Abstract
Alzheimer's disease (AD) is the most common form of dementia and its prevalence is increasing. Recent developments in AD management provide improved ways of supporting patients and their caregivers throughout the disease continuum. Managing cardiovascular risk factors, maintaining an active lifestyle (with regular physical, mental and social activity) and following a Mediterranean diet appear to reduce AD risk and may slow cognitive decline. Pharmacologic therapy for AD should be initiated upon diagnosis. All of the currently available cholinesterase inhibitors (ChEIs; donepezil, galantamine, and rivastigmine) are indicated for mild-to-moderate AD. Donepezil (10 and 23 mg/day) and rivastigmine transdermal patch (13.3 mg/24 h) are indicated for moderate-to-severe AD. Memantine, an N-methyl-d-aspartate receptor antagonist, is approved for moderate-to-severe AD. ChEIs have been shown to improve cognitive function, global clinical status and patients' ability to perform activities of daily living. There is also evidence for reduction in emergence of behavioral symptoms with ChEI therapy. Treatment choice (e.g., oral vs. transdermal) should be based on patient or caregiver preference, ease of use, tolerability, and cost. Treatment should be individualized; patients can be switched from one ChEI to another if the initial agent is poorly tolerated or ineffective. Memantine may be introduced in moderate-to-severe disease stages. Clinicians will regularly monitor symptoms and behaviors, manage comorbidities, assess function, educate and help caregivers access information and support, evaluate patients' fitness to drive or own firearms, and provide advice about the need for legal and financial planning. Review of caregiver well-being and prompt referral for support is vital.
Introduction
Alzheimer's disease (AD) is the most common form of dementia, accounting for 60–80% of cases.1 In the United States, one in nine people aged ≥65 years has AD (two-thirds of whom are women), and one person develops AD every 67 sec.1 Alzheimer's Disease International estimates that the prevalence of AD will increase by 225% by 2050, affecting more than 115 million people globally,2 and more than 13.8 million people in the United States.1
AD is associated with initial memory loss, followed by impairments in cognitive function, language, visuospatial skills and executive function, coupled with behavioral changes.3 Terminally, patients may become bedridden, incontinent and unable to communicate.4 AD imposes an intolerable burden on healthcare systems, society, patients and their families, and is one of the leading contributors to disability among elderly people.5 Most patients require assistance with activities of daily living (ADL), and many eventually require full-time care and supervision.2,4 Caring for a patient with AD can be stressful, especially if the patient displays neuropsychiatric symptoms, such as irritability, dysphoria or delusions.6
Clinicians play a key role in the medical management of AD, and provide recommendations and advice to patients and families/caregivers on a broad range of issues, including psychosocial problems, and legal and financial resources.7 Specialists involved in AD diagnosis and management include neurologists, geriatricians and geriatric psychiatrists.
Therapeutic nihilism (disbelief in the efficacy or clinical value of a therapy) is a key issue in dementia management, particularly among clinicians, due to negative associations and stigma associated with this progressive illness.8 These perceptions should be overcome as they may delay diagnosis, referral, and treatment.8
This review provides recommendations on managing AD based on current knowledge and available pharmacologic agents. Support for patients, families, and caregivers is also discussed, from early recognition to difficult decisions around end-stage care. The goal of this approach is to maximize quality of life throughout the course of this complex disease. Data sources include pivotal clinical studies of donepezil, galantamine, rivastigmine, memantine, and medical foods identified using PubMed in 2014 and the relevant United States prescribing information. English-language articles considered of relevance to primary care physicians in relation to AD, its diagnosis and management, are included. Previous guidelines and management recommendations were reviewed. The recent meta-analysis of brain health activities by Alzheimer's Disease International was a key document.9
General Brain Health and Wellness
Modifying risk factors for cognitive dysfunction
There are potential risk factors for, or protective factors against, cognitive dysfunction. It is reasonable to recommend modifying these, based on the available evidence. Recommendations for maintaining brain health in adults with and without AD are summarized in Box 1.
Box 1. Recommendations for maintaining brain health in elderly patients with and without AD.
Consider following a Mediterranean-style diet, with fish, vegetables, legumes, fruit, cereals, unsaturated fatty acids (e.g., olive oil), and a limited amount of meat or dairy products.
Consider taking supplements containing omega-3 (particularly docosahexaenoic acid), B-complex vitamins (including B12, B6, folic acid), and vitamin E.
Keep alcohol intake to a low-to-moderate level (e.g., one glass of wine per day with dinner).
Engage in regular physical activity.
Maintain leisure and social activities – keep socially engaged.
Continue or take up activities that help to stimulate the brain, e.g., Tai Chi, dancing, puzzles.
Become educated about AD and seek support from others with AD, e.g., the Alzheimer's Association, Alzheimer's Foundation of America, Keep Memory Alive, and other community groups.
Include music in daily life – listening to music, playing an instrument, singing.
Maintain regular sleep patterns.
Manage stress – stop doing things if they are becoming too stressful (e.g., volunteer work, answering the telephone), keep to a regular daily schedule, include relaxing activities (e.g., playing with pets, massage, and aromatherapy).
Observational studies point to a protective role for certain nutrients and dietary patterns (Mediterranean diet). However, data from randomized controlled trials have been inconsistent. Whether factors such as cooking processes and other dietary components explain these inconsistencies is uncertain. The potential mechanisms by which certain nutrients may protect brain health also remain to be established.10
Long-term studies on the relationship between physical activity and the incidence of noncommunicable diseases in general is limited. However, the few available studies indicate that physical activity in healthy people is an important factor preventing the development of cognitive impairment and AD.11
Depression is associated with cognitive decline,12 supporting the management of depressive symptoms in the elderly. Further, there is clinical rationale to actively manage hyperlipidemia and diabetes.13 Untreated hypertension is associated with rapid decline in cognitive function in vulnerable individuals.14
A family history of AD is a risk factor for the disorder; family members should be encouraged to adopt a brain-healthy lifestyle.
Lifestyle considerations for patients with AD
Despite evidence that omega-3 fatty acids slow cognitive decline in the elderly, findings in patients with AD are inconsistent.15–17 One trial reported benefits of omega-3 fatty acid and lipoic acid treatment on cognition and ADL performance.16 However, due to small sample sizes, further studies are required.16 Folic acid and vitamin B supplements may help to preserve brain function.18 A small, double-blind, randomized, controlled trial reported that folic acid supplementation may improve response to cholinesterase inhibitor (ChEI) therapy.19 Time to institutionalization and daily living functioning have improved following vitamin E supplementation.20,21 As with all interventions, the benefit–risk ratio of supplements should be considered. A recent meta-analysis concluded that high blood levels of docosahexaenoic acid and eicosapentaenoic acid are possibly associated with increased risks of high-grade prostate cancer.22 These findings should be interpreted with caution due to the multifactorial etiology of prostate cancer, and the complex metabolism of long-chain omega-3 fatty acids.22
Dementia symptoms are stressful for patients, care-givers, and families.23–26 Patients who believe nothing can be done about their illness are more likely to experience depression,27 and clinicians should educate patients about steps they can take to preserve function (Box 1). Learning about AD and seeking support early in the disease course can help reduce stress and improve coping and health-related behaviors.28 Artistic pursuits may help maintain a sense of identity and self-expression, improve aspects of behavior, and enhance communication.29–31 Music therapy reportedly improves language functions in patients with dementia32 and symptoms of anxiety and depression in patients with mild-to-moderate AD.33 Interactions with pets may reduce agitation and anxiety in some patients with dementia.34 Patients and caregivers can also reduce stress by adjusting activities to suit the patient's abilities, and keeping to a regular daily routine that includes relaxing activities.35
Clinicians should urge patients with AD to participate in leisure activities, where possible, preserving function and quality of life.23,25,36 Cognitive training may improve function, so patients should be encouraged to learn new skills or hobbies and undertake activities that stimulate mental activity.12,37 Social interactions may be difficult for patients with AD, and they may withdraw due to self-consciousness, depression, or apathy.23,25 Participation in specialized adult day programs for patients with dementia may enhance the benefits of drug therapy and improve sleep quality, by keeping the patient engaged and reducing inactivity.38 Specialized memory rehabilitation programs for patients with AD have also proven valuable,39 but are not widely available.
