Abstract
Objective
Cellular inclusions of hyperphosphorylated tau are a hallmark of tauopathies, which are neurodegenerative disorders that include Alzheimer's disease (AD). Active and passive immunization against hyperphosphorylated tau has been shown to attenuate phenotypes in model mice. We developed new monoclonal antibodies to hyperphosphorylated tau and sought high therapeutic efficacy for future clinical use.
Methods
Using more than 20 antibodies, we investigated which sites on tau are phosphorylated early and highly in the tauopathy mouse models tau609 and tau784. These mice display tau hyperphosphorylation, synapse loss, memory impairment at 6 months, and tangle formation and neuronal loss at 15 months. We generated mouse monoclonal antibodies to selected epitopes and examined their effects on memory and tau pathology in aged tau609 and tau784 mice by the Morris water maze and by histological and biochemical analyses.
Results
Immunohistochemical screening revealed that pSer413 is expressed early and highly. Monoclonal antibodies to pSer413 and to pSer396 (control) were generated. These antibodies specifically recognized pathological tau in AD brains but not normal tau in control brains according to Western blots. Representative anti-pSer413 and anti-pSer396 antibodies were injected intraperitoneally into 10–11- or 14-month-old mice once a week at 0.1 or 1 mg/shot 5 times. The anti-pSer413 antibody significantly improved memory, whereas the anti-pSer396 antibodies showed less effect. The cognitive improvement paralleled a reduction in the levels of tau hyperphosphorylation, tau oligomer accumulation, synapse loss, tangle formation, and neuronal loss.
Interpretation
These results indicate that pSer413 is a promising target in the treatment of tauopathy.
Introduction
Neuronal and glial inclusions of hyperphosphorylated tau aggregates are hallmarks of tauopathies, which are neurodegenerative disorders that include Alzheimer's disease (AD), Pick's disease, corticobasal degeneration, progressive supranuclear palsy, argyrophilic grain disease, and frontotemportal dementia and parkinsonism linked to chromosome 17 (FTDP-17).1 FTDP-17 is an inherited tauopathy, and a number of exonic and intronic mutations in the tau gene have been identified. Many mouse models of tauopathies have been generated by introducing the human tau gene with or without mutations.2 We previously generated tau transgenic (Tg) mice expressing both three-repeat and four-repeat human tau by inserting tau intronic sequences into both sides of tau exon 10 in the transgene.3 These mice, originally referred to as lines 609 and 784 and hereafter termed tau609 and tau784, dominantly express four-repeat human tau in adult age by the presence of the FTDP-17-related tau intron 10 + 16C → T mutation. They exhibited abnormal tau phosphorylation, synapse loss, and memory impairment at 6 months, and neurofibrillary tangle (NFT) formation and neuronal loss at 24 months. More recently, we found that these mice start to display NFTs and neuronal loss at 15 months in layer II/III of the entorhinal cortex (EC-II/III) and cingulated cortex.
Active and passive immunization against hyperphosphorylated tau has been shown to attenuate phenotypes in model mice. For example, active immunization with tau partial peptides phosphorylated at Ser396/404,4–7 Ser202/Thr205, Thr212/Ser214, Thr231,8 or Ser4229 decreased the level of hyperphosphorylated tau and rescued motor/cognitive dysfunction. Immunization with human paired helical filaments (PHFs) composed of hyperphosphorylated tau aggregates also reduced NFTs.10 Meanwhile, some studies cautioned that active tau immunization may cause neuroinflammation in the brain.11,12 Thus, passive immunization would seem safer than active immunization, as the former only compensates humoral immunity, whereas the latter activates both humoral and cellular immunity making it difficult to manage adverse effects. Additionally, passive immunization with PHF-1 (anti-pSer396/404) or MC1 (anti-pathological conformation) antibody decreased the level of hyperphosphorylated tau and improved motor function.13–15 These passive immunization studies, however, lean to the prevention rather than therapy of tauopathy, as they used young mice before or just after the disease onset. To evaluate clinical efficacy, immunization should be performed in aged mice with overt neuropathology.
For future clinical use in the treatment of tauopathy, we decided to develop new monoclonal antibodies to hyperphosphorylated tau with higher therapeutic efficacy than those of existing anti-tau monoclonal antibodies. To determine the target epitopes, we initially studied which sites on tau are phosphorylated early and highly in our model mice, tau609 and tau784. Immunohistochemical screening with more than 20 commercially available antibodies revealed that Ser413 is such a site. We generated mouse monoclonal antibodies to pSer413 and to pSer396, our control, and compared their effects in aged tau609 and tau784 mice. Our results indicate that pSer413 is a promising target in the treatment of tauopathy.
Materials and Methods
Immunohistochemical screening for target epitopes
Antibodies used in the immunohistochemical screening for target epitopes are listed in Table S1. Brain sections were prepared from tau609, tau784, and non-Tg mice at 6 and 24 months.3 Immunohistochemical staining was performed as described previously3 but the pretreatment of sections under an acidic condition was not performed.
Peptide synthesis
Tau partial peptides used in immunization and antibody screening are listed in Table S2.
Generation of polyclonal antibody
Anti-pSer413 polyclonal antibody was generated by immunizing a rabbit with the pSer413 peptide, as detailed in Data S1.
Generation of monoclonal antibodies
Anti-pSer413 and anti-pSer396/404 monoclonal antibodies were generated by immunizing mice with the corresponding peptides, as detailed in Data S1.
SPR analysis
The binding affinity of monoclonal antibodies was evaluated by surface plasmon resonance (SPR), as detailed in Data S1.
Western blot analysis with AD samples
Brain samples were obtained by autopsy from patients with AD and from nondemented control subjects with informed consent. The samples were processed according to the methods of Berger et al.16 and subjected to Western blot to study the specificity of the monoclonal antibodies. Details are given in Data S1.
Passive immunization
Passive immunization was performed in male tau609 and tau784 mice. These two lines showed no apparent differences in the levels of tau expression or tauopathy phenotypes. Monoclonal antibodies were diluted in the buffers indicated in the figure legends. Anti-Pseudomonas aeruginosa lipopolysaccharide (LPS) mouse monoclonal antibodies 4C10F4 and 6F11B6 were generated at Teijin Pharma Limited, whereas anti-Shiga like-toxin II (Stx2) mouse monoclonal antibody 11F11 was obtained from ATCC. These antibodies were used as the control. Mice were divided into two groups (n = 8–10 each) so that the mean body weights were not significantly different between groups. One group was exposed to anti-pSer413 or anti-pSer396 antibody and the other to the control antibody. Anti-pSer413 (Ta1505) and anti-pSer396 (Ta4) antibodies were injected intraperitoneally into 14-month-old tau784 mice once a week at 1 mg/shot 5 times, whereas another anti-pSer396 antibody (Ta9) was injected into age-matched tau609 mice in the same protocol. In additional experiments, Ta1505 and Ta9 antibodies were injected into 10–11-month-old tau784 mice once a week at 0.1 mg/shot 5 times. The buffer used for antibody dilution was injected into age-matched non-Tg littermates (n = 8–10) as a normal control. Mice were subjected to behavioral tests in the week after the last injection. All animal experiments were approved by the ethics committee of Osaka City University (Osaka, Japan) and were performed in accordance with the Guide for Animal Experimentation, Osaka City University.
Behavioral tests
Spatial reference memory in antibody-treated mice was assessed using the Morris water maze as described previously17 except for intertrial intervals of 5 min. Locomotor activities of the mice were examined by an open-field test.17
Histological analysis
After the behavioral tests, antibody-treated mice were divided into two groups: one (n = 5–6) for histological analysis and the other (n = 3–4) for Western blot analysis. Brain sections were prepared and immunohistochemical and Gallyas silver staining were performed as described previously.3 PHF-1 antibody was a kind gift from Dr. Peter Davies, Albert Einstein College of Medicine, whereas AT8 (Thermo Scientific, Waltham, MA), anti-tau oligomer (T22; EMD Millipore, Temecula, CA),18 antisynaptophysin (SVP-38; Sigma-Aldrich, St. Louis, MO), and anti-NeuN (Chemicon, Temecula, CA) antibodies were purchased. Tau hyperphosphorylation and tau oligomer accumulation were evaluated by quantifying the staining intensities of phospho-tau-positive and T22-positive areas in each photograph of the hippocampal CA2-3 region. Analysis was performed using NIH ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/nih-image/) on individual sections (3 sections per animal). Synapse loss was assessed by quantifying synaptophysin fluorescence intensities in the apical dendritic-somata field (30 × 60 μm) of the hippocampal CA3 region using NIH ImageJ software (2 sections per animal). The level of NFT formation was determined by counting Gallyas silver positive cells in an area (220 × 160 μm) of the EC-II/III region, whereas neuronal loss was estimated by counting NeuN-positive cells in an area within 1000 μm along the EC-II (3 sections per animal).
