Abstract
The latest discoveries and advanced knowledge in the fields of stem cell biology and developmental cardiology hold great promise for cardiac regenerative medicine, enabling researchers to design novel therapeutic tools and approaches to regenerate cardiac muscle for diseased hearts. However, progress in this arena has been hampered by a lack of reproducible and convincing evidence, which at best has yielded modest outcomes and is still far from clinical practice. To address current controversies and move cardiac regenerative therapeutics forward, it is crucial to gain a deeper understanding of the key cellular and molecular programs involved in human cardiogenesis and cardiac regeneration. In this review, we consider the fundamental principles that govern the “programming” and “reprogramming” of a human heart cell and discuss updated therapeutic strategies to regenerate a damaged heart.
Keywords: cardiac progenitor cell, cardiac regeneration, cardiomyocyte proliferation, embryonic heart field, reprogramming
Introduction
Heart disease is the leading cause of mortality in the industrialized world, with insufficient therapeutic options and poor prognosis (Lopez et al, 2006). The adult mammalian heart cannot sufficiently regenerate or replace damaged cardiac tissue with new functional muscle after injury. Given the drastic shortage of donor hearts for transplantation, this calls for an urgent need to develop novel regenerative therapies to repair severely diseased hearts (Hansson et al, 2009). In this regard, cell transplantation approaches are attractive, due to the potential of various stem cell populations to promote cardiac regeneration and repair in experimental models of heart disease and to their feasibility of use in the clinics (Sanganalmath & Bolli, 2013). There have been a number of attempts to transplant cells to diseased hearts using a wide range of cell types, such as autologous/allogenic non-cardiac somatic stem cells and putative endogenous cardiac progenitor cells (CPCs). However, they have at best yielded mixed results and are still far from clinical practice (Ptaszek et al, 2012).
Meanwhile, recent revolutionary work in the fields of stem cell biology and cardiac regenerative medicine has progressively moved our understanding of human cardiac development and homeostasis forward, opening novel paths toward cardiac regeneration. For example, since the breakthrough discovery that fully differentiated mouse and human fibroblasts can be reprogrammed into pluripotent stem cells by retroviral transduction of four defined factors (Oct3/4, Sox2, c-Myc, and Klf4) (Takahashi & Yamanaka, 2006; Takahashi et al, 2007), modifications to this original protocol have been developed to directly reprogram somatic cells into cardiac lineage cells, bypassing the pluripotent state. Pioneering this field, the Srivastava group showed successful direct conversion of murine fibroblasts to cardiomyocyte-like cells in vitro and in vivo by a specific combination of cardiac transcriptional factors (Gata4, Mef2c, and Tbx5) (Ieda et al, 2010; Qian et al, 2012). Despite these encouraging results, much more work will be needed to optimize the technology before it is transferred to clinical testing. Some of the critical issues that need to be resolved include the low reprogramming efficiency and the possible risk of viral transduction-mediated tumorigenesis, which remains a subject of debate.
Post-natal cardiomyocyte renewal/turnover in mammals is another recent discovery in this field (Garbern & Lee, 2013). Over the last decade, the classical 20th-century paradigm that the human heart is a post-mitotic and terminally developed organ with no cell renewal/replication capability has been overturned. Recent studies from several laboratories have demonstrated that cardiomyocyte turnover occurs throughout life in mammals, including humans (Bergmann et al, 2009; Kajstura et al, 2010; Mollova et al, 2013; Senyo et al, 2013). Although the estimated rate of mammalian cardiomyocyte renewal varies from study to study depending on the method used to measure it, most reports find a remarkably low annual turnover rate of approximately 1%, which increases modestly after injury but declines with age. This demonstrates afresh that the inherent capability in humans to regenerate myocardium with aging or after injury in adulthood is entirely insufficient, encouraging researchers to investigate strategies to increase human cardiomyocyte renewal.
So where are we now on the translational road map from stem cell biology to true regenerative therapeutics for heart disease? Importantly, even though much has been uncovered over the decades, the key programs governing human heart development and regeneration/replication remain undetermined. To address current controversies and achieve authentic cardiac regeneration in the clinical setting, it is mandatory for us to understand cardiac development (“programming” a heart) and regeneration (“reprogramming” a diseased heart) on a much deeper level, by employing rigorous research to elucidate the core mechanisms underlying these processes. In this review, we discuss the fundamental principles that govern the “programming” of a developing heart at the cellular/molecular level. We then provide an overview of current and novel therapeutic strategies for heart regeneration in humans, including stem cell transplantation and cellular “reprogramming” approaches, some of which are being tested clinically. Finally, we consider current controversies and issues to be addressed, and show where the field of cardiac regenerative biology and medicine is headed.