Diagnosis
In clinical trials, the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria are commonly used to diagnose dementia of the Alzheimer's type.40 Recently, an updated version, DSM-5, was published; key updates are the change in dementia to the newly named entity “major neurocognitive disorder (NCD)”, and recognition of a less severe level of cognitive impairment, termed “mild NCD”.41
The most recent diagnostic guidelines from the National Institute on Aging and Alzheimer's Association define three stages of AD:42
Preclinical phase: neuropathologic changes occur, no overt (or only subtle) symptoms.
Phase of mild cognitive impairment: symptoms become apparent; ADL are preserved; the patient does not have dementia.
Dementia phase: ADL are impaired.
There may be preclinical neurologic changes in the form of cerebrospinal fluid or amyloid imaging biomarkers.43 However, AD diagnosis is principally based on clinical criteria (Fig.1);44,45 biomarkers can be used to define AD as the probable underlying cause of cognitive impairment, but are not strictly necessary for diagnosis.42,45 The accuracy of diagnosis is enhanced by integration of biomarkers. Table1 outlines the recommended diagnostic steps.46
Figure 1.
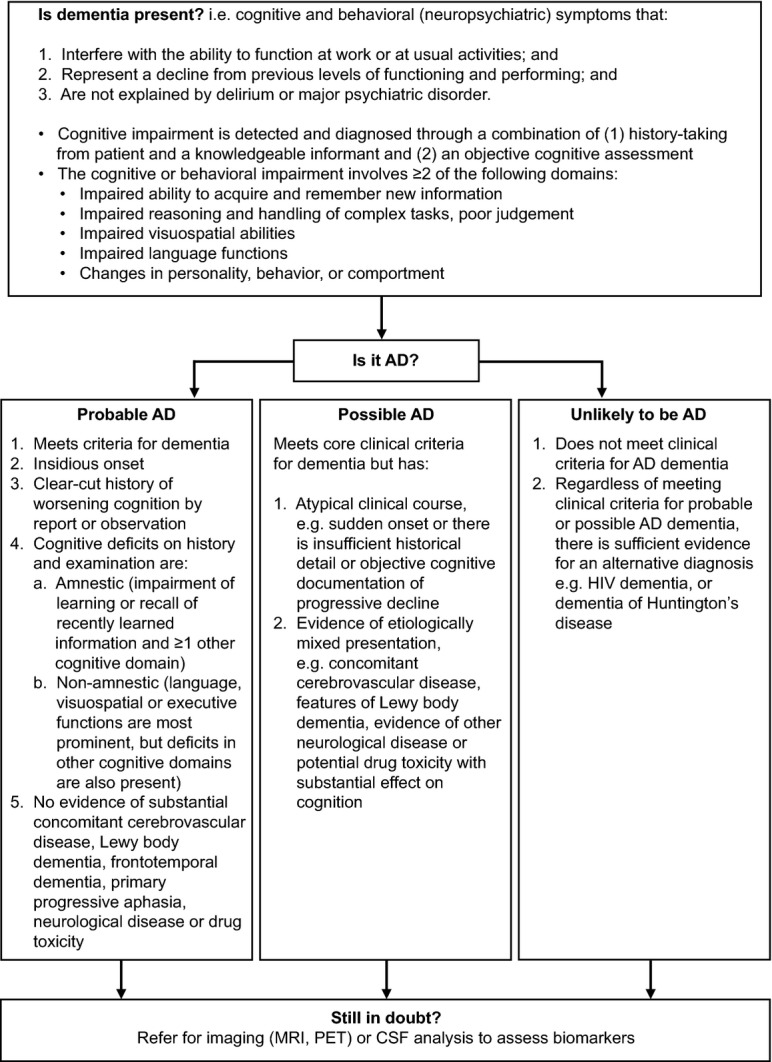
Diagnostic criteria for AD.45 Permission to reproduce text used in this figure was kindly provided by Elsevier Limited. AD, Alzheimer's disease; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography.
Table 1.
Steps in the diagnosis and assessment of a patient with suspected AD in primary care46
| Step | Purpose | Tools/information required |
|---|---|---|
| Step 1: Prediagnostic tests | Identify risks for neurocognitive disorders |
|
| Step 2: Assess performance | Cognitive assessment | |
| Step 3: Assess daily functioning | Determine level of independence and degree of disability |
|
| Step 4: Assess behavioral symptoms | Determine presence and degree of behavioral symptoms |
|
| Step 5: Identify caregiver and assess needs | Identify primary caregiver and assess adequacy of family and other support systems |
|
| Other considerations | Identify cultural differences, language, and literacy level of patient and caregiver |
|
AD, Alzheimer's disease; AD8, 8-item Ascertain Dementia tool; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living scale; Mini-cog, Mini Cognitive Assessment Instrument; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NPI-Q, Neuropsychiatric Inventory Questionnaire; SIB-8, Severe Impairment Battery (8-item).
Reproduced by permission of the American Board of Family Medicine.
Pharmacotherapy
There are a number of Food and Drug Administration (FDA)-approved pharmacotherapies for AD (Table2). These may improve symptoms or delay decline; none impact the underlying neurodegenerative process.47 It is important for patients and their caregivers to understand this, so they can make informed treatment decisions and have realistic expectations regarding the impact of treatment.48
Table 2.