Western blot analysis
For Western blot analysis, anti-pSer396 antibody-treated brain samples were obtained from 15-month-old mice (n = 3–4) after the behavioral tests, whereas anti-pSer413 antibody-treated brain samples were newly prepared by injecting anti-pSer413 and control 11F11 antibodies into 12-month-old tau784 mice (n = 4 each) once a week at 1 mg/shot 5 times. The separate preparations were carried out because we had used up anti-pSer413 antibody-treated 15-month-old brain samples in different experiments not shown here. Brain tissues were homogenized and fractionated into Tris buffered saline (TBS)-, sarkosyl-, and GuHCl-soluble fractions as described previously3 with minor modification. That is, sarkosyl-insoluble precipitates were dissolved in four volumes of tissue weight of 4 mol/L GuHCl. After centrifugation, the supernatants were dialyzed against TBS using a Slide-A-Lyzed G2 Dialysis Cassette with 20K cut-off membrane (Thermo Scientific). The TBS-soluble and dialyzed GuHCl-soluble fractions were subjected to Western blot with anti-tau and anti-actin (Sigma-Aldrich) antibodies, as described previously.3 To measure tau oligomers, brain homogenates were centrifuged at 13,000g and 4°C for 15 min. The supernatants were collected as total tau extracts. Both TBS-soluble fractions and total tau extracts were subjected to Western blot with human tau-specific Tau12 antibody (Abcam, Cambridge, UK).
Statistical analysis
All data are given as mean ± SEM. Comparisons of means between two groups were performed using Student's t-test, whereas comparisons of means among more than two groups were performed using analysis of variance (ANOVA) or two-factor repeated measures ANOVA (for the behavioral tests), followed by Fisher's protected least significant difference test. Differences with a P value of <0.05 were considered significant.
Results
Immunohistochemical screening for target epitopes
In our previous study, both tau609 and tau784 mice began to exhibit abnormal tau phosphorylation in hippocampal mossy fibers from 6 months and in neuronal cell bodies of the hippocampus and cerebral cortex from 18 months,3 suggesting the hippocampal mossy fibers are most susceptible to tau hyperphosphorylation in these mice. Therefore, we focused on this region during immunohistochemical screening for target epitopes. We first examined 24-month-old mouse brains. Of the more than 20 commercially available antibodies, 13 antibodies clearly stained the hippocampal mossy fibers (Table1). None of the 13 antibodies, except AT100, showed positive staining in 24-month-old non-Tg mouse brains. AT100 antibody reacted with the nuclei of hippocampal pyramidal neurons in both Tg and non-Tg mice. The remaining 12 antibodies were further tested with 6-month-old mouse brains, and eight antibodies showed positive staining in the hippocampal mossy fibers. These include PHF-1 (anti-pSer396/404), MC1 (anti-pathological conformation), anti-pSer46, anti-pSer199, anti-pSer400, anti-pSer412, anti-pSer413, and anti-pSer422 antibodies. According to the intensity of staining, we finally selected pSer413 as a target epitope.
Table 1.
Expression levels of tau phospho-epitopes in tau694 and tau784 mice
| Antibody | Working conc. (μg/mL) | 24 months | 6 months | ||||
|---|---|---|---|---|---|---|---|
| tau609 | tau784 | non-Tg | tau609 | tau784 | non-Tg | ||
| PHF-1 | 2000 × dil. | +++ | +++ | – | ++ | ++ | – |
| MC1 | 200 × dil. | +++ | +++ | – | ++ | ++ | – |
| AT270 | 0.1 | ++ | ++ | – | – | + | – |
| AT8 | 5 | ++ | ++ | – | – | + | – |
| AT100 | 5 | (++) | (++) | (–) | ND | ND | ND |
| AT180 | 5 | – | – | – | ND | ND | ND |
| anti-pSer46 | 0.4 | ++ | ++ | – | + | ++ | – |
| anti-pSer184 | 1 | – | – | – | ND | ND | ND |
| anti-pSer198 | 0.4 | – | – | – | ND | ND | ND |
| anti-pSer199 | 1000 × dil. | ++ | ++ | – | + | ++ | – |
| anti-pThr217 | 0.4 | + | + | – | – | + | – |
| anti-pThr231 | 10 | ++ | + | – | + | + | – |
| anti-pSer235 | 0.4 | – | – | – | ND | ND | ND |
| anti-pSer237 | 0.4 | – | – | – | ND | ND | ND |
| anti-pSer238 | 0.4 | – | – | – | ND | ND | ND |
| anti-pSer262 | 2 | – | – | – | ND | ND | ND |
| anti-pSer356 | 2 | – | – | – | ND | ND | ND |
| anti-pSer400 | 0.4 | ++ | ++ | – | + | ++ | – |
| anti-pSer409 | 50 × dil. | – | – | – | ND | ND | ND |
| anti-pSer412 | 0.4 | +++ | +++ | – | + | ++ | – |
| anti-pSer413 | 0.5 | +++ | ++ | – | ++ | ++ | – |
| anti-pSer422 | 50 × dil. | ++ | ++ | – | + | + | – |
Brain sections were stained with each antibody at the concentrations/dilutions indicated, and the staining intensity in the hippocampal mossy fibers was judged as +++ (strong), ++ (moderate), + (weak), or – (none). AT100 antibody reacted with the nuclei of neurons in addition to the mossy fibers. ND, not determined.
Generation and in vivo evaluation of anti-pSer413 polyclonal antibody
Preliminary to generating monoclonal antibodies, we tested the validity of pSer413 as a target epitope using a polyclonal antibody. Anti-pSer413 polyclonal antibody was injected intraperitoneally into 9–11-month-old tau609 mice once a week at 1 mg/shot 5 times. As the control, phosphate buffered saline (PBS) was injected into age-matched tau609 mice. The antibody significantly improved the memory of Tg mice to the same level as that of non-Tg littermates (Fig. S1). Thus, pSer413 was shown to be a good target for passive immunotherapy.
Generation of anti-pSer413 and anti-pSer396 monoclonal antibodies
We established at least seven clones of hybridomas producing anti-pSer413 antibody and 10 clones producing anti-pSer396/404 antibody. We tested the specificity of the seven anti-pSer413 antibodies by ELISA. All antibodies showed a strong reactivity to the pSer413 peptide and no reactivity to peptides phosphorylated at other sites (Table S3). We also examined the exact epitope of the 10 anti-pSer396/404 antibodies by ELISA. Six antibodies preferentially reacted with the pSer396 peptide, whereas the others reacted with the pSer404 peptide (Table S4). We chose anti-pSer396 antibodies as control, because two representative anti-pSer396 antibodies (Ta4 and Ta9) showed higher immunoreactivity than an anti-pSer404 antibody (Ta115) in immunohistochemistry using our mouse brain sections (data not shown). Then we compared the binding affinity of the seven anti-pSer413 and two representative anti-pSer396 antibodies by SPR. Of the anti-pSer413 antibodies tested, Ta1505 showed the highest binding affinity with the lowest KD value to the antigen peptide (Table S5). On the other hand, the two anti-pSer396 antibodies showed a higher binding affinity to their antigen peptide than did the anti-pSer413 antibodies. We therefore chose Ta1505 as a representative anti-pSer413 antibody for further evaluation.
Specificity of monoclonal antibodies to pathological tau in AD brains
We examined whether our monoclonal antibodies specifically recognized pathological tau in AD brains. Initially, we compared four samples from AD patients and two samples from nondemented control subjects (Table S6) for pathological tau species by Western blot with human tau-specific G2 antibody.3 The control brains showed only monomers of tau with molecular sizes of 45–65 kDa, whereas AD brains exhibited not only monomers but also dimers and/or trimers with molecular sizes of 120–220 kDa (Fig.1). Two AD samples and one control sample were selected for the analysis of the specificity of the monoclonal antibodies. Anti-human tau monoclonal antibody Tau12 recognized all tau species in both AD and control brains, whereas Ta1505, Ta4, and Ta9 antibodies recognized only tau species in AD brains but not in control brains.
Figure 1.
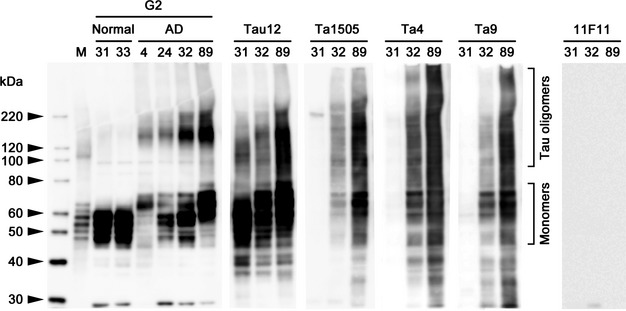
Specificity of monoclonal antibodies to pathological tau in Alzheimer's disease (AD) brains. Four samples from AD patients (no. 4, 24, 32, 89) and two samples from nondemented control subjects (no. 31, 33) were stained with G2 antibody, a rabbit polyclonal antibody specific to human tau.3 M, six isoforms of recombinant human tau. Two AD (no. 32, 89) samples and one control (no. 31) sample were selected for the analysis of the specificity of monoclonal antibodies. Tau12, anti-human tau antibody; Ta1505, anti-pSer413 antibody; Ta4 and Ta9, anti-pSer396 antibodies; 11F11, anti-Stx2 antibody.