Fundamental principles of cardiac development and regeneration
Embryonic heart fields and multipotent CPCs
First and second heart field CPCs
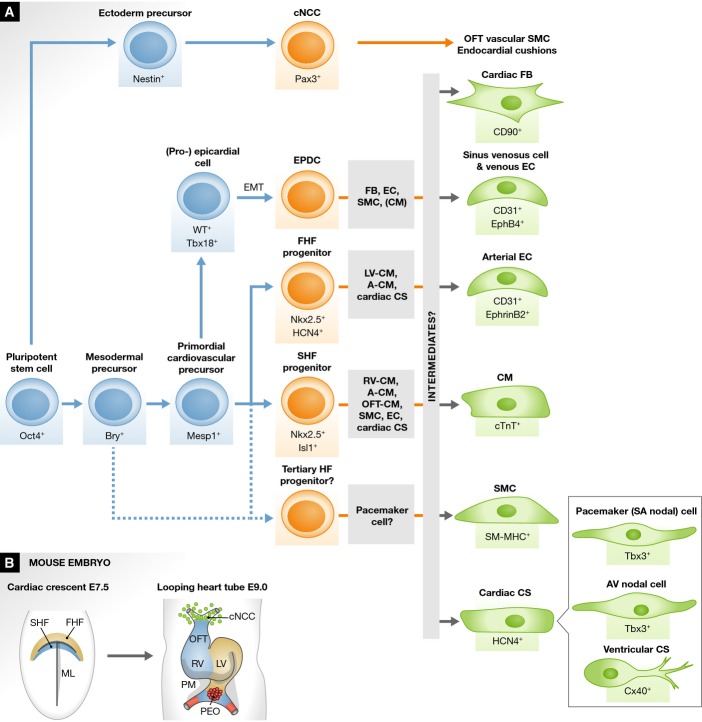
The human heart is a complex organ system and is composed of highly diverse cell types, including cardiomyocytes, conductive cells of the cardiac conduction system (CS), vascular smooth muscle cells (SMCs), and endothelial cells (ECs). All of these cells must be assembled into discrete anatomic and functional structures at the earliest embryonic stages (Hansson et al, 2009; Vincent & Buckingham, 2010). This assembly is a complicated and sequential morphogenetic process that depends on the spatiotemporally regulated contribution of multipotent CPCs (Buckingham et al, 2005; Moretti et al, 2006; Wu et al, 2006; Musunuru et al, 2010). The first differentiated myocardial cells are detected in the cardiac crescent in the splanchnic mesoderm at murine embryonic day (E) 7.5 (Fig1). The crescent region is referred to as the first heart field (FHF) and is marked by expression of a broad heart field marker gene, Nkx2-5 (Wu et al, 2006; Brade et al, 2013), and also by expression of the ion channel HCN4 (hyperpolarization-activated cyclic nucleotide-gated channel 4) (Liang et al, 2013; Spater et al, 2013). The crescent/FHF then fuses at the midline to form the primitive heart tube that begins to pump blood. The second heart field (SHF) is instead specifically marked by Isl1 expression (Cai et al, 2003) and lies medially and posteriorly to the crescent/FHF (Fig1). The SHF progenitors then migrate behind the heart tube and extend anteriorly and posteriorly into the pharyngeal mesoderm to form the looping heart tube at E9.0 in concert with the FHF progenitors (Laugwitz et al, 2005; Moretti et al, 2006; Nakano et al, 2008; Bu et al, 2009). As the embryo grows, FHF derivatives give rise to left ventricular myocardium, with partial contribution to the atria, whereas SHF derivatives contribute to myocardium of the right ventricle, parts of the atria, and the outflow tract, with some minor mutual contribution of FHF cells to the right ventricle and SHF cells to the left ventricle (Buckingham et al, 2005; Vincent & Buckingham, 2010). Lineage tracing experiments in vivo and clonal analyses in vitro demonstrated that the Isl1+ SHF progenitors can give rise to various cardiac lineages, including cardiomyocytes, conductive cells, vascular SMCs, and ECs (Moretti et al, 2006; Sun et al, 2007; Bu et al, 2009). In contrast, the HCN4+ FHF progenitors appear to be committed toward cardiomyocytes of the left ventricle and parts of the atria, and conductive cells of the atrio-ventricular (AV) node and ventricular CS (Liang et al, 2013; Spater et al, 2013). There is still controversy around the embryonic origin of pacemaker cells in the sino-atrial (SA) node. Interestingly, a recent study in chick embryo found that chick pacemaker cells arise from a discrete region outside the FHF/SHF, a so-called tertiary heart field (Fig1A) (Bressan et al, 2013).
Figure 1.