(A) Food and Drug Administration-approved AD therapies and (B) medical foods
| Approved/intended indication | Administration | ||||
|---|---|---|---|---|---|
| Mechanism | Route | Dosing | Frequency | ||
| (A) Pharmacologic agents | |||||
| Donepezil (Aricept®)49 | ChEI | Mild-to-moderate AD
|
PO (tablet) | Titration:
|
Once daily |
| Galantamine (Razadyne®)50 | ChEI | Mild-to-moderate AD | PO (tablet/oral solution) | Titration:
|
Twice daily, with food |
| Galantamine ER (Razadyne® ER)50 | ChEI | Mild-to-moderate AD | PO (capsule) | Titration:
|
Once daily, in morning, with food |
| Rivastigmine (Exelon®)51 | ChEI | Mild-to-moderate AD
|
PO (capsules/oral solution) | Titration:
|
Twice daily |
| Rivastigmine patch (Exelon® Patch)52 | ChEI | Mild-to-moderate AD
|
TD patch | Titration:
|
Apply new patch once every 24 h |
| Memantine (Namenda®)66 | NMDA receptor antagonist | Moderate-to-severe AD | PO (tablet/oral solution) | Titration:
|
Twice daily |
| Memantine (Namenda®) XR67 | NMDA receptor antagonist | Moderate-to-severe AD | PO (capsules) | Titration:
|
Once daily |
| (B) Medical foods | |||||
| Caprylidene (Axona®)72 | Medical food – nutritionally supports brain metabolism | Mild-to-moderate AD | PO (powder to be added to 4–8 oz of liquid) | Titration:
|
Once daily after food |
| l-methylfolate/methylcobalamin/N-acetylcysteine (CerefolinNAC®, Triveen-CF NAC®)73 | Medical food – supports the brain's metabolic balance | Mild or moderate cognitive impairment | PO (caplet) | 1 caplet/day | Once daily |
| Phosphatidylserine/docosahexaenoic acid/eicosapentaenoic acid (Vayacog®) | Medical food – supports management of lipid imbalances | Early memory impairment | PO (capsule) | 1 capsule/day | Once daily |
| Omega-3 fatty acids, uridine, choline, vitamins C, E, B6, and B12, selenium, and folic acid (Souvenaid®) | Medical food – supports synaptic integrity | Early AD | PO (liquid) | 1 bottle (125 mL) per day | Once daily |
In contrast to FDA-approved drugs, no premarket review process exists for medical foods: data supporting their effectiveness (if such data exist) have not undergone the same rigorous scientific scrutiny as approved drugs. AD, Alzheimer's disease; ChEI, cholinesterase inhibitor; ER, extended release; NMDA, N-methyl-d-aspartate; PDD, Parkinson's disease dementia; PO, per os (oral administration); TD, transdermal.
ChEIs
Three ChEIs (donepezil [Aricept®; Eisai Inc., Woodcliff Lake, New Jersey], galantamine [Razadyne®; Janssen Pharmaceuticals Inc, Titusville, New Jersey] and rivastigmine [Exelon® capsules and Exelon® Patch; Novartis Pharmaceuticals Corporation, East Hanover, New Jersey]) are indicated for mild-to-moderate AD in the United States; donepezil and rivastigmine transdermal patch are also indicated for severe AD.49–52 All three ChEIs are approved in oral formulations;49–51 rivastigmine is the only ChEI also approved for delivery via a transdermal patch.52 Approval of high-dose 23 mg/day donepezil for moderate-to-severe AD was based on a randomized clinical trial that demonstrated greater cognitive efficacy versus the standard dose (10 mg/day).53 The high-dose (13.3 mg/24 h) rivastigmine patch was approved for mild-to-moderate and severe AD, based on positive findings in the OPTIMA (OPtimising Transdermal Exelon In Mild-to-moderate Alzheimer's disease) and ACTION (ACTivities of daily living and cognitION) studies, respectively.54,55
All three ChEIs have demonstrated clinical benefits on cognitive function, global clinical status,54–57 and performance of ADL.54–56,58 There are no proven clinically meaningful differences between the agents in terms of efficacy.56,57,59 Efficacy and tolerability associated with ChEIs are dose dependent,60 so while high doses may be efficacious, adverse events (AEs) can be dose limiting. The agents have similar tolerability profiles, with nausea, vomiting, and diarrhea being the most common AEs.56
Gastrointestinal (GI) AEs typically arise due to peaks in plasma drug concentrations.61 Strategies that reduce the dosing frequency, lower peak plasma concentrations, and reduce the rate at which peak concentrations are reached may improve tolerability.62 A sustained-release formulation of donepezil was developed to provide access to high therapeutic doses, while avoiding the rapid rises in peak drug plasma concentrations.53 Donepezil 23 mg/day is associated with greater cognitive benefits in moderate-to-severe AD than donepezil 10 mg/day IR; however, there is an dose-related increase in AEs.53
A pharmacokinetic analysis predicted lower peaks in plasma drug concentrations, but a similar area under the curve, with extended-release (ER) galantamine compared with the IR formulation.63 During a clinical study, both once-daily galantamine ER and twice-daily galantamine IR (flexible dosing of 16 or 24 mg/day) demonstrated superiority to placebo on cognition in patients with mild-to-moderate AD, with a similar incidence of AEs observed in all three groups.64
By providing sustained delivery over a 24-h period, transdermal delivery may improve GI tolerability and permit easier access to high-dose efficacy compared with oral dosing.62 Relative to 6 mg twice-daily oral rivastigmine, 9.5 mg/24 h rivastigmine transdermal patch was associated with comparable efficacy and two-thirds fewer GI AEs.65 Rivastigmine patch treatment is initiated with the 4.6 mg/24 h dose, with up-titration to the minimum effective dose of 9.5 mg/24 h patch after at least 4 weeks, assuming good tolerability.52 After an additional 4 weeks, if well-tolerated, the dose may be increased to 13.3 mg/24 h patch.52 Continued use of the 4.6 mg/24 h dose can be considered in patients weighing less than 50 kg with tolerability concerns, or patients with mild-to-moderate hepatic impairment.52
Transdermal delivery may be associated with application site reactions in some patients; the occurrence can be minimized by following the recommendations in the rivastigmine patch United States prescribing information.52 These include: rotating the application site; applying the patch only to clean, dry, hairless skin that is free of redness, irritation, cuts, or burns; and ensuring the skin is free of creams, lotions, or powders prior to patch application.52
After initiating ChEI treatment, patients should be assessed after 2–4 weeks for the development of AEs,7 and after 3–6 months for effects on cognitive function and other behaviors/abilities.7 ChEI doses should be up-titrated according to the prescribing information (Table2).49–52 Regular follow-up appointments should be established to assess disease progression. Patients should be switched to another ChEI if they develop intolerable or nontransient AEs, if they do not respond to the initial agent (i.e., continued deterioration at pretreatment rate),7 or if caregiver or patient preferences change. The principle of switching is to transition the patient from one ChEI to another in an attempt to improve clinical outcomes. Switching should follow the recommendations outlined in the relevant prescribing information.49–52
NMDA receptor antagonist
Memantine (Namenda®; Forest Pharmaceuticals Inc., St Louis, Missouri) is an N-methyl-d-aspartate (NMDA) receptor antagonist indicated for moderate-to-severe AD (Table2).66,67 Clinical studies have demonstrated efficacy of memantine (20 mg/day) versus placebo on cognition, global function, and ADL performance.68,69 A high-dose (28 mg/day) once-daily ER formulation has demonstrated cognitive efficacy, and an acceptable tolerability profile, compared with placebo in patients with moderate-to-severe AD receiving ChEIs.70 Memantine can be used as monotherapy71 or in combination with a ChEI.7,47 Combining two agents with different mechanisms of action, may improve efficacy relative to single-agent therapy, and the use of memantine with oral ChEIs may ameliorate ChEI-related GI AEs.47
Medical foods
Several medical foods are available in the United States for management of AD and/or cognitive impairment, including:
Caprylidene (Axona®; Accera Inc., Broomfield, California), a proprietary formulation of medium-chain triglycerides, intended for dietary management of mild-to-moderate AD.72
Cerefolin NAC® (PAMLAB, LLC, Covington, Louisiana), a combination of folic acid, vitamin B12, and N-acetylcysteine, intended for dietary management of mild cognitive impairment.73