In vivo evaluation of monoclonal antibodies
Effects on memory
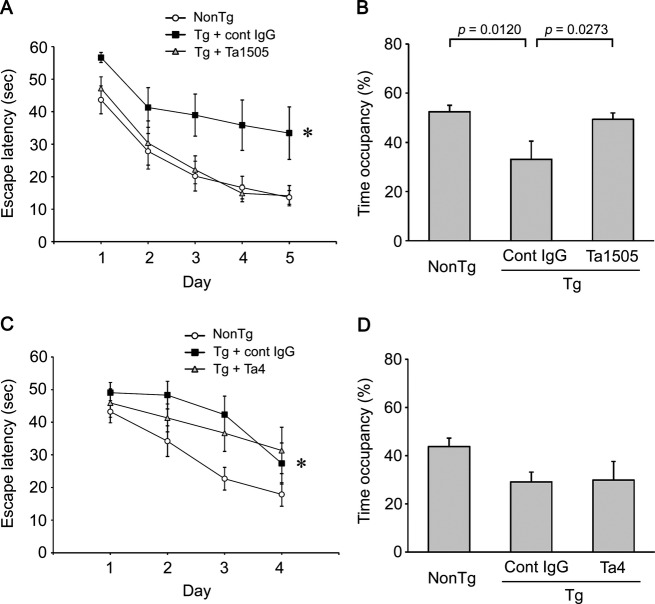
We compared the effects of monoclonal antibodies on memory in tau609 and tau784 mice. Ta1505 and control 4C10F4 antibodies were injected intraperitoneally into 14-month-old tau784 mice once a week at 1 mg/shot 5 times. Ta1505 antibody significantly improved the memory of Tg mice to the same level as non-Tg littermates, whereas 4C10F4 antibody did not (Fig.2A). Probe trials revealed that memory retention was significantly improved by Ta1505 injection to a level similar to that in non-Tg littermates (Fig.2B). No differences in locomotor activities were observed among the three groups (not shown). Next, Ta4 and control 4C10F4 antibodies were administered to 14-month-old tau784 mice once a week at 1 mg/shot 5 times. Ta4 antibody showed a tendency to improve the memory of Tg mice, but the difference between Ta4- and 4C10F4-treated groups was not significant (Fig.2C). In probe trials, Ta4 antibody showed no significant improvement in memory retention (Fig.2D). We repeated the experiments with another anti-pSer396 antibody, Ta9. Ta9 and control 6F11B6 antibodies were injected into 14-month-old tau609 mice once a week at 1 mg/shot 5 times. Ta9 antibody significantly improved the memory of Tg mice, whereas 6F11B6 antibody did not (Fig. S2A). While the effect of Ta9 antibody was weaker than that of Ta1505 antibody, memory retention was significantly improved by Ta9 injection to a level similar to that in non-Tg littermates (Fig. S2B).
Figure 2.
Effects of monoclonal antibodies on the memory of tauopathy mice. (A) Anti-pSer413 Ta1505 antibody (IgG2a) in 0.1 mol/L citrate buffer (pH5.0) was injected intraperitoneally into 14-month-old tau784 mice (n = 9) once a week at 1 mg/shot 5 times. As the control, antilipopolysaccharide (LPS) mouse monoclonal antibody 4C10F4 (IgG2b) in the same buffer was injected into age-matched tau784 mice (n = 9). The buffer used for the antibody dilution was injected into age-matched non-Tg littermates (n = 8) as the normal control. Spatial reference memory was assessed at 15 months of age by the Morris water maze. *P = 0.0110 versus non-Tg, P = 0.0152 versus Ta1505. No significant difference between non-Tg and Ta1505 was observed. (B) Probe trials with the platform removed were carried out on day 6. Each bar represents the mean time occupancy in the target quadrant for 30 sec. Again, no significant difference between non-Tg and Ta1505 was observed. (C) Anti-pSer396 Ta4 antibody (IgG2b) in PBS was injected intraperitoneally into 14-month-old tau784 mice (n = 9) once a week at 1 mg/shot 5 times. As the control, IgG subtype-matched 4C10F4 antibody in PBS was injected into age-matched tau784 mice (n = 8). PBS was injected into age-matched non-Tg littermates (n = 9) as the normal control. *P = 0.0254 versus non-Tg. (D) Probe trials were carried out on day 5. No significant differences among three groups were observed.
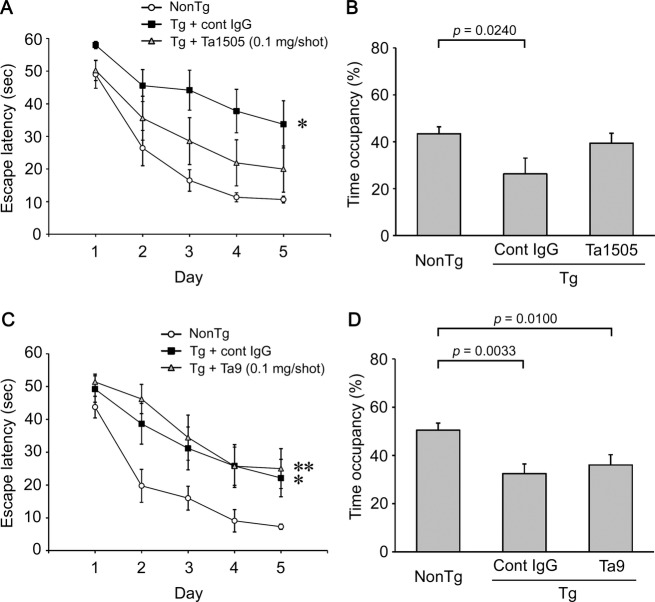
The effects of monoclonal antibodies were further studied at a lower dose. Ta1505 and control 11F11 antibodies were injected into 10–11-month-old tau784 mice once a week at 0.1 mg/shot 5 times. Even at such a low dose, Ta1505 antibody significantly improved the memory of Tg mice to a level between that of 11F11-injected Tg mice and PBS-injected non-Tg littermates (Fig.3A and B). Next, Ta9 and control 6F11B6 antibodies were administered to 10–11-month-old tau784 mice once a week at 0.1 mg/shot 5 times. In contrast to Ta1505 antibody, Ta9 antibody showed no significant effect on memory (Fig.3C and D).
Figure 3.
Effects of lower doses of monoclonal antibody on the memory of tauopathy mice. (A) Anti-pSer413 Ta1505 antibody (IgG2a) in PBS was injected intraperitoneally into 10–11-month-old tau784 mice (n = 10) once a week at 0.1 mg/shot 5 times. As the control, IgG subtype-matched anti-Stx2 mouse monoclonal antibody 11F11 (IgG2a) in PBS was injected into age-matched tau784 mice (n = 10). PBS was injected into age-matched non-Tg littermates (n = 9) as the normal control. Spatial reference memory was assessed at 11–12 months of age. *P = 0.0025 versus non-Tg, P = 0.0493 versus Ta1505. No significant difference between non-Tg and Ta1505 was observed. (B) Probe trials were carried out on day 6. Again, no significant difference between non-Tg and Ta1505 was observed. (C) Anti-pSer396 Ta9 antibody (IgG3) in PBS was injected intraperitoneally into 10–11-month-old tau784 mice (n = 10) once a week at 0.1 mg/shot 5 times. As the control, IgG subtype-matched antilipopolysaccharide (LPS) mouse monoclonal antibody 6F11B6 (IgG3) in PBS was injected into age-matched tau784 mice (n = 8). PBS was injected into age-matched non-Tg littermates (n = 8) as a normal control. *P = 0.0065 versus non-Tg; **P = 0.0044 versus non-Tg. (D) Probe trials were carried out on day 6.
Effects on tau hyperphosphorylation
Brain sections prepared from 15-month-old antibody-treated mice (at 1 mg/shot) were stained with Ta1505, PHF-1, and AT8 antibodies. Compared with control antibody-treated mice, Ta1505-treated mice showed a significant reduction in Ta1505-, PHF-1-, and AT8-positive staining in the hippocampal mossy fibers (Fig.4A and C). In contrast, Ta4- and Ta9-treated mice exhibited a significant reduction in PHF-1-positive staining but little in Ta1505- and AT8-staining (Fig.4B and C and Fig. S3A and B). We also examined brain sections of 11–12-month-old antibody-treated mice (at 0.1 mg/shot). Ta1505-treated mice showed an apparent reduction in Ta1505- and PHF-1-positive staining (Fig. S4A and C), whereas Ta9-treated mice exhibited no effect (Fig. S4B and C).
