Abstract
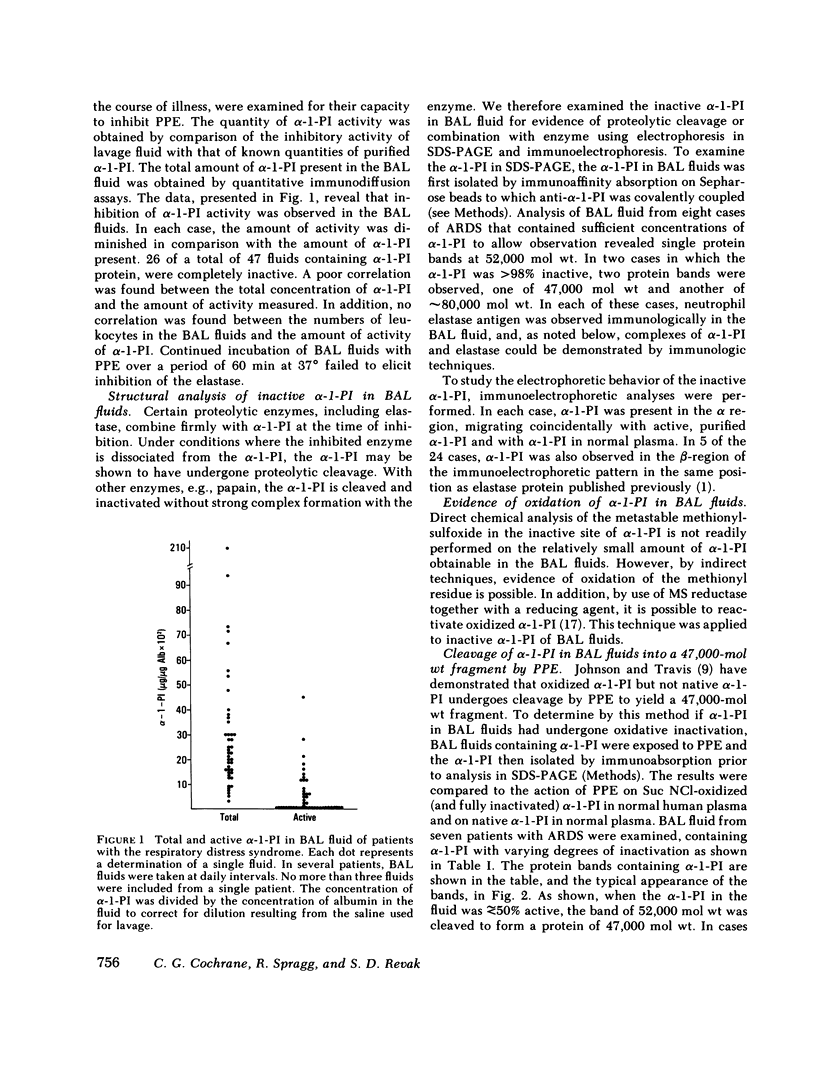
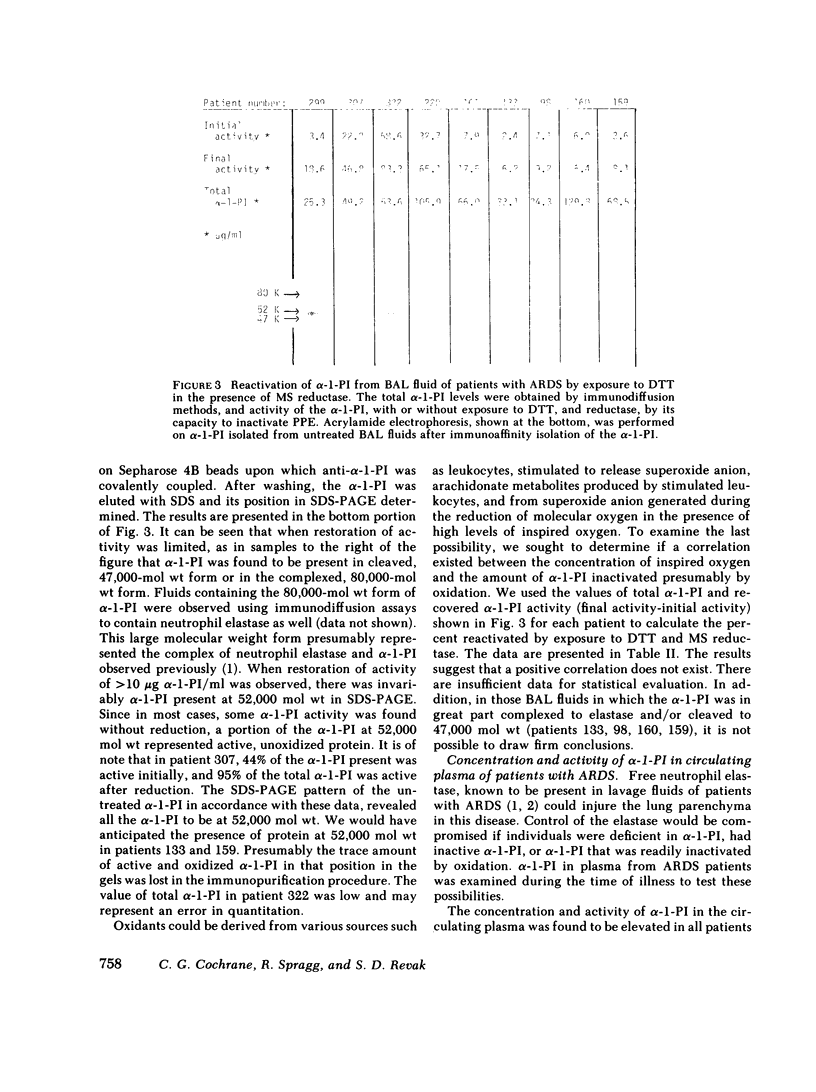
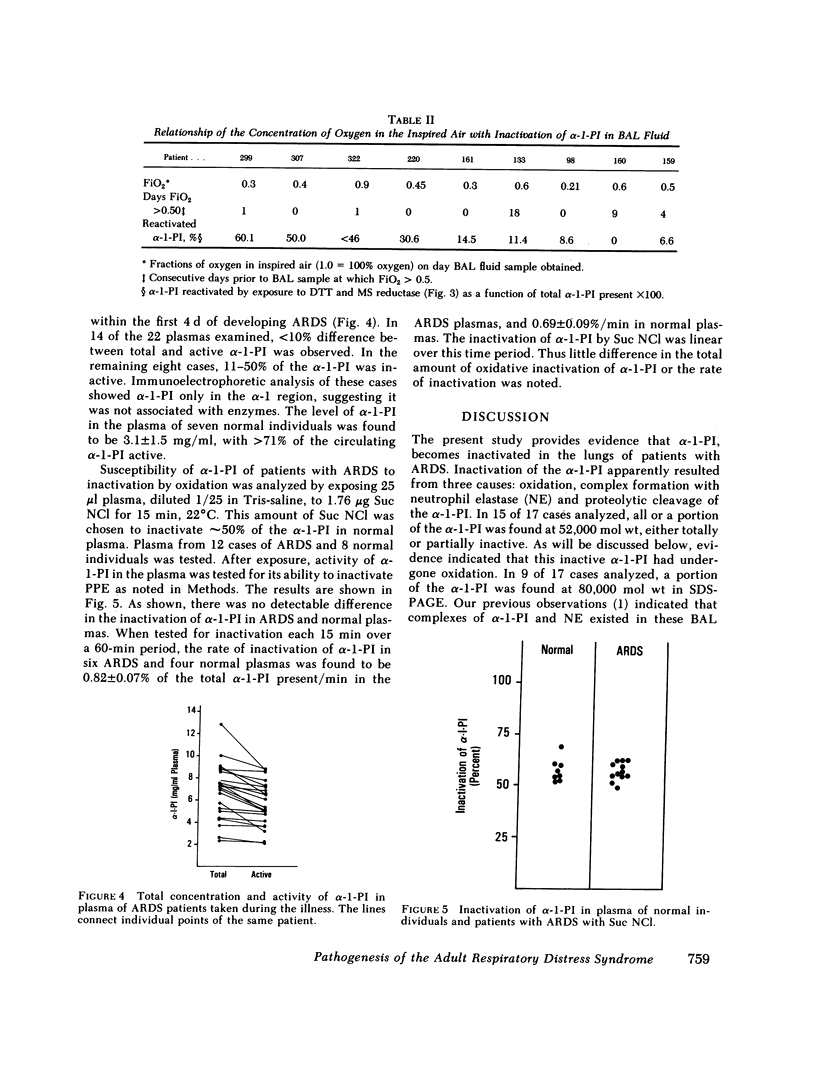
Evidence is presented indicating that oxidants are generated in lungs of patients with the adult respiratory distress syndrome (ARDS). The evidence was derived from observations that alpha-1-PI, recovered in bronchoalveolar lavage (BAL) fluid, had been inactivated by oxidation, presumably oxidation of the methionyl residue in the reaction site of the molecule. This was indicated by findings that activity of the alpha-1-PI could be restored by exposure to the reducing agent, dithiothreitol in the presence of methionyl sulfoxide peptide reductase. The amount of activity restored was proportional to the amount of inactive alpha-1-PI present at 52,000 D. Oxidation of the 52,000-D alpha-1-PI was also revealed by the finding that the inactive molecule was subject to proteolytic cleavage to 47,000 D when exposed to porcine pancreatic elastase, a characteristic of alpha-1-PI with oxidized methionyl residues in the reactive site. Inactivation of the alpha-1-PI in vivo also resulted from complexing to an active enzyme, shown previously to be neutrophil elastase, and from proteolytic cleavage in vivo, that produced a fragment of 47,000 mol wt. In contrast to that in BAL fluids, the alpha-1-PI in