Vayacog® (VAYA Pharma Inc., Greenville, South Carolina), a combination of phosphatidylserine, docosahexaenoic acid, and eicosapentaenoic acid, intended for dietary management of lipid imbalances associated with early memory impairment.74
Noncarriers of the apolipoprotein ε4 allele have shown significant improvements in cognitive function with caprylidene.75 Currently, there is limited published evidence to support using folic acid, vitamin B12, and N-acetylcysteine,76 except in the setting of hyperhomocysteinemia. However, in older adults receiving folic acid supplementation for 3 years, plasma homocysteine levels were reduced and memory function improved compared with placebo.77
A 15-week, randomized, double-blind study reported that phosphatidylserine-containing omega-3 fatty acids improved cognitive performance in elderly with memory complaints without dementia, although further studies are required to confirm these findings.78
Souvenaid® (Nutricia Advanced Medical Nutrition, Schiphol, The Netherlands) a combination of omega-3 fatty acids, uridine, choline, vitamins C, E, B6, and B12, selenium, and folic acid is not yet available in the United States. Souvenaid® is available in Europe (Food for Special Medical Purpose) as a dietary means of supporting synaptic integrity. In clinical trials of patients with mild AD, this combination demonstrated a significant improvement in delayed verbal memory,79,80 but not global cognition,79 or performance of ADL.81
No single agent or combination will suit every patient, so clinicians should construct a comprehensive, integrated, multifaceted treatment plan that reflects the preferences of the patient and caregiver, and the most recent scientific guidance. Clinicians should be prepared to reassess their approach to achieve optimal therapeutic benefit/outcomes. The optimal duration of ChEI treatment has not been established, but patients in clinical trials have shown benefit for up to 4 years,82 so continuing treatment as long as patients tolerate the medication and families support treatment is reasonable. Maintaining open discussions with the family on disease progression and treatment outcomes may guide the clinician regarding whether or not to maintain treatment, and help the family to prepare for the eventual need for long-term residential placement.
Managing Comorbidities
In one study of 679 patients with AD in the United States, 61% had ≥3 medical comorbidities, and the number increased with advancing dementia severity.83 After controlling for other variables, higher medical comorbidity was associated with worse cognitive function and poorer self-care.83 Other data show that patients with AD are 55% more likely to be admitted to hospital than people without AD.84 Common medical comorbidities in patients with AD include cardiovascular disease, thyroid dysfunction, sleep apnea, osteoporosis, glaucoma, cancer, falls, depression, infections, anorexia, rheumatoid conditions, and incontinence.85,86 One key treatable comorbidity is sleep apnea. In addition to being a risk factor for hypertension, stroke, and mortality,87,88 sleep apnea has been reported as a significant risk for dementia in older women, nearly doubling the risk for mild cognitive impairment and dementia over 5 years.89 It is therefore important for clinicians to evaluate the presence of sleep disordered breathing as another modifiable risk.
It is important for clinicians to manage conditions comorbid to AD (Box 2). This can limit cognitive and functional decline,85 and reduce the risk of hospital admission, which can be disorienting, distressing, and lead to medication discontinuation. Moreover, medical illness and pain are common triggers for agitation or aggression in patients with AD,47 so early recognition and treatment of comorbid medical conditions can limit neuropsychiatric and behavioral symptoms.
Box 2. Management of comorbidities.
Assess regularly for medical and neuropsychiatric comorbidities.
Manage comorbidities, particularly cardiovascular risk factors, with lifestyle recommendations and medications to reduce risk of cognitive decline.
Investigate potential medical, somatic, or medication-related reasons for any new change in function or behaviors.
Promptly treat acute medical illnesses to reduce the risk of hospital admission.
Offer caregiver education and support to help manage neuropsychiatric or behavioral symptoms (Table3).
Initiate psychoactive medication as necessary, balancing the benefits and risks, and document a discussion with the patient and/or family members in the medical record.
Be aware of adverse impact of polypharmacy and potential for drug–drug interactions.
Encourage adherence – simplify medication regimen, where possible.
Neuropsychiatric symptoms are a key component of AD, affecting almost all patients over the disease course.90 Studies suggest that more than 90% of patients with AD show behavioral or neuropsychiatric symptoms, including depression, agitation, anxiety, psychosis, hallucinations, apathy, eating disorders, disinhibition, and sleep disturbances.91–93 The first step in management is to rule out potential medical and/or somatic causes, for example, lower urinary tract infection,7,86,94 and recommend nonpharmacologic interventions (Table3).7 ChEIs or memantine may delay or relieve symptoms,7,86,95 but some patients may require targeted pharmacotherapy, such as antipsychotics or antidepressants. Although antidepressant agents may be efficacious in treating depression in patients with AD, the supporting evidence is limited, and no agent has been approved specifically for depression or psychosis of AD.96,97 A cross-sectional study reported that treatment of neuropsychiatric symptoms in elderly patients with dementia residing in care homes is generally not syndrome specific, and included use of neuroleptics and other substances not indicated for dementia.98 Antipsychotic use has been associated with an increased risk of death due to pneumonia in elderly patients with dementia,94 highlighting the importance of investigating the underlying cause of behavioral symptoms prior to prescribing treatment.
Table 3.
Nonpharmacologic approaches to manage common behavioral symptoms and mood disorders7
| Behavioral symptom | Nonpharmacologic intervention |
|---|---|
| Apathy | Stimulation/activities
|
| Sleep disturbances | Take steps to maintain regular, good quality sleep
|
| Irritability/agitation | Break down tasks into simple steps
|
| Wandering | Visual cues
|
| Mood disorders | Exercise |
| Psychotic disorders | Reassurance
|
| Eating/appetite disorders | Offering simple, finger foods
|
Permission to reproduce this table was kindly provided by the Alzheimer's Disease Program, California Department of Public Health.
Patients with AD exhibiting agitation and other neuropsychiatric symptoms often receive anticonvulsants, such as divalproex, as a second-line treatment.99,100 However, data from controlled clinical studies suggest divalproex accelerated brain atrophy, and did not benefit patients with moderate AD in terms of neuropsychiatric symptoms, functional abilities, and cognition.101,102
There is significant potential for polypharmacy in patients with AD.103 Clinicians should be aware of potential drug interactions when introducing new medications and of difficulties in maintaining compliance/adherence with complex drug regimens.103 Attempts should be made to simplify the medication regimen, for example, use of alternate modes of delivery that are more user friendly.103
Societal and Financial Impact
Residential care is a major contributor to the financial impact of AD. In several studies ChEI treatment was associated with a delay in time to nursing home placement,104 an effect that is reportedly dose dependent.105 Similarly, evidence suggests cost-savings with memantine compared with nonpharmacological treatment in moderately severe-to-severe AD,106 as well as some benefit for combined ChEI/memantine therapy based on health-care data from France.107
Nonpharmaceutical interventions in the management of behavioral symptoms and mood disorders have also been linked to improved cognitive and functional ability, enhanced patient and caregiver quality of life and delayed institutionalization.7
Other Considerations
Clinicians responsible for patients with AD should pay attention to several areas that may impact well-being, and not only medication (Box 3).