Figure 4.
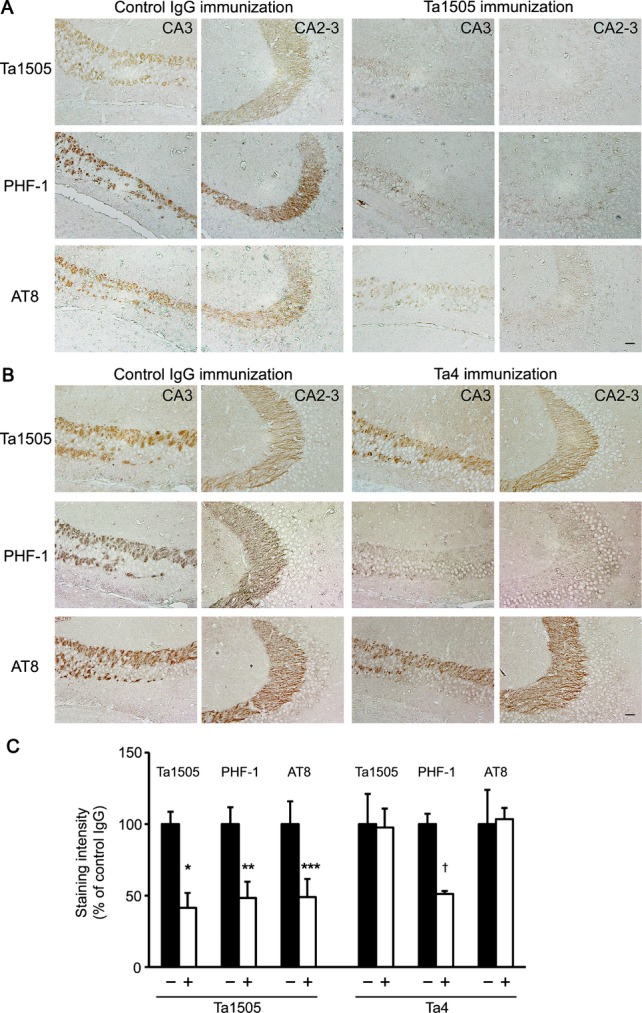
Effects of monoclonal antibodies on hyperphosphorylated tau in tauopathy mice based on immunohistochemistry. (A and B) Brain sections from 15-month-old antibody-treated tau784 mice were stained with Ta1505, PHF-1, and AT8 antibodies. Ta1505-treated mice showed an apparent reduction of hyperphosphorylated tau in hippocampal mossy fibers (A). In contrast, Ta4-treated mice exhibited reduced PHF-1-staining but not Ta1505- or AT8-staining (B). CA3 and CA2-3, hippocampal CA3 and CA2-3 regions. Scale bar, 30 μm. (C) Ta1505-, PHF-1-, and AT8-positive areas in each photograph of the hippocampal CA2-3 region were quantified using NIH ImageJ software. n = 5 for each group, except for control IgG in the Ta4 experiment where n = 3. *P = 0.0026, **P = 0.0133, ***P = 0.0364, and †P = 0.0002 versus control IgG.
TBS-soluble and GuHCl-soluble (sarkosyl-insoluble) brain fractions prepared from 13- and 15-month-old antibody-treated mice (at 1 mg/shot) were subjected to Western blot with G2, pool-2 (anti-human/mouse tau),3 Ta1505, PHF-1, and AT8 antibodies. Ta1505-treated mice showed significantly lower levels of total (i.e., pool-2-positive) and Ta1505-, PHF-1-, and AT8-positive tau than did control antibody-treated mice in the TBS-soluble and GuHCl-soluble fractions (Fig.5A and B). In contrast, Ta4-treated mice exhibited a significant reduction only of PHF-1-positive tau in the GuHCl-soluble fraction (Fig.5C and D). Finally, Ta9-treated mice showed significantly reduced levels of PHF-1-positive tau in the TBS-soluble and GuHCl-soluble fractions, and Ta1505- and AT8-positive tau in either fraction, but the effects were weaker than those in Ta1505-treated mice (Fig. S5A and B).
Figure 5.
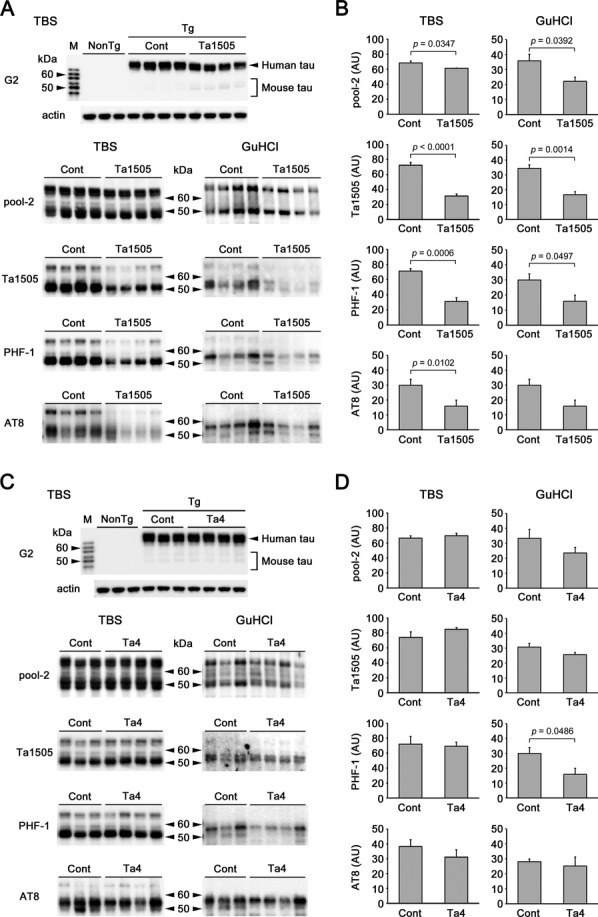
Effects of monoclonal antibodies on hyperphosphorylated tau in tauopathy mice based on Western blots. Ta4-treated brain samples were obtained from 15-month-old tau784 mice (n = 3–4) after behavioral tests, whereas Ta1505-treated brain samples were newly prepared by injecting Ta1505 and control 11F11 antibodies in PBS into 12-month-old tau784 mice (n = 4 each) once a week at 1 mg/shot 5 times. Brain tissues were homogenized and fractionated into TBS-, sarkosyl-, and GuHCl-soluble fractions. (A and C) TBS-soluble and GuHCl-soluble fractions were subjected to Western blot with G2 (human tau-specific), pool-2 (anti-human/mouse tau), Ta1505, PHF-1, and AT8 antibodies. M, six isoforms of recombinant human tau. Actin was the loading control. (B and D) Signals were quantified using a LAS-3000 luminescent image analyzer (Fujifilm) and are shown in arbitrary units (AU). Ta1505-treated mice showed significantly lower levels of total and Ta1505-, PHF-1-, and AT8-positive tau than did control antibody-treated mice in TBS-soluble and GuHCl-soluble fractions (B). In contrast, Ta4-treated mice exhibited a significant reduction only of PHF-1-positive tau in GuHCl-soluble fraction (D). n = 4 for each group, except for control IgG in the Ta4 experiment where n = 3.
Effects on tau oligomer accumulation
Recent evidence suggests that tau oligomers are the toxic species in tauopathy. For example, tau oligomers isolated from AD brains were shown to inhibit synaptic plasticity and disrupt memory in mice.19 Thus, we studied the involvement of tau oligomers in our mice by immunohistochemical staining with tau oligomer-specific T22 antibody. Both tau609 and tau784 mice displayed an apparent accumulation of tau oligomers in hippocampal neurons at 6 months (Fig. S6A), at which age these mice showed synaptic and cognitive dysfunction.3 The effects of antibodies on tau oligomer accumulation were examined with 15-month-old brain sections. Ta1505 significantly reduced the levels of tau oligomers, but Ta4 and Ta9 did not (Fig. S6B and C). This finding was confirmed by Western blot with Tau12 antibody. The levels of tau oligomers in both TBS-soluble brain fractions and total tau extracts were significantly decreased by Ta1505 injection (Fig. S6D and E).
Effects on synapse loss, NFT formation, and neuronal loss
We next examined synapse loss in 15-month-old antibody-treated mice. Ta1505-treated mice showed significant recovery in synaptophysin levels in the hippocampal CA3 region (Fig.6A and B), whereas Ta4-treated mice did not (Fig.6C and D). Ta9-treated mice showed only slight recovery of synapses (Fig. S7A and B).
Figure 6.