plasma of patients with respiratory distress syndrome was found to be greater than 90% active in 14 of 22 cases and 50-90% active in 8 cases. This suggested that for the most part, alpha-1-PI was inactivated after leaving the vessels and entering the lung. The circulating alpha-1-PI in patients with the respiratory distress syndrome was found to be equally susceptible to oxidative inactivation as alpha-1-PI from normal individuals. It seems improbable therefore that patients develop ARDS because of labile alpha-1-PI inhibitor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams W. R., Weinbaum G., Weissbach L., Weissbach H., Brot N. Enzymatic reduction of oxidized alpha-1-proteinase inhibitor restores biological activity. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7483–7486. doi: 10.1073/pnas.78.12.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Werb Z. Limited proteolysis by macrophage elastase inactivates human alpha 1-proteinase inhibitor. J Exp Med. 1980 Dec 1;152(6):1563–1570. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Fox R. B., Hoidal J. R., Brown D. M., Repine J. E. Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis. 1981 May;123(5):521–523. doi: 10.1164/arrd.1981.123.5.521. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Carp H., Lee D. K., Drew R. T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979 Dec 14;206(4424):1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. Inactivation of human alpha 1-proteinase inhibitor by thiol proteinases. Biochem J. 1977 Jun 1;163(3):639–641. doi: 10.1042/bj1630639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- McGuire W. W., Spragg R. G., Cohen A. B., Cochrane C. G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982 Mar;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 1976 Nov;58(5):1174–1184. doi: 10.1172/JCI108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman H. A., Fridovich I. Editorial: Oxygen toxicity. Introduction to a protective enzyme: superoxide dismutase. Circulation. 1973 Nov;48(5):921–923. doi: 10.1161/01.cir.48.5.921. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Bowman C. M., Fox R. B., Harada R. M., Tate R. M., Repine J. E. Angiotensin converting enzyme concentrations in the lung lavage of normal rabbits and rabbits treated with nitrogen mustard exposed to hyperoxia. Am Rev Respir Dis. 1981 Aug;124(2):202–203. doi: 10.1164/arrd.1981.124.2.202. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Vanbenthuysen K. M., Tate R. M., Shasby S. S., McMurtry I., Repine J. E. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982 Apr;125(4):443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]