Box 3. Checklist for clinicians caring for patients with AD.
Health
Have you provided the patient/caregiver with nutritional advice?120
If using a transdermal patch, have you provided the patient/caregiver with guidance on best patch use, including skin care?52
Are you regularly assessing your patient's clinical and functional state?
Safety
Have you or has someone else (caregiver, social worker) assessed the safety of the patient's home?113
Have you asked your patient and their caregiver(s) about firearms in the home?113
Have you talked to your patient and their caregiver(s) about the likely need to stop driving?121
Legal considerations
Have you advised your patient and their caregiver(s) to seek legal advice with regard to planning for future incapacity?122
Are you aware of the state requirements regarding medical fitness to drive for patients with AD?121
Are you aware of your legal obligations/appropriate steps to take if you suspect abuse/neglect in the patient or caregiver?123
Support
Safety
Keeping the person with AD safe is important, to prevent injuries, maximize function, minimize stress and agitation, and reduce caregiver burden.7 A home assessment may be useful to identify hazards that could increase the risk of falls. Patients with AD may no longer remember how to use appliances correctly, identify toxic substances, or handle sharp objects, so caregivers should ensure that potentially harmful objects are removed.7 The Alzheimer's Association, Alzheimer Foundation of America, and other advocacy groups have resources on how to assess and manage patient safety in the home.108
Approximately 60% of US households that have a family member with dementia have a gun.109 Few laws regulate gun ownership in relation to age or cognitive function.110 Since patients with dementia may develop aggressive behaviors toward themselves and others as the disease progresses,111 firearms pose a risk of serious injury.110 When it comes to gun ownership, clinicians need to balance the patient's right to autonomy against the need for safety.7,112
Patients with AD will have to stop driving when their limitations make it unsafe. Discussing this possibility early in the disease course can help planning.113 Clinicians should be familiar with their state's regulations regarding minimum cognitive requirements for licensure, and legal requirements for reporting the diagnosis. The American Occupational Therapy Association website (http://www.aota.org) identifies resources for people with AD and their families, including a national database of driving specialists, who can evaluate and offer strategies to reduce driving risk.
Ongoing follow-up
Clinicians need to regularly assess the patient for changes in daily functioning, cognitive status, comorbidities, behavioral symptoms, medication requirements, and care needs; any sudden changes require attention.7 Weight loss occurs in ∽40% of patients with AD.114 Patients showing signs of weight loss should be offered nutritional supplementation and advice on increasing daily calorie intake. Dosing modifications may also be appropriate for these patients.
Abuse
Clinicians are well placed to identify potential patient abuse and neglect, or signs of overwhelming stress and abuse in caregivers.7 It is important to recognize that abuse may be exerted by the patient on the caregiver or vice versa.7 Effective management of aggressive symptoms or psychiatric comorbidities is essential, as is timely referral to support services. Across the United States, a number of elder abuse multidisciplinary teams exist, which provide expert consultation to service providers, and identify service gaps and systems problems when managing cases of abuse.115
Residential care and legal aspects
The most common reasons for patients to move to residential care are the need for more skilled care than the caregiver can provide, the caregiver's health, and the patient's dementia-related behaviors.116 Discussing the potential future need for residential care early in the disease course, and identifying the threshold for such decisions, can ease the process. Clinicians should advise patients and their caregivers to plan for when the patient will have diminished capacity, and seek legal advice to put in place the necessary powers of attorney, as well as documentation around end-of-life preferences, such as wills and advance directives/living wills.7
Caregiver well-being
The importance of shaping policy and practice to support the caregiver cannot be overemphasized.117 Demands on caregivers increase as the disease progresses, and may include managing bathing, dressing, feeding, and toileting.86 Caregivers must cope with sometimes challenging behaviors while dealing with the emotional impact of the changes to their relationship with the patient. They are essential in providing information to healthcare professionals about symptomatic and functional changes in the patient.118 Clinicians should pay careful attention to the physical and psychological health of caregivers.119 Education, support, counseling, respite care, and referral to other services may be required. Involving a social worker early in the course of the disease can help caregivers gain access to local support services and provide advice around financial assistance.7
Summary
AD is one of the most challenging chronic conditions to manage. Treatment must be individualized for the symptoms, functional status, comorbidities, behaviors, and the psychosocial situation of each patient, and requires regular reassessments for changes in the patient's and caregiver's medical, mental, and psychological states. Figure2 shows an algorithm for how to manage AD in primary care. Early diagnosis, referral to specialists, and treatment initiation are key; as are understanding and conveying realistic expectations of treatment. Patients respond individually to different interventions, so clinicians should be willing to change medications to maximize patient and caregiver quality of life, and to engage the assistance of other healthcare professionals (e.g., geriatricians, neurologists, psychiatrists, psychologists, physical or occupational therapists, social workers). It should be emphasized to patients and caregivers that, while the progressive course of AD cannot be reversed, lifestyle changes and medication can delay the progression of cognitive and functional symptoms of AD and maximize quality of life.
Figure 2.
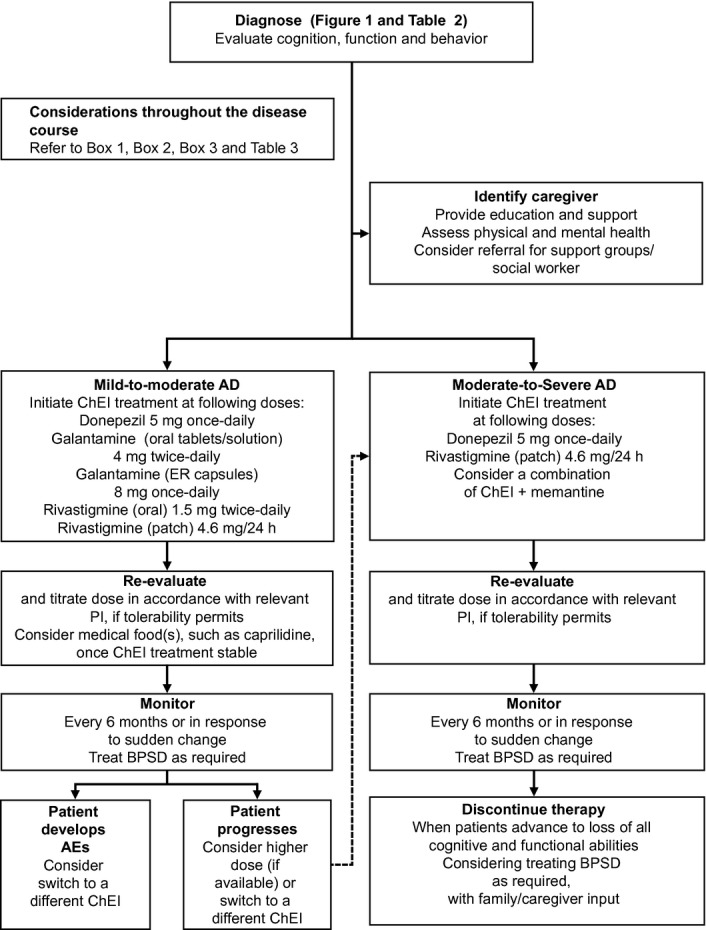
Treatment and management of AD.47 Permission to reproduce this figure was kindly granted by Physician's Weekly. AD, Alzheimer's disease; BPSD, behavioral and psychological symptoms of dementia; ChEI, cholinesterase inhibitor; ER, extended release; PI, prescribing information.