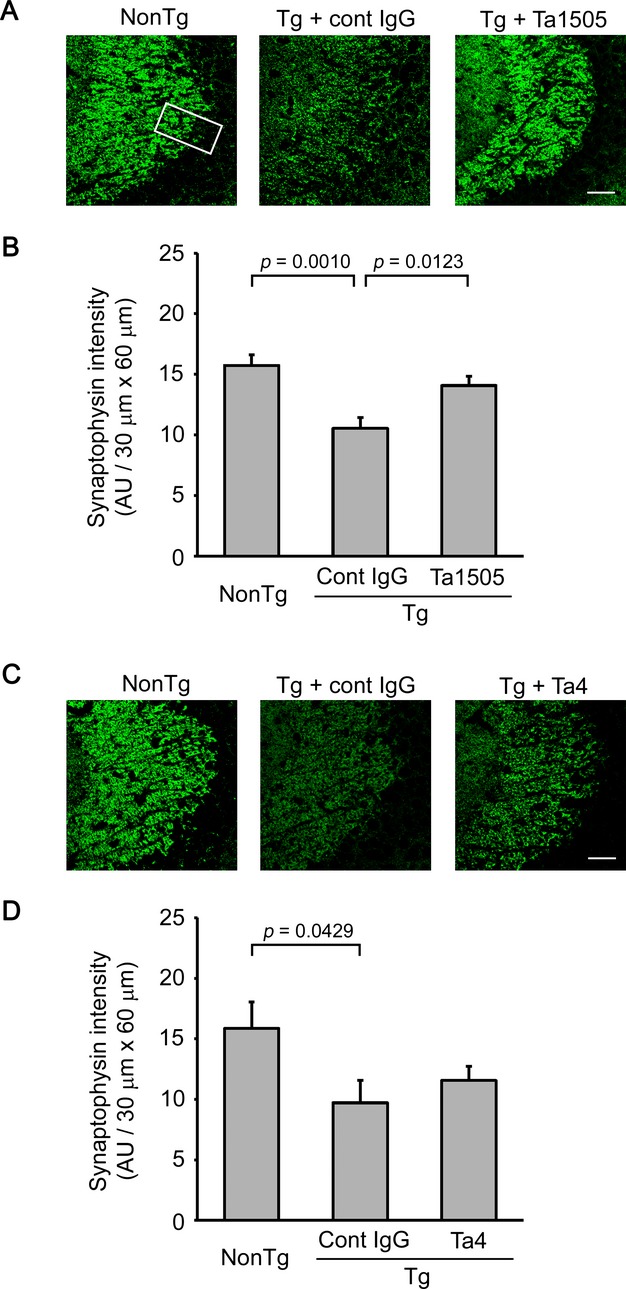
Effects of monoclonal antibodies on synapse loss in tauopathy mice. (A and C) Brain sections from 15-month-old antibody-treated tau784 mice were stained with antisynaptophysin antibody. Ta1505-treated mice showed apparent recovery of synaptophysin levels (A), whereas Ta4-treated mice did not (C). All images were taken from the hippocampal CA3 region. Scale bar, 30 μm. (B and D) Synaptophysin fluorescence intensity in the apical dendritic-somata field (30 × 60 μm, rectangle) of the hippocampal CA3 region was quantified and is shown in arbitrary units (AU). (B) n = 5 for each group. (D) n = 6 for non-Tg, n = 4 for cont IgG, and n = 5 for Ta4.
We also assessed NFTs and neuronal loss in 15-month-old antibody-treated mice, as our mice start to display NFTs and neuronal loss at this age. Ta1505-treated mice displayed significantly decreased levels of NFTs in the EC-II/III region (Fig.7A and B), whereas Ta4- and Ta9-treated mice showed little changes (Fig.7C and D and Fig. S8A and B). Furthermore, Ta1505 partially prevented neuronal loss in the EC-II (Fig.8A and B), whereas Ta4 and Ta9 did not (Fig.8C and D and Fig. S9A and B).
Figure 7.
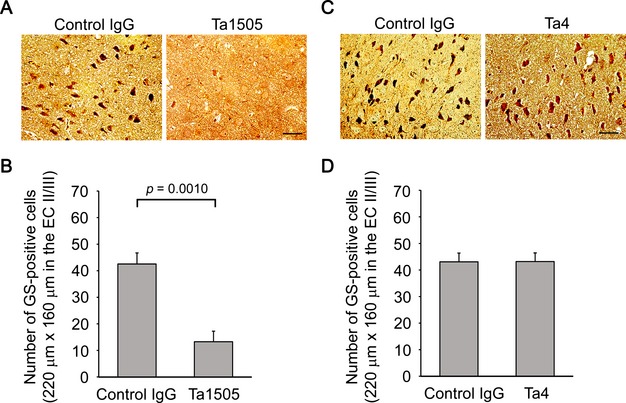
Effects of monoclonal antibodies on NFT formation in tauopathy mice. (A and C) Brain sections from 15-month-old antibody-treated tau784 mice were examined for NFTs by Gallyas silver staining. Ta1505-treated mice showed apparently reduced levels of NFTs (A), whereas Ta4-treated mice exhibited no changes (C). All images were taken from the EC-II/III region. Scale bar, 30 μm. (B and D) Gallyas silver positive cells in an area (220 × 160 μm) of the EC-II/III region were counted. (B) n = 5 for each group. (D) n = 4 for each group. NFT, neurofibrillary tangle; EC, entorhinal cortex.
Figure 8.
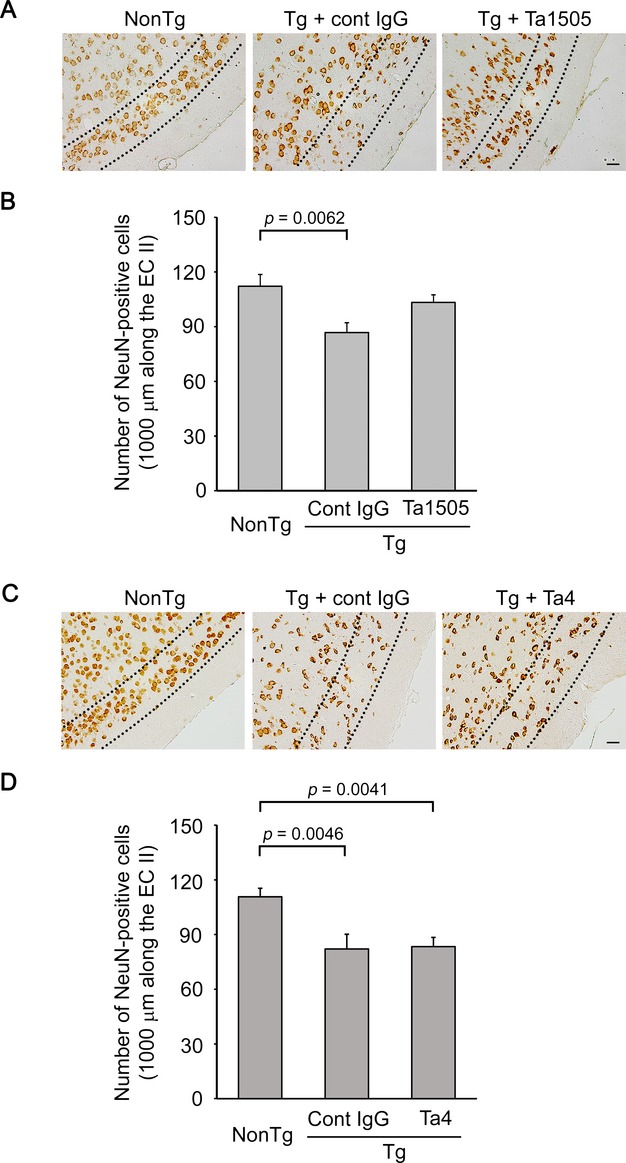
Effects of monoclonal antibodies on neuronal loss in tauopathy mice. (A and C) Brain sections from 15-month-old antibody-treated tau784 mice were stained with anti-NeuN antibody. Ta1505 injection partially prevented neuronal loss (A), whereas Ta4 injection did not (C). All images were taken from the entorhinal cortex (EC)-II/III region. Scale bar, 30 μm. (B and D) NeuN-positive cells in an area within 1000 μm along the EC-II (the region between the two broken lines) were counted. (B) n = 5 for each group. (D) n = 6 for non-Tg, n = 4 for cont IgG, and n = 5 for Ta4.
