365-2605.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46:502–510. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O'Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784:106–115. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Belles-Isles M, Gagnon C. Characteristics of a seminal plasma inhibitor of sperm motility. Ann N Y Acad Sci USA. 1984;438:125–131. doi: 10.1111/j.1749-6632.1984.tb38281.x. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Lamothe G, Villemure M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic Biol Med. 2009;46:1420–1427. doi: 10.1016/j.freeradbiomed.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22:643–656. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY. Nitric oxide and cyclic nucleotides: their roles in junction dynamics and spermatogenesis. Adv Exp Med Biol. 2008;636:172–185. doi: 10.1007/978-0-387-09597-4_10. [DOI] [PubMed] [Google Scholar]
- Lefièvre L, De Lamirande E, Gagnon C. The cyclic GMP-specific phosphodiesterase inhibitor, sildenafil, stimulates human sperm motility and capacitation but not acrosome reaction. J Androl. 2000;21:929–937. [PubMed] [Google Scholar]
- Lewis SE, Donnelly ET, Sterling ES, Kennedy MS, Thompson W, Chakravarthy U. Nitric oxide synthase and nitrite production in human spermatozoa: evidence that endogenous nitric oxide is beneficial to sperm motility. Mol Hum Reprod. 1996;2:873–878. doi: 10.1093/molehr/2.11.873. [DOI] [PubMed] [Google Scholar]
- Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue Y, Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: evidence from null mutant mice. Endocrinology. 2003;144:3092–3100. doi: 10.1210/en.2002-0142. [DOI] [PubMed] [Google Scholar]
- Luiking YC, Weusten BL, Portincasa P, Van Der Meer R, Smout AJ, Akkermans LM. Effects of long-term oral L-arginine on esophageal motility and gallbladder dynamics in healthy humans. Am J Physiol. 1998;274:984–991. doi: 10.1152/ajpgi.1998.274.6.G984. [DOI] [PubMed] [Google Scholar]
- MacLeod J. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol. 1943;138:512–518. [Google Scholar]
- Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 2004;19:2267–2276. doi: 10.1093/humrep/deh416. [DOI] [PubMed] [Google Scholar]
- Marsh N, Marsh A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol. 2000;27:313–319. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia E, Rullo ML, Bosia A, Massobrio M, Revelli A, Ghigo D. Stimulation of the nitric oxide/cyclic guanosine monophosphate signaling pathway elicits human sperm chemotaxis in vitro. Fertil Steril. 2007;87:1059–1063. doi: 10.1016/j.fertnstert.2006.07.1540. [DOI] [PubMed] [Google Scholar]
- Miraglia E, De Angelis F, Gazzano E, Hassanpour H, Bertagna A, Aldieri E, et al. Nitric oxide stimulates human sperm motility via activation of the cyclic GMP/protein kinase G signaling pathway. Reproduction. 2011;141:47–54. doi: 10.1530/REP-10-0151. [DOI] [PubMed] [Google Scholar]
- Mishra DP, Shaha C. Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway: role of superoxide and nitric oxide. J Biol Chem. 2005;280:6181–6196. doi: 10.1074/jbc.M405970200. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989;38:1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nobunaga T, Tokugawa Y, Hashimoto K, Kubota Y, Sawai K, Kimura T, et al. Elevated nitric oxide concentration in the seminal plasma of infertile males: nitric oxide inhibits sperm motility. Am J Reprod Immunol. 1996;36:193–197. doi: 10.1111/j.1600-0897.1996.tb00162.x. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Zini A, Cheng CY, Schlegel PN. Human sperm endothelial nitric oxide synthase expression: correlation with sperm motility. Fertil Steril. 1998;70:1143–1147. doi: 10.1016/s0015-0282(98)00382-3. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41:528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Otasevic V, Korac A, Buzadzic B, Stancic A, Jankovic A, Korac B. Nitric oxide and thermogenesis-challenge in molecular cell physiology. Front Biosci (Schol Ed) 2011;3:1180–1195. doi: 10.2741/219. [DOI] [PubMed] [Google Scholar]