Acknowledgments
Medical writing and editorial assistance in the development of this manuscript were provided by Katy Cooke at Fishawack Communications Ltd, Oxford, UK, and this service was supported by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
Conflicts of Interest
Jeffrey L. Cummings has provided consultation to Acadia, ADAMAS, Anavex, Avanir, Avid, Baxter, Bristol-Myers Squibb, Eisai, Elan, EnVivo, GE, Genentech, Lilly, Lundbeck, MedAvante, Merck, Neuronix, Novartis, Otsuka, Pain Therapeutics, Pfizer, Prana, QR, Sanofi, Takeda, Toyama and UBC. Jeffrey L. Cummings owns stock in ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, Neurokos, and QR pharma. Jeffrey L. Cummings has participated as a speaker/lecturer for Eisai, Forest, Janssen, Novartis, Pfizer, and Lundbeck. Jeffrey L. Cummings owns the copyright of the Neuropsychiatric Inventory. Jeffrey L. Cummings has provided expert witness consultation regarding olanzapine and ropinirole. Richard Isaacson has served as a scientific advisor/consultant for Novartis and Accera in the last year. Frederick A. Schmitt has received research funding in the past from Pfizer and Forest Laboratories, and presently serves on a data safety monitoring committee for Pfizer. Drew Velting is an employee and stock holder of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
References
- Alzheimer's Association. 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Wimo A, Prince M. World Alzheimer Report 2010: the global economic impact of dementia. London, UK: Alzheimer's Disease International; 2010. [Google Scholar]
- Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287:2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- Lopez OL. The growing burden of Alzheimer's disease. Am J Manag Care. 2011;17(suppl 13):S339–S345. [PubMed] [Google Scholar]
- Alzheimer's Disease International. World Alzheimer Report. 2009. . Available at http://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf (accessed 21 November 2014) [Google Scholar]
- Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- California Workshop on Guidelines for Alzheimer's Disease Management. Guidelines for Alzheimer's disease management. Final report 2008.
- Iliffe S, Wilcock J, Haworth D. Obstacles to shared care for patients with dementia: a qualitative study. Fam Pract. 2006;23:353–362. doi: 10.1093/fampra/cmi116. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Disease International. World Alzheimer Report 2014. Available at http://www.alz.co.uk/research/world-report-2014 (accessed 21 November 2014)
- Otaegui-Arrazola A, Amiano P, Elbusto A, et al. Diet, cognition, and Alzheimer's disease: food for thought. Eur J Nutr. 2014;53:1–23. doi: 10.1007/s00394-013-0561-3. [DOI] [PubMed] [Google Scholar]
- Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity–a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi: 10.1186/1471-2458-13-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Plassman BL, Burke J, et al. Preventing Alzheimer's disease and cognitive decline. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2010. . Contract No.: 10-E005. [Google Scholar]
- Nelson PT, Smith CD, Abner EA, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–469. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki M, Luo X, Schmeidler J, et al. Hypertension is associated with cognitive decline in elderly people at high risk for dementia. Am J Geriatr Psychiatry. 2012;20:179–187. doi: 10.1097/JGP.0b013e31820ee833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto L, Quinn J, Montine T, et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer's disease. J Alzheimers Dis. 2014;38:111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- Douaud G, Refsum H, de Jager CA, et al. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci USA. 2013;110:9523–9528. doi: 10.1073/pnas.1301816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly PJ, Prentice NP, Cousland G, Bonham J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer's disease. Int J Geriatr Psychiatry. 2008;23:155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311:33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Chua ME, Sio MC, Sorongon MC, Morales ML., Jr The relevance of serum levels of long chain omega-3 polyunsaturated fatty acids and prostate cancer risk: a meta-analysis. Can Urol Assoc J. 2013;7:E333–E343. doi: 10.5489/cuaj.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadell LS, Clare L. I'm still the same person: the impact of early-stage dementia on identity. Dementia. 2011;10:379–398. [Google Scholar]
- Judge KS, Menne HL, Whitlatch CJ. Stress process model for individuals with dementia. Gerontologist. 2010;50:294–302. doi: 10.1093/geront/gnp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney A, Dahlke S, Purves B. Shifting patterns of everyday activity in early dementia: experiences of men and their families. J Fam Nurs. 2013;19:348–374. doi: 10.1177/1074840713486727. [DOI] [PubMed] [Google Scholar]
- Van Dijkhuizen M, Clare L, Pearce A. Striving for connection: appraisal and coping among women with early-stage Alzheimer's disease. Dementia. 2006;5:73–94. [Google Scholar]
- Clare L, Goater T, Woods B. Illness representations in early-stage dementia: a preliminary investigation. Int J Geriatr Psychiatry. 2006;21:761–767. doi: 10.1002/gps.1558. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Silverio E. Evaluation of an education and support program for early-stage Alzheimer's disease. J Appl Gerontol. 2009;28:419–435. [Google Scholar]
- Beard R. Art therapies and dementia care: a systematic review. Dementia. 2012;11:633–656. [Google Scholar]
- Koger SM, Chapin K, Brotons M. Is music therapy an effective intervention for dementia? A meta-analytic review of literature. J Music Ther. 1999;36:2–15. doi: 10.1093/jmt/36.1.2. [DOI] [PubMed] [Google Scholar]
- Gotell E, Brown S, Ekman SL. The influence of caregiver singing and background music on vocally expressed emotions and moods in dementia care: a qualitative analysis. Int J Nurs Stud. 2009;46:422–430. doi: 10.1016/j.ijnurstu.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Brotons M, Koger SM. The impact of music therapy on language functioning in dementia. J Music Ther. 2000;37:183–195. doi: 10.1093/jmt/37.3.183. [DOI] [PubMed] [Google Scholar]
- Guetin S, Portet F, Picot MC, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer's type dementia: randomised, controlled study. Dement Geriatr Cogn Disord. 2009;28:36–46. doi: 10.1159/000229024. [DOI] [PubMed] [Google Scholar]
- Filan SL, Llewellyn-Jones RH. Animal-assisted therapy for dementia: a review of the literature. Int Psychogeriatr. 2006;18:597–611. doi: 10.1017/S1041610206003322. [DOI] [PubMed] [Google Scholar]
- Holt FE, Birks TPH, Thorgrimsen LM, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;3:CD003150. doi: 10.1002/14651858.CD003150. [DOI] [PubMed] [Google Scholar]
- Öhman A, Nygård L. Meaning and motives for engagement in self-chosen daily life occupations among individuals with Alzheimer's disease. OTJR. 2005;25:89–97. [Google Scholar]
- Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer's disease: a meta-analysis of the literature. Acta Psychiatr Scand. 2006;114:75–90. doi: 10.1111/j.1600-0447.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- Femia EE, Zarit SH, Stephens MA, Greene R. Impact of adult day services on behavioral and psychological symptoms of dementia. Gerontologist. 2007;47:775–788. doi: 10.1093/geront/47.6.775. [DOI] [PubMed] [Google Scholar]