Discussion
In this study, we generated new monoclonal antibodies to hyperphosphorylated tau and showed their therapeutic effects in our model mice, tau609 and tau784. While many have previously reported passive immunization with anti-tau monoclonal antibodies, these studies examined mostly preventative not therapeutic measures. For example, Boutajangout et al.13 injected PHF-1 antibody to 2–3-month-old JNPL3 mice for 3 months and observed its beneficial effects at 5–7 months. As JNPL3 mice show phenotypes at 6.5 months in hemizygotes and 4.5 months in homozygotes,20 this treatment is preventive. Similarly, Chai et al.14 administered PHF-1 and MC1 antibodies to 2-month-old JNPL3 and P301S mice for 3–4 months, but since P301S mice show phenotypes at 5–6 months in homozygotes,21 this treatment is also preventive. D'Abramo et al.15 injected MC1 antibody into 3- or 7-month-old JNPL3 mice for 4 months. They described the immunization of 3-month-old mice as preventive and that of 7-month-old mice as therapeutic. Here, we treated 14-month-old tau609 and tau784 mice with Ta1505 antibody for 1 month. As our mice showed phenotypes at 6 months,3 the results indicate that Ta1505 antibody has curative effects against tauopathy.
The phosphorylation of tau at Ser413 is mediated by GSK3β,22 and PHF-tau extracted from AD brains has been shown to be phosphorylated there.23 Immunohistochemical analysis revealed that pSer413-tau was detected along with GSK3β within NFTs, neuropil threads, neuritic plaques, and neurons without NFTs in the hippocampal CA1 region in the early stages of AD.23 Our behavioral, histological, and biochemical analyses of antibody-treated mice indicated that pSer413 is a more promising target than pSer396. The differences in therapeutic efficacy between anti-pSer413 and anti-pSer396 antibodies cannot be explained by the difference in their binding affinities, as SPR analysis demonstrated that the affinities of Ta4 and Ta9 antibodies were higher than that of Ta1505 antibody. It is known that pathological tau in AD brains is phosphorylated at more than 40 sites24 and that phosphorylation at several sites occurs sequentially.25–27 Our observation that Ta1505 antibody suppressed not only Ta1505- but also PHF-1-staining in mouse brains whereas Ta4 and Ta9 antibodies only reduced PHF-1-staining suggests that tau phosphorylation occurs first at Ser413 and then at Ser396. This notion supports our conclusion that anti-pSer413 antibody has higher therapeutic efficacy than anti-pSer396 antibody. However, the pSer413 epitope might have been more prominent in our mice, which could have contributed to our observations. To determine whether our results apply to other tauopathies, further studies with different models may be necessary. Our results indicate that Ta9 antibody has higher therapeutic efficacy than Ta4 antibody despite them recognizing the same epitope. This property is probably because Ta9 antibody has a higher affinity to the antigen peptide than Ta4 antibody. Furthermore, the IgG subclass is different between the two antibodies: Ta4 antibody is IgG2b while Ta9 antibody is IgG3. These differences may have differently affected the antibody efficacy in vivo.
Recent studies suggest that soluble tau oligomers, rather than insoluble tau aggregates in NFTs, are the toxic species in tauopathy. In a conditional mouse model of tauopathy (rTg4510), suppression of tau expression resulted in the recovery of memory and halting of neuronal loss despite the continued accumulation of NFTs.28 In this mouse model, two forms of tau multimers (140 and 170 kDa) were identified as pathological tau species and had levels that correlated with memory loss.16 These oligomers were also detected in JNPL3 mice, patients with AD and FTDP-17,16 and our tauopathy mice (Fig. S6). Studies with tau oligomer-specific antibodies revealed that tau oligomers (primarily dimers and trimers) are markedly elevated in AD brains and are detected in neuropil threads and pretangles.18,29 Additionally, a biochemical study demonstrated that hyperphosphorylated tau oligomers accumulate at synapses in AD brains.30 Although tau is primarily a cytoplasmic protein, recent evidence indicates that tau is secreted into the extracellular space depending on neuronal activity.31 It may be that tau oligomers are released from affected neurons and that these oligomers impair synaptic function. In fact, tau oligomers isolated from AD brains were shown to inhibit synaptic plasticity in hippocampal brain slices, and when injected into the hippocampus of wild-type mice, brain-derived tau oligomers, but not PHFs, disrupted memory and induced the aggregation and propagation of endogenous mouse tau.19 These findings suggest that extracellular tau oligomers contribute to the intercellular propagation of tau pathology, which is consistent with a hypothesis for the pathogenesis.32–34
If so, the depletion of extracellular tau oligomers could prevent the progression of tauopathy. Yanamandra et al.35 developed new anti-tau monoclonal antibodies that block the seeding activity of tau aggregates in vitro and tested them in P301S mice, a different mouse line36 from the one described above. Intracerebroventricular infusion of the antibodies from 6 months of age for 3 months reduced hyperphosphorylated tau and improved memory. Castillo-Carranza et al.37 generated a new monoclonal antibody to tau oligomers and tested it in JNPL3 mice. A single intravenous or intracerebroventricular injection of the antibody into 8-month-old mice reversed both locomotor and memory deficits 4–6 days after injection without affecting NFTs or tau monomers. These two studies demonstrate that passive immunization targeting extracellular tau aggregates/oligomers has curative effects against tauopathy even after the disease onset. Because our Ta1505 antibody strongly reacted with tau oligomers in AD brains (Fig.1B) and decreased the levels of tau oligomers in our tauopathy mice (Fig. S6), it is likely that it exhibited therapeutic effects by sequestering extracellular tau oligomers. Peripherally administered antibodies have been shown to enter the brain and be detected within neurons.4 The entry likely occurs via endocytosis, which allows the internalized antibodies to localize within the endosomes/lysosomes isolated from tau in the cytoplasm. Nevertheless, intracellular pathological tau, including tau oligomers, was cleared by immunization in many studies including ours. The clearance of extracellular tau by passive immunization may shift the equilibrium between the extracellular and intracellular tau pools, leading to the removal of intracellular tau.37 Another plausible mechanism to the clearance of pathological tau could be lysosomes absorbing tau aggregates in the cytoplasm (via, e.g., autophagy), where internalized antibodies may bind to and unravel tau aggregates for degradation by lysosomal enzymes.4
Our goal is to develop new monoclonal antibodies with higher therapeutic efficacy than existing antibodies for future clinical use in the treatment of tauopathy. To this end, a selected mouse monoclonal antibody should be humanized to avoid immunological rejection. The properties of our Ta1505 antibody suggest that it could be used as a prototype. To evaluate the antibodies, we used tau609 and tau784 mice that express only human tau. However, if we consider the antibody for the treatment of AD, one of the most studied tauopathies, animal models showing both amyloid and tau pathology38 would be required for more precise evaluation of the clinical efficacy. Whether passive immunization with an anti-tau antibody is effective at improving memory and tau pathology even in the presence of amyloid pathology remains to be studied.
Acknowledgments
This study was supported by funding from Teijin Pharma Limited. We thank Naoko Namiki, Shinobu Tani, Naomi Sakama, Reina Fujita and Maiko Mori for technical assistance, Naruhiko Sahara for discussion, and Peter Karagiannis for reading the manuscript.
Author Contributions
Y. K., Y. M., T. Ta., H. M., and T. To. conceived and/or designed the study, T. U., H. E. and T. To. performed the experiments, T. U., H. E., Y. K., Y. M., and T. To. analyzed the data, and T. To. wrote the manuscript.
Conflict of Interest
This study was supported by funding from Teijin Pharma Limited, developed the anti-pSer413 and anti-pSer396 antibodies tested in this study. Dr. Eguchi, Dr. Kunori and Dr. Matsumoto, has a patent WO 2013/180238 A1 pending. Dr. Mori and Dr. Tomiyama reports grants from Teijin Pharma Limited, during the conduct of the study. In addition, Dr. Mori and Dr. Tomiyama has a patent WO2013180238 A1 pending. Dr. Umeda reports grants from Teijin Pharma Limited, during the conduct of the study.
Supporting Information
Data S1. Materials and methods.
Table S1. Antibodies used in immunohistochemical screening for target epitopes.
Table S2. Tau partial peptides used in immunization and antibody screening. The peptides were synthesized and purified by HPLC at Medical & Biological Laboratories (MBL, Nagoya, Japan), GL Biochem (Shanghai, China) and Bio-Synthesis Inc. (Lewisville, TX). Underlines represent additional amino acids coupled onto the tau peptides.
Table S3. The specificity of anti-pSer413 monoclonal antibodies. The reactivity of the antibodies to tau partial peptides phosphorylated at different sites was determined by ELISA with plates coated with each peptide.
Table S4. The specificity of anti-pSer396/404 monoclonal antibodies. The reactivity of the antibodies to tau partial peptides phosphorylated at Ser396 and/or Ser404 was determined by ELISA with plates coated with each peptide.
Table S5. The binding affinity of anti-pSer413 and anti-pSer396 monoclonal antibodies. Binding affinity of antibodies to the pSer413(Im) or pSer396/pSer404 peptide was determined by SPR using Biacore 3000. No bindings of the antibodies to the NonP-Ser413(L) or NonP-Ser396/pSer404 peptide were detected. The negative control mouse monoclonal antibody MAB003 showed no significant interaction with the pSer413(Im), NonP-Ser413(L), pSer396/pSer404, or NonP-Ser396/pSer404 peptide.