- Otasevic V, Korac A, Vucetic M, Macanovic B, Garalejic E, Ivanovic-Burmazovic I, et al. Is manganese (II) pentaazamacrocyclic superoxide dismutase mimic beneficial for human sperm mitochondria function and motility? Antioxid Redox Signal. 2013;18:170–178. doi: 10.1089/ars.2012.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;16:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Parinaud J, Le Lannou D, Vieitez G, Griveau JF, Milhet P, Richoilley G. Enhancement of motility by treating spermatozoa with an antioxidant solution (Sperm-Fit) following ejaculation. Hum Reprod. 1997;12:2434–2436. doi: 10.1093/humrep/12.11.2434. [DOI] [PubMed] [Google Scholar]
- Park KG. The Sir David Cuthbertson Medal Lecture 1992. The immunological and metabolic effects of L-arginine in human cancer. Proc Nutr Soc. 1993;52:387–401. doi: 10.1079/pns19930080. [DOI] [PubMed] [Google Scholar]
- Patel AB, Srivastava S, Phadke RS, Govil G. Arginine activates glycolysis of goat epididymal spermatozoa: an NMR study. Biophys J. 1998;75:1522–1528. doi: 10.1016/S0006-3495(98)74071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera DM, Katz M, Heenbanda SR, Marchant S. Nitric oxide synthase inhibitor NG-monomethyl-L-arginine preserves sperm motility after swim-up. Fertil Steril. 1996;66:830–833. doi: 10.1016/s0015-0282(16)58645-2. [DOI] [PubMed] [Google Scholar]
- Petrović V, Korac A, Buzadzic B, Korac B. The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J Exp Biol. 2005;208:4263–4271. doi: 10.1242/jeb.01895. [DOI] [PubMed] [Google Scholar]
- Petrović V, Korac A, Buzadzic B, Vasilijevic A, Jankovic A, Micunovic K, et al. Nitric oxide regulates mitochondrial re-modelling in interscapular brown adipose tissue: ultrastructural and morphometric-stereologic studies. J Microsc. 2008;232:542–548. doi: 10.1111/j.1365-2818.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- Petrović V, Buzadzic B, Korac A, Vasilijevic A, Jankovic A, Korac B. NO modulates the molecular basis of rat interscapular brown adipose tissue thermogenesis. Comp Biochem Physiol C. 2010a;152:147–159. doi: 10.1016/j.cbpc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Petrović V, Buzadžić B, Korać A, Korać B. Antioxidative defense and mitochondrial thermogenic response in brown adipose tissue. Genes Nutr. 2010b;5:225–235. doi: 10.1007/s12263-009-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover SJ, Giojalas LC, Teves ME, de Oliveira GS, Garcia AA, Barratt CL, et al. Ca2+ signaling in the control of motility and guidance in mammalian sperm. Front Biosci. 2008;13:5623–5637. doi: 10.2741/3105. [DOI] [PubMed] [Google Scholar]
- Revelli A, Soldati G, Costamagna C, Pellerey O, Aldieri E, Massobrio M, et al. Follicular fluid proteins stimulate nitric oxide (NO) synthesis in human sperm: a possible role for NO in acrosomal reaction. J Cell Physiol. 1999;178:85–92. doi: 10.1002/(SICI)1097-4652(199901)178:1<85::AID-JCP11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Revelli A, Costamagna C, Moffa F, Aldieri E, Ochetti S, Bosia A, et al. Signaling pathway of nitric oxide-induced acrosome reaction in human spermatozoa. Biol Reprod. 2001;64:1708–1712. doi: 10.1095/biolreprod64.6.1708. [DOI] [PubMed] [Google Scholar]
- Roessner C, Paasch U, Glander HJ, Grunewald S. Activity of nitric oxide synthase in mature and immature human spermatozoa. Andrologia. 2010;42:132–137. doi: 10.1111/j.1439-0272.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–1033. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- Rossato M. Endocannabinoids, sperm functions and energy metabolism. Mol Cell Endocrinol. 2008;286:S31–S35. doi: 10.1016/j.mce.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Dubey RK, Imthurn B, Macas E, Keller PJ. Effects of nitric oxide on human spermatozoa: evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum Reprod. 1995;10:1786–1790. doi: 10.1093/oxfordjournals.humrep.a136174. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Diez C, Lapeña AC, Pérez-Martos A, Montoya J, Alvarez E, et al. Correlation of sperm motility with mitochondrial enzymatic activities. Clin Chem. 1998;44:1616–1620. [PubMed] [Google Scholar]
- Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide dismutase mimetics. Pulm Pharmacol Ther. 2002;15:439–447. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- Schaad NC, Zhang XQ, Campana A, Schorderet-Slatkine S. Human seminal plasma inhibits brain nitric oxide synthase activity. Hum Reprod. 1996;11:561–565. doi: 10.1093/humrep/11.3.561. [DOI] [PubMed] [Google Scholar]
- Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, et al. L-arginine therapy in myo-cardial infarction. The Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88–95. doi: 10.1161/CIRCULATIONAHA.110.944603. [DOI] [PubMed] [Google Scholar]
- Scibona M, Meschini P, Capparelli S, Pecori C, Rossi P, Menchini Fabris GF. L-arginine and male infertility. Minerva Urol Nefrol. 1994;46:251–253. [PubMed] [Google Scholar]
- Sengoku K, Tamate K, Yoshida T, Takaoka Y, Miyamoto T, Ishikawa M. Effects of low concentrations of nitric oxide on the zona pellucida binding ability of human spermatozoa. Fertil Steril. 1998;69:522–527. doi: 10.1016/s0015-0282(97)00537-2. [DOI] [PubMed] [Google Scholar]
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–882. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66. doi: 10.1186/1477-7827-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Peluffo G, Radi R. Protein tyrosine nitration-functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Desai P, Coutinho E, Govil G. Protective effect of L-arginine against lipid peroxidation in goat epididymal spermatozoa. Physiol Chem Phys Med NMR. 2000;32:127–135. [PubMed] [Google Scholar]
- Stancic A, Korac A, Buzadzic B, Otasevic V, Jankovic A, Vucetic M, et al. L-Arginine in nutrition: multiple beneficial effects in the etiopathology of diabetes. J Nutr Ther. 2012;1:114–131. [Google Scholar]
- Stanislavov R, Nikolova V, Rohdewald P. Improvement of seminal parameters with Prelox: a randomized, double-blind, placebo-controlled, cross-over trial. Phytother Res. 2009;23:297–302. doi: 10.1002/ptr.2592. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu H, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukcharoen N, Keith J, Irvine DS, Aitken RJ. Prediction of the in-vitro fertilization (IVF) potential of human spermatozoa using sperm function tests: the effect of the delay between testing and IVF. Hum Reprod. 1996;11:1030–1034. doi: 10.1093/oxfordjournals.humrep.a019291. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Takano H, Lim HB, Miyabara Y, Ichinose T, Yoshikawa T, Sagai M. Oral administration of L-arginine potentiates allergen-induced airway inflammation and expression of interleukin-5 in mice. J Pharmacol Exp Ther. 1998;286:767–771. [PubMed] [Google Scholar]
- Tankersley RW. Amino acid requirements of herpes simplex virus in human cells. J Bacteriol. 1964;87:609–613. doi: 10.1128/jb.87.3.609-613.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome LA, Yu L, de Castro I, Campos SB, Seguro AC. Beneficial and harmful effects of L-arginine on renal ischaemia. Nephrol Dial Transplant. 1999;14:1139–1145. doi: 10.1093/ndt/14.5.1139. [DOI] [PubMed] [Google Scholar]
- Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev. 2006;18:25–38. doi: 10.1071/rd05120. [DOI] [PubMed] [Google Scholar]
- Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat Rev Drug Discov. 2002;1:939–950. doi: 10.1038/nrd960. [DOI] [PubMed] [Google Scholar]
- Vasilijevic A, Buzadzic B, Korac A, Petrovic V, Jankovic A, Korac B. Beneficial effects of L-arginine nitric oxide-producing pathway in rats treated with alloxan. J Physiol. 2007;584:921–933. doi: 10.1113/jphysiol.2007.140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilijevic A, Vojcic L, Dinulovic I, Buzadzic B, Korac A, Petrovic V, et al. Expression pattern of thermogenesis-related factors in interscapular brown adipose tissue of alloxan-treated rats: beneficial effect of L-arginine. Nitric Oxide. 2010;23:42–50. doi: 10.1016/j.niox.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Vera Y, Erkkilä K, Wang C, Nunez C, Kyttänen S, Lue Y, et al. Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol. 2006;20:1597–1609. doi: 10.1210/me.2005-0395. [DOI] [PubMed] [Google Scholar]