- De Vreese LP, Neri M, Fioravanti M, et al. Memory rehabilitation in Alzheimer's disease: a review of progress. Int J Geriatr Psychiatry. 2001;16:794–809. doi: 10.1002/gps.428. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- APA. Highlights of changes from DSM-IV-TR to DSM-5. Arlington, Virginia: American Psychiatric Association; 2013. [Google Scholar]
- Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Sadowsky CH. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25:367–382. doi: 10.3122/jabfm.2012.03.100181. [DOI] [PubMed] [Google Scholar]
- Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350–366. doi: 10.3122/jabfm.2012.03.100183. [DOI] [PubMed] [Google Scholar]
- Lindstrom HA, Smyth KA, Sami SA, et al. Medication use to treat memory loss in dementia: perspectives of persons with dementia and their caregivers. Dementia. 2006;5:27–50. [Google Scholar]
- 2010. Donepezil (Aricept®) US Prescribing Information. Available at http://www.aricept.com/docs/pdf/aricept_PI.pdf (accessed 21 November 2014)
- ®. 2005. Galantamine (Razadyne ER® and Razadyne ) Prescribing Information. Available at: http://www.razadyneer.com/sites/default/files/shared/pi/razadyne_er.pdf#zoom=100 (accessed 21 November 2014)
- 2006. Rivastigmine (Exelon®) US Prescribing Information. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020823s016,021025s008lbl.pdf (accessed 21 November 2014)
- Rivastigmine patch (Exelon Patch®. 2009. US Prescribing Information. Revised 2012 and 2013. Available at http://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf (accessed 21 November 2014)
- Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32:1234–1251. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Froelich L, Black SE, et al. Randomized, double-blind, parallel-group, 48-week study for efficacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm2 in Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33:341–353. doi: 10.1159/000340056. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Grossberg GT, Sadowsky CH, et al. A 24-week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6 mg/24 h in severe Alzheimer's dementia. CNS Neurosci Ther. 2013;19:745–752. doi: 10.1111/cns.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;1:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS. Treatment guidelines for Alzheimer's disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9:113–121. doi: 10.4088/pcc.v09n0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabins PV, Blacker D, Rovner BW, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Second edition. Am J Psychiatry. 2007;164:5–56. [PubMed] [Google Scholar]
- Anand R, Messina J, Hartman R. Dose-response effect of rivastigmine in the treatment of Alzheimer's disease. Int J Geriatr Psychopharmacol. 2000;2:68–72. [Google Scholar]
- Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–739. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]
- Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology. 2007;69:S4–S9. doi: 10.1212/01.wnl.0000281845.40390.8b. [DOI] [PubMed] [Google Scholar]
- Hing JP, Piotrovsky V, Kimko H, et al. Pharmacokinetic simulation for switching from galantamine immediate-release to extended-release formulation. Curr Med Res Opin. 2005;21:483–488. doi: 10.1185/030079905X38213. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Corey-Bloom J, Potocnik FC, et al. Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;20:120–132. doi: 10.1159/000086613. [DOI] [PubMed] [Google Scholar]
- Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer's disease–rivastigmine patch versus capsule. Int J Geriatr Psychiatry. 2007;22:456–467. doi: 10.1002/gps.1788. [DOI] [PubMed] [Google Scholar]
- Memantine (Namenda®. 2013. tablets/oral solution. US Prescribing Information. Available at http://pi.actavis.com/data_stream.asp?product_group=1901&p=pi&language=E (accessed 21 November 2014)
- 2010. Memantine (Namenda®) XR US Prescribing Information. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022525s000lbl.pdf (accessed 21 November 2014)
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- Grossberg GT, Manes F, Allegri RF, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer's disease taking cholinesterase inhibitors. CNS Drugs. 2013;27:469–478. doi: 10.1007/s40263-013-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Snow V, Cross JT, Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370–378. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 2012. Caprylidene US Prescribing Information. Available at http://www.about-axona.com/assets/files/us-en/global/pdf/Axona-PrescribingInformation.pdf (accessed 21 November 2014)
- 2012. CerefolinNAC® Caplets US Prescribing Information. Available at http://www.drugs.com/cdi/cerefolin-with-nac.html (accessed 21 November 2014)
- 2012. Vayacog® US Prescribing Information. Available at http://vayacog.com/wp-content/uploads/2012/09/Vayacog-Sample-Outsert-Rev.-8-copy.pdf (accessed 21 November 2014)
- Henderson ST, Vogel JL, Barr LJ, et al. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE. Optimizing diagnosis and management in mild-to-moderate Alzheimer's disease. Neurodegener Dis Manag. 2012;2:291–304. doi: 10.2217/nmt.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- Vakhapova V, Cohen T, Richter Y, et al. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord. 2010;29:467–474. doi: 10.1159/000310330. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Kamphuis PJ, Verhey FR, et al. Efficacy of a medical food in mild Alzheimer's disease: a randomized, controlled trial. Alzheimers Dement. 2010;6:1–10.e11. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Twisk JW, Blesa R, et al. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31:225–236. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- Kamphuis PJ, Verhey FR, Olde Rikkert MG, et al. Effect of a medical food on body mass index and activities of daily living in patients with Alzheimer's disease: secondary analyses from a randomized, controlled trial. J Nutr Health Aging. 2011;15:672–676. doi: 10.1007/s12603-011-0339-3. [DOI] [PubMed] [Google Scholar]
- Lyle S, Grizzell M, Willmott S, et al. Treatment of a whole population sample of Alzheimer's disease with donepezil over a 4-year period: lessons learned. Dement Geriatr Cogn Disord. 2008;25:226–231. doi: 10.1159/000114450. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Leon J, Cummings JL, et al. Prevalence and impact of medical comorbidity in Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2002;57:M173–M177. doi: 10.1093/gerona/57.3.m173. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kuo TC, Weir S, et al. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer's. BMC Health Serv Res. 2008;8:108. doi: 10.1186/1472-6963-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie A, Chew D, Soiza RL. Non-psychiatric comorbidity associated with Alzheimer's disease. QJM. 2011;104:913–920. doi: 10.1093/qjmed/hcr118. [DOI] [PubMed] [Google Scholar]
- Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1–e26. doi: 10.1111/j.1468-1331.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimers Dement. 2013;9:602–608. doi: 10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Gobartt AL, Balana M. Behavioural symptoms in patients with Alzheimer's disease and their association with cognitive impairment. BMC Neurol. 2010;10:87. doi: 10.1186/1471-2377-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alberca JM, Pablo Lara J, Gonzalez-Baron S, et al. Prevalence and comorbidity of neuropsychiatric symptoms in Alzheimer's disease. Actas Esp Psiquiatr. 2008;36:265–270. [PubMed] [Google Scholar]
- Youn JC, Lee DY, Jhoo JH, et al. Prevalence of neuropsychiatric syndromes in Alzheimer's disease (AD) Arch Gerontol Geriatr. 2011;52:258–263. doi: 10.1016/j.archger.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry. 2012;169:900–906. doi: 10.1176/appi.ajp.2012.12030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbo E, Ligori LD. Differential effects of current specific treatments on behavioral and psychological symptoms in patients with Alzheimer's disease: a 12-month, randomized, open-label trial. J Alzheimers Dis. 2014;39:477–485. doi: 10.3233/JAD-131190. [DOI] [PubMed] [Google Scholar]
- Bains J, Birks JS, Dening TR. The efficacy of antidepressants in the treatment of depression in dementia. Cochrane Database Syst Rev. 2002;4:CD003944. doi: 10.1002/14651858.CD003944. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc. 2011;59:577–585. doi: 10.1111/j.1532-5415.2011.03355.x. [DOI] [PubMed] [Google Scholar]
- Majic T, Pluta JP, Mell T, et al. The pharmacotherapy of neuropsychiatric symptoms of dementia: a cross-sectional study in 18 homes for the elderly in Berlin. Dtsch Arztebl Int. 2010;107:320–327. doi: 10.3238/arztebl.2010.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner AV, O'Brien JG, Schoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647–652. [PubMed] [Google Scholar]
- Tariot PN, Schneider LS, Mintzer JE, et al. Safety and tolerability of divalproex sodium in the treatment of signs and symptoms of mania in elderly patients with dementia: results of a double-blind, placebo-controlled trial. Curr Ther Res. 2001;61:51–67. [Google Scholar]
- Fleisher AS, Truran D, Mai JT, et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77:1263–1271. doi: 10.1212/WNL.0b013e318230a16c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Schneider LS, Cummings J, et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry. 2011;68:853–861. doi: 10.1001/archgenpsychiatry.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small G, Dubois B. A review of compliance to treatment in Alzheimer's disease: potential benefits of a transdermal patch. Curr Med Res Opin. 2007;23:2705–2713. doi: 10.1185/030079907x233403. [DOI] [PubMed] [Google Scholar]
- Beusterien KM, Thomas SK, Gause D, et al. Impact of rivastigmine use on the risk of nursing home placement in a US sample. CNS Drugs. 2004;18:1143–1148. doi: 10.2165/00023210-200418150-00008. [DOI] [PubMed] [Google Scholar]
- Wattmo C, Wallin AK, Londos E, Minthon L. Risk factors for nursing home placement in Alzheimer's disease: a longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. Gerontologist. 2011;51:17–27. doi: 10.1093/geront/gnq050. [DOI] [PubMed] [Google Scholar]
- Jones RW, McCrone P, Guilhaume C. Cost effectiveness of memantine in Alzheimer's disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging. 2004;21:607–620. doi: 10.2165/00002512-200421090-00005. [DOI] [PubMed] [Google Scholar]
- Touchon J, Lachaine J, Beauchemin C, et al. The impact of memantine in combination with acetylcholinesterase inhibitors on admission of patients with Alzheimer's disease to nursing homes: cost-effectiveness analysis in France. Eur J Health Econ. 2014;15:791–800. doi: 10.1007/s10198-013-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association . Available at http://www.alz.org/care/alzheimers-dementia-home-safety.asp#safetytips (accessed 21 November 2014)
- Spangenberg KB, Wagner MT, Hendrix S, Bachman DL. Firearm presence in households of patients with Alzheimer's disease and related dementias. J Am Geriatr Soc. 1999;47:1183–1186. doi: 10.1111/j.1532-5415.1999.tb05197.x. [DOI] [PubMed] [Google Scholar]
- Greene E, Bornstein BH, Dietrich H. Granny, (don't) get your gun: competency issues in gun ownership by older adults. Behav Sci Law. 2007;25:405–423. doi: 10.1002/bsl.766. [DOI] [PubMed] [Google Scholar]
- Kunik ME, Snow AL, Davila JA, et al. Consequences of aggressive behavior in patients with dementia. J Neuropsychiatry Clin Neurosci. 2010;22:40–47. doi: 10.1176/jnp.2010.22.1.40. [DOI] [PubMed] [Google Scholar]
- Kapp MB. Geriatric patients, firearms, and physicians. Ann Intern Med. 2013;159:421–422. doi: 10.7326/0003-4819-159-5-201309030-00682. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. Staying safe: steps to take for a person with dementia. Available at http://www.alz.org/national/documents/brochure_stayingsafe.pdf (accessed 21 November 2014)
- Guerin O, Andrieu S, Schneider SM, et al. Different modes of weight loss in Alzheimer disease: a prospective study of 395 patients. Am J Clin Nutr. 2005;82:435–441. doi: 10.1093/ajcn.82.2.435. [DOI] [PubMed] [Google Scholar]
- Teaster P, Nerenberg L. A national look at elder abuse multidisciplinary teams. J Elder Abuse Negl. 2003;15:91–107. [Google Scholar]
- Buhr GT, Kuchibhatla M, Clipp EC. Caregivers' reasons for nursing home placement: clues for improving discussions with families prior to the transition. Gerontologist. 2006;46:52–61. doi: 10.1093/geront/46.1.52. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Teaster P. The family caregiving career: implications for community-based long-term care practice and policy. J Aging Soc Policy. 2006;18:141–154. doi: 10.1300/J031v18n03_10. [DOI] [PubMed] [Google Scholar]
- Haley WE. The family caregiver's role in Alzheimer's disease. Neurology. 1997;48:S25–S29. doi: 10.1212/wnl.48.5_suppl_6.25s. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Rosenheck R, Lyketsos CG, Schneider LS. Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am J Geriatr Psychiatry. 2010;18:917–927. doi: 10.1097/JGP.0b013e3181d5745d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. Food, eating and Alzheimer's. Available at http://www.alz.org/care/alzheimers-food-eating.asp (accessed 21 November 2014)
- Alzheimer's Association. Dementia & driving resource center. Available at http://www.alz.org/care/alzheimers-dementia-and-driving.asp (accessed 21 November 2014)
- Alzheimer's Association. Financial and legal planning. Available at http://www.alz.org/care/alzheimers-dementia-planning-ahead.asp (accessed 21 November 2014)
- Alzheimer's Association. Abuse. Available at http://www.alz.org/care/alzheimers-dementia-elder-abuse.asp (accessed 21 November 2014)
- Alzheimer's Association. Caregiver stress check. Available at http://www.alz.org/care/alzheimers-dementia-stress-check.asp (accessed 21 November 2014)
- Alzheimer's Association. Caregiver support groups. Available at http://www.alz.org/care/alzheimers-dementia-support-groups.asp (accessed 21 November 2014)
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Saxton JA, Xu Y, et al. A brief instrument to assess treatment response in the patient with advanced Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:377–383. doi: 10.1097/WAD.0b013e3181ac9cc1. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Coats MA, Morris JC. Patient's rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64:725–730. doi: 10.1001/archneur.64.5.725. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]