Table S6. Human brain samples.
Figure S1. Effects of anti-pSer413 polyclonal antibody on the memory of tauopathy mice. The antibody was diluted in PBS and injected intraperitoneally into 9–11-month-old tau609 mice (n = 10) once a week at 1 mg/shot 5 times. As the control, PBS was injected into age-matched tau609 mice (n = 9) and non-Tg littermates (n = 8). Three days after the last injection, we started behavioral tests to examine spatial reference memory using the Morris water maze. *P = 0.0071 versus non-Tg, P = 0.0029 versus pSer413. No significant difference between non-Tg and pSer413 was observed. (B) Probe trials with the platform removed were carried out on day 5.
Figure S2. Effects of anti-pSer396 Ta9 antibody on the memory of tauopathy mice. (A) Ta9 antibody (IgG3) in 20 mmol/L citrate buffer (pH6.0) was injected intraperitoneally into 14-month-old tau609 (n = 10) once a week at 1 mg/shot 5 times. As the control, IgG subtype-matched 6F11B6 antibody (IgG3) in the same buffer was injected into age-matched tau609 mice (n = 9). The buffer used for the antibody dilution was injected into age-matched non-Tg littermates (n = 10) as the normal control. *P = 0.0003 versus non-Tg, P = 0.0402 versus Ta9, **P = 0.0460 versus non-Tg. (B) Probe trials were carried out on day 6.
Figure S3. Effects of anti-pSer396 Ta9 antibody on hyperphosphorylated tau in tauopathy mice based on immunohistochemistry. (A) Brain sections from 15-month-old antibody-treated tau609 mice were stained with Ta1505, PHF-1, and AT8 antibodies. Ta9-treated mice showed reduced PHF-1-staining but not Ta1505- or AT8-staining in hippocampal mossy fibers. Scale bar, 30 μm. (B) Ta1505-, PHF-1-, and AT8-positive areas in each photograph of the hippocampal CA2-3 region were quantified using NIH ImageJ software. n = 5 for each group. *P = 0.0351 versus control IgG.
Figure S4. Effects of lower doses of monoclonal antibodies on hyperphosphorylated tau in tauopathy mice based on immunohistochemistry. (A and B) Brain sections from 11–12-month-old antibody-treated (at 0.1 mg/shot) tau784 mice were stained with Ta1505, PHF-1, and AT8 antibodies. Ta1505-treated mice showed an apparent reduction of hyperphosphorylated tau in hippocampal mossy fibers (A). In contrast, Ta9-treated mice exhibited no effects (B). Scale bar, 30 μm. (C) Ta1505-, PHF-1-, and AT8-positive areas in each photograph of the hippocampal CA2-3 region were quantified using NIH ImageJ software. In Ta1505 experiments, n = 5 for control IgG, n = 6 for Ta1505 in Ta1505- and PHF-1-staining, and n = 4 for each group in AT8-staining. *P = 0.0467, **P = 0.0430 versus control IgG. In Ta9 experiments, n = 4 for control IgG and n = 5 for Ta9 in all staining.
Figure S5. Effects of anti-pSer396 Ta9 antibody on hyperphosphorylated tau in tauopathy mice based on Western blots. Ta9-treated brain samples were obtained from 15-month-old tau609 mice (n = 4) after behavioral tests. (A) TBS-soluble and GuHCl-soluble fractions were subjected to Western blot with G2 (human tau-specific), pool-2 (anti-human/mouse tau), Ta1505, PHF-1, and AT8 antibodies. M, 6 isoforms of recombinant human tau. Actin was the loading control. (B) Signals were quantified using a LAS-3000 luminescent image analyzer and are shown in arbitrary units (AU). Ta9-treated mice showed significantly reduced levels of PHF-1-positive tau in TBS-soluble and GuHCl-soluble fractions. n = 4 for both control IgG and Ta9.
Figure S6. Effects of monoclonal antibodies on tau oligomer accumulation in tauopathy mice. (A) Brain sections from 4-, 6-, and 8-month-old non-Tg, tau609, and tau784 mice were stained with tau oligomer-specific T22 antibody. All images were taken from the hippocampal CA2-3 region. Scale bar, 30 μm. (B) Brain sections from 15-month-old antibody-treated tau784 and tau609 mice were stained with T22 antibody. Ta1505- and, to a lesser extent, Ta9-treated mice showed reduced levels of tau oligomers, but Ta4-treated mice did not. (C) T22-positive areas in each photograph were quantified using NIH ImageJ software. n = 5 for each group, except for control IgG in the Ta4 experiment where n = 3. (D) TBS-soluble brain fractions and total tau extracts were subjected to Western blot with Tau12 antibody. M, six isoforms of recombinant human tau. (E) Signals of tau oligomers (arrows in D) were quantified using a LAS-3000 luminescent image analyzer and are shown in arbitrary units (AU). Ta1505 significantly decreased the levels of tau oligomers in both TBS-soluble fractions and total tau extracts, but Ta4 and Ta9 did not. n = 4 for each group, except for control IgG in the Ta4 experiment where n = 3.
Figure S7. Effects of anti-pSer396 Ta9 antibody on synapse loss in tauopathy mice. (A) Brain sections from 15-month-old antibody-treated tau609 mice were stained with antisynaptophysin antibody. All images were taken from the hippocampal CA3 region. Scale bar, 30 μm. (B) Synaptophysin fluorescence intensity in the apical dendritic-somata field (30 × 60 μm, rectangle) of the hippocampal CA3 region was quantified and is shown in arbitrary units (AU). Ta9-treated mice showed slight recovery of synaptophysin levels. n = 4 for non-Tg, n = 5 for control IgG and Ta9.
Figure S8. Effects of anti-pSer396 Ta9 antibody on NFT formation in tauopathy mice. (A) Brain sections from 15-month-old antibody-treated tau609 mice were examined for NFTs by Gallyas silver staining. All images were taken from the EC-II/III region. Scale bar, 30 μm. (B) Gallyas silver positive cells in an area (220 × 160 μm) of the EC-II/III region were counted. Ta9 exhibited little effect on NFT formation. n = 5 for each group.
Figure S9. Effects of anti-pSer396 Ta9 antibody on neuronal loss in tauopathy mice. (A) Brain sections from 15-month-old antibody-treated tau609 mice were stained with anti-NeuN antibody. All images were taken from the EC-II/III region. Scale bar, 30 μm. (B) NeuN-positive cells in an area within 1000 μm along the EC-II (the region between the two broken lines) were counted. Ta9 exhibited no effects on neuronal loss. n = 5 for each group.
References
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Frank S, Clavaguera F, Tolnay M. Tauopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:39–53. doi: 10.1007/s00401-007-0291-9. [DOI] [PubMed] [Google Scholar]
- Umeda T, Yamashita T, Kimura T, et al. Neurodegenerative disorder FTDP-17-related tau intron 10 + 16C → T mutation increases tau exon 10 splicing and causes tauopathy in transgenic mice. Am J Pathol. 2013;183:211–225. doi: 10.1016/j.ajpath.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi M, Ittner A, Ke YD, et al. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One. 2011;6:e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome-based vaccine against protein tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8:e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, et al. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Troquier L, Caillierez R, Burnouf S, et al. Targeting phospho-Ser422 by active tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9:397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Kabova A, Stygelbout V, et al. Vaccination with sarkosyl insoluble PHF-tau decrease neurofibrillary tangles formation in aged tau transgenic mouse model: a pilot study. J Alzheimers Dis. 2014;40:S135–145. doi: 10.3233/JAD-132237. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Grigoriadis N, Karussis D, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006;63:1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- Rozenstein-Tsalkovich L, Grigoriadis N, Lourbopoulos A, et al. Repeated immunization of mice with phos-phorylated-tau peptides causes neuroinflammation. Exp Neurol. 2013;248:451–456. doi: 10.1016/j.expneurol.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray TK, et al. Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Abramo C, Acker CM, Jimenez HT, Davies P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One. 2013;8:e62402. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, et al. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J. 2012;26:1946–1959. doi: 10.1096/fj.11-199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Omori A, Takamatsu M, et al. Phosphorylation sites on tau by tau protein kinase I, a bovine derived kinase generating an epitope of paired helical filaments. Neurosci Lett. 1992;148:202–206. doi: 10.1016/0304-3940(92)90839-y. [DOI] [PubMed] [Google Scholar]
- Shiurba RA, Ishiguro K, Takahashi M, et al. Immunocytochemistry of tau phosphoserine 413 and tau protein kinase I in Alzheimer pathology. Brain Res. 1996;737:119–132. doi: 10.1016/0006-8993(96)00717-2. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Li T, Paudel HK. Glycogen synthase kinase 3beta phosphorylates Alzheimer's disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochemistry. 2006;45:3125–3133. doi: 10.1021/bi051634r. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Plouffe V, Sénéchal P, Leclerc N. The pattern of human tau phosphorylation is the result of priming and feedback events in primary hippocampal neurons. Neuroscience. 2010;168:323–334. doi: 10.1016/j.neuroscience.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KR, Remmers C, Fu Y, et al. Characterization of prefibrillar tau oligomers in vitro and in Alzheimer disease. J Biol Chem. 2011;286:23063–23076. doi: 10.1074/jbc.M111.237974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HC, Serrano-Pozo A, Hashimoto T, et al. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Sengupta U, Guerrero-Muñoz MJ, et al. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014;34:4260–4272. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda T, Maekawa S, Kimura T, et al. Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice. Acta Neuropathol. 2014;127:685–698. doi: 10.1007/s00401-014-1259-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and methods.