- Visioli F, Hagen TM. Antioxidants to enhance fertility: role of eNOS and potential benefits. Pharmacol Res. 2011;64:431–437. doi: 10.1016/j.phrs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Visioli F, Smith A, Zhang W, Keaney JF, Jr, Hagen T, Frei B. Lipoic acid and vitamin C potentiate nitric oxide synthesis in human aortic endothelial cells independently of cellular glutathione status. Redox Rep. 2002;7:223–227. doi: 10.1179/135100002125000604. [DOI] [PubMed] [Google Scholar]
- Vucetic M, Otasevic V, Korac A, Stancic A, Jankovic A, Markelic M, et al. Interscapular brown adipose tissue metabolic reprogramming during cold acclimation: interplay of HIF-1α and AMPKα. Biochim Biophys Acta. 2011;1810:1252–1261. doi: 10.1016/j.bbagen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Ou JX, Chen GW, Wu JP, Shi HJ, O WS, et al. Does prohibiting expression regulate sperm mitochondrial membrane potential, sperm motility, and male fertility? Antioxid Redox Signal. 2012;17:513–519. doi: 10.1089/ars.2012.4514. [DOI] [PubMed] [Google Scholar]
- Wang Y, Newton DC, Miller TL, Teichert AM, Phillips MJ, Davidoff MS, et al. An alternative promoter of the human neuronal nitric oxide synthase gene is expressed specifically in Leydig cells. Am J Pathol. 2002;160:369–380. doi: 10.1016/S0002-9440(10)64380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Doty E, Bonaventura J, Haney AF. Nitric oxide inhibition of human sperm motility. Fertil Steril. 1995;64:408–413. doi: 10.1016/s0015-0282(16)57743-7. [DOI] [PubMed] [Google Scholar]
- Wu TP, Huang BM, Tsai HC, Lui MC, Liu MY. Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryonic development. Arch Androl. 2004;50:173–179. doi: 10.1080/01485010490425494. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Ajonuma LC, Rowlands DK, Tsang LL, Ho LS, Lam SY, et al. The role of inducible nitric oxide synthase in gamete interaction and fertilization: a comparative study on knockout mice of three NOS isoforms. Cell Biol Int. 2005;29:785–791. doi: 10.1016/j.cellbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ, Risley GL, Brunson ME. Depletion of dietary arginine inhibits growth of metastatic tumor. Arch Surg. 1991;126:1376–1382. doi: 10.1001/archsurg.1991.01410350066010. [DOI] [PubMed] [Google Scholar]
- Yeoman RR, Jones WD, Rizk BM. Evidence for nitric oxide regulation of hamster sperm hyperactivation. J Androl. 1998;19:58–64. [PubMed] [Google Scholar]
- Zhang H, Zhou QM, Li XD, Xie Y, Duan X, Min FL, et al. Ginsenoside R(e) increases fertile and asthenozoospermic infertile human sperm motility by induction of nitric oxide synthase. Arch Pharm Res. 2006;29:145–151. doi: 10.1007/BF02974276. [DOI] [PubMed] [Google Scholar]
- Zini A, De Lamirande E, Gagnon C. Low levels of nitric oxide promote human sperm capacitation in vitro. J Androl. 1995;16:424–431. [PubMed] [Google Scholar]
- Zini A, O'Bryan MK, Magid MS, Schlegel PN. Immunohistochemical localization of endothelial nitric oxide synthase in human testes, epididymis, and vas deferens suggests a possible role for nitric oxide in spermatogenesis, sperm maturation, and programmed cell death. Biol Reprod. 1996;55:935–941. doi: 10.1095/biolreprod55.5.935. [DOI] [PubMed] [Google Scholar]
- Zini A, Abitbol J, Girardi SK, Schulsinger D, Goldstein M, Schlegel PN. Germ cell apoptosis and endothelial nitric oxide synthase (eNOS) expression following ischemia-reperfusion injury to testes. Arch Androl. 1998;41:57–65. doi: 10.3109/01485019808988547. [DOI] [PubMed] [Google Scholar]
- Zini A, Abitbol J, Schulsinger D, Goldstein M, Schlegel PN. Restoration of spermatogenesis after scrotal replacement of experimentally cryptorchid rat testes: assessment of germ cell apoptosis and eNOS expression. Urology. 1999;53:223–227. doi: 10.1016/s0090-4295(98)00415-4. [DOI] [PubMed] [Google Scholar]