Table S1. Antibodies used in immunohistochemical screening for target epitopes.
Table S2. Tau partial peptides used in immunization and antibody screening. The peptides were synthesized and purified by HPLC at Medical & Biological Laboratories (MBL, Nagoya, Japan), GL Biochem (Shanghai, China) and Bio-Synthesis Inc. (Lewisville, TX). Underlines represent additional amino acids coupled onto the tau peptides.
Table S3. The specificity of anti-pSer413 monoclonal antibodies. The reactivity of the antibodies to tau partial peptides phosphorylated at different sites was determined by ELISA with plates coated with each peptide.
Table S4. The specificity of anti-pSer396/404 monoclonal antibodies. The reactivity of the antibodies to tau partial peptides phosphorylated at Ser396 and/or Ser404 was determined by ELISA with plates coated with each peptide.
Table S5. The binding affinity of anti-pSer413 and anti-pSer396 monoclonal antibodies. Binding affinity of antibodies to the pSer413(Im) or pSer396/pSer404 peptide was determined by SPR using Biacore 3000. No bindings of the antibodies to the NonP-Ser413(L) or NonP-Ser396/pSer404 peptide were detected. The negative control mouse monoclonal antibody MAB003 showed no significant interaction with the pSer413(Im), NonP-Ser413(L), pSer396/pSer404, or NonP-Ser396/pSer404 peptide.
Table S6. Human brain samples.
Figure S1. Effects of anti-pSer413 polyclonal antibody on the memory of tauopathy mice. The antibody was diluted in PBS and injected intraperitoneally into 9–11-month-old tau609 mice (n = 10) once a week at 1 mg/shot 5 times. As the control, PBS was injected into age-matched tau609 mice (n = 9) and non-Tg littermates (n = 8). Three days after the last injection, we started behavioral tests to examine spatial reference memory using the Morris water maze. *P = 0.0071 versus non-Tg, P = 0.0029 versus pSer413. No significant difference between non-Tg and pSer413 was observed. (B) Probe trials with the platform removed were carried out on day 5.
Figure S2. Effects of anti-pSer396 Ta9 antibody on the memory of tauopathy mice. (A) Ta9 antibody (IgG3) in 20 mmol/L citrate buffer (pH6.0) was injected intraperitoneally into 14-month-old tau609 (n = 10) once a week at 1 mg/shot 5 times. As the control, IgG subtype-matched 6F11B6 antibody (IgG3) in the same buffer was injected into age-matched tau609 mice (n = 9). The buffer used for the antibody dilution was injected into age-matched non-Tg littermates (n = 10) as the normal control. *P = 0.0003 versus non-Tg, P = 0.0402 versus Ta9, **P = 0.0460 versus non-Tg. (B) Probe trials were carried out on day 6.
Figure S3. Effects of anti-pSer396 Ta9 antibody on hyperphosphorylated tau in tauopathy mice based on immunohistochemistry. (A) Brain sections from 15-month-old antibody-treated tau609 mice were stained with Ta1505, PHF-1, and AT8 antibodies. Ta9-treated mice showed reduced PHF-1-staining but not Ta1505- or AT8-staining in hippocampal mossy fibers. Scale bar, 30 μm. (B) Ta1505-, PHF-1-, and AT8-positive areas in each photograph of the hippocampal CA2-3 region were quantified using NIH ImageJ software. n = 5 for each group. *P = 0.0351 versus control IgG.
Figure S4. Effects of lower doses of monoclonal antibodies on hyperphosphorylated tau in tauopathy mice based on immunohistochemistry. (A and B) Brain sections from 11–12-month-old antibody-treated (at 0.1 mg/shot) tau784 mice were stained with Ta1505, PHF-1, and AT8 antibodies. Ta1505-treated mice showed an apparent reduction of hyperphosphorylated tau in hippocampal mossy fibers (A). In contrast, Ta9-treated mice exhibited no effects (B). Scale bar, 30 μm. (C) Ta1505-, PHF-1-, and AT8-positive areas in each photograph of the hippocampal CA2-3 region were quantified using NIH ImageJ software. In Ta1505 experiments, n = 5 for control IgG, n = 6 for Ta1505 in Ta1505- and PHF-1-staining, and n = 4 for each group in AT8-staining. *P = 0.0467, **P = 0.0430 versus control IgG. In Ta9 experiments, n = 4 for control IgG and n = 5 for Ta9 in all staining.
Figure S5. Effects of anti-pSer396 Ta9 antibody on hyperphosphorylated tau in tauopathy mice based on Western blots. Ta9-treated brain samples were obtained from 15-month-old tau609 mice (n = 4) after behavioral tests. (A) TBS-soluble and GuHCl-soluble fractions were subjected to Western blot with G2 (human tau-specific), pool-2 (anti-human/mouse tau), Ta1505, PHF-1, and AT8 antibodies. M, 6 isoforms of recombinant human tau. Actin was the loading control. (B) Signals were quantified using a LAS-3000 luminescent image analyzer and are shown in arbitrary units (AU). Ta9-treated mice showed significantly reduced levels of PHF-1-positive tau in TBS-soluble and GuHCl-soluble fractions. n = 4 for both control IgG and Ta9.
Figure S6. Effects of monoclonal antibodies on tau oligomer accumulation in tauopathy mice. (A) Brain sections from 4-, 6-, and 8-month-old non-Tg, tau609, and tau784 mice were stained with tau oligomer-specific T22 antibody. All images were taken from the hippocampal CA2-3 region. Scale bar, 30 μm. (B) Brain sections from 15-month-old antibody-treated tau784 and tau609 mice were stained with T22 antibody. Ta1505- and, to a lesser extent, Ta9-treated mice showed reduced levels of tau oligomers, but Ta4-treated mice did not. (C) T22-positive areas in each photograph were quantified using NIH ImageJ software. n = 5 for each group, except for control IgG in the Ta4 experiment where n = 3. (D) TBS-soluble brain fractions and total tau extracts were subjected to Western blot with Tau12 antibody. M, six isoforms of recombinant human tau. (E) Signals of tau oligomers (arrows in D) were quantified using a LAS-3000 luminescent image analyzer and are shown in arbitrary units (AU). Ta1505 significantly decreased the levels of tau oligomers in both TBS-soluble fractions and total tau extracts, but Ta4 and Ta9 did not. n = 4 for each group, except for control IgG in the Ta4 experiment where n = 3.
Figure S7. Effects of anti-pSer396 Ta9 antibody on synapse loss in tauopathy mice. (A) Brain sections from 15-month-old antibody-treated tau609 mice were stained with antisynaptophysin antibody. All images were taken from the hippocampal CA3 region. Scale bar, 30 μm. (B) Synaptophysin fluorescence intensity in the apical dendritic-somata field (30 × 60 μm, rectangle) of the hippocampal CA3 region was quantified and is shown in arbitrary units (AU). Ta9-treated mice showed slight recovery of synaptophysin levels. n = 4 for non-Tg, n = 5 for control IgG and Ta9.
Figure S8. Effects of anti-pSer396 Ta9 antibody on NFT formation in tauopathy mice. (A) Brain sections from 15-month-old antibody-treated tau609 mice were examined for NFTs by Gallyas silver staining. All images were taken from the EC-II/III region. Scale bar, 30 μm. (B) Gallyas silver positive cells in an area (220 × 160 μm) of the EC-II/III region were counted. Ta9 exhibited little effect on NFT formation. n = 5 for each group.
Figure S9. Effects of anti-pSer396 Ta9 antibody on neuronal loss in tauopathy mice. (A) Brain sections from 15-month-old antibody-treated tau609 mice were stained with anti-NeuN antibody. All images were taken from the EC-II/III region. Scale bar, 30 μm. (B) NeuN-positive cells in an area within 1000 μm along the EC-II (the region between the two broken lines) were counted. Ta9 exhibited no effects on neuronal loss. n = 5 for each group.