Fate map of cardiac cell lineages during development
(A) A cellular flow chart shows the stepwise commitment of pluripotent stem cells via various cardiac progenitor cells, including the first and second heart field (FHF and SHF) progenitors, epicardium-derived progenitor cells (EPDCs) and so-called tertiary heart field (HF) progenitors, and putative intermediates toward mature cardiac cell types during heart development. Mature cardiac cells include cardiomyocytes (CMs), vascular smooth muscle cells (SMCs), arterial and venous endothelial cells (ECs), fibroblasts (FBs), and conductive cells of the cardiac conduction system (CS), which include pacemaker (sino-atrial [SA] nodal) cells, atrio-ventricular (AV) nodal cells, and the ventricular CS cells (ex. Purkinje fibers). The gray boxes in the middle indicate the major mature cell types that each cardiac progenitor differentiates into. Cardiac neural crest cells (cNCCs) originating from the ectoderm also contribute to vascular SMCs of the outflow tract (OFT) and thereby to OFT separation and patterning. (B) Cardiac development at the early stage of the mouse embryo. Developing hearts at murine embryonic day (E) 7.5 and 9.0 are shown. A, atria; EMT, epithelial-to-mesenchymal transition; LV, left ventricle; ML, midline; PEO, proepicardial organ; PM, pharyngeal mesoderm; and RV, right ventricle.
The molecular cues that spatiotemporally regulate embryonic CPC populations and promote their differentiation into diverse cell types through putative intermediates are still under investigation (Fig1A) (Soh et al, 2014). Given that the embryonic FHF/SHF CPCs are multipotent, these CPCs are attractive therapeutic targets for cardiac regeneration (Domian et al, 2009). However, Isl1+ SHF progenitors are no longer present in the adult heart (Laugwitz et al, 2005) and in addition, there is no convincing evidence that they can be reactivated post-natally in situ to produce sufficient quantities of cardiomyocytes to repair the injured heart (Weinberger et al, 2012). In light of this, understanding the mechanisms that regulate CPC behavior during embryogenesis and identifying the specific signals that govern the transition between multipotent CPCs and fully differentiated cardiac cells is essential to establish novel therapeutic avenues for heart regeneration.
EPDCs and cNCCs
In addition to FHF and SHF CPCs, other cell populations also contribute to the formation of the heart. The proepicardial organ (PEO) is a transitory mesenchymal structure that forms near the posterior end of the heart tube at around E9.0 (Fig1B) and then develops into the epicardium, the outer layer of the heart (Manner et al, 2001; Schlueter & Brand, 2012). Some epicardial cells undergo epithelial-to-mesenchymal transition (EMT) and enter the heart as epicardium-derived progenitor cells (EPDCs), which contribute to SMCs, cardiac fibroblasts, and possibly to ECs of the coronary vasculature (Fig1A) (Christoffels et al, 2009; Katz et al, 2012). Whether the EPDCs can also contribute to myocardium is controversial. The PEO and epicardium are marked by expression of Wt1 and Tbx18 (Cai et al, 2008; Zhou et al, 2008a). Whereas previous fate-mapping studies in chick or mouse have shown no EPDC contribution to the myocardium (Winter & Gittenberger-de Groot, 2007), more recent reports using a Wt1-Cre or Tbx18-Cre conditional reporter mouse line suggest that EPDCs might contribute to a small population of cardiomyocytes (Cai et al, 2008; Zhou et al, 2008a). It should, however, be noted that Wt1 and Tbx18 expression may not be specific to the epicardium alone, thus making it difficult to unequivocally interpret the results of these fate-mapping experiments (Christoffels et al, 2009; Ruiz-Villalba et al, 2013). Nevertheless, the suggestion that embryonic EPDCs may contribute to a certain extent not only to the coronary SMCs/ECs but also to the myocardium is important. Unlike Isl1+ SHF progenitors, EPDCs are maintained throughout life in the adult heart and may potentially represent an endogenous source of newly formed cardiomyocytes following injury.
Cardiac neural crest cells (cNCCs) originate from the dorsal neural tube and migrate through the posterior pharyngeal arches to the arterial pole of the heart tube at around E9.5 (Fig1). cNCCs and their derivatives give rise to SMCs of the pharyngeal arch arteries and the outflow tract of the heart, contributing to septum and valve formation and thereby resulting in outflow tract separation and patterning into the pulmonary trunk and aorta (Hutson & Kirby, 2007; Hildreth et al, 2008).
Adult endogenous CPCs
Cardiac progenitor cells are usually defined as self-renewing, clonogenic, and multipotent cells that can differentiate into cardiomyocytes, SMCs, and ECs both in vitro and in vivo (Beltrami et al, 2003; Garbern & Lee, 2013; Sanganalmath & Bolli, 2013). To date, various kinds of putative endogenous CPCs have been isolated from adult rodent and human hearts, although the magnitude of their contribution to heart homeostasis and repair remains controversial (Chong et al, 2014a). The presence of the tyrosine kinase receptor c-kit is often used to identify CPCs (Beltrami et al, 2003; Bearzi et al, 2007). c-kit+ cardiac cells isolated from adult human heart and injected into infarcted rodent myocardium were shown to promote functional and structural cardiac improvement (Bearzi et al, 2007). However, whether endogenous c-kit+ cardiac cells can contribute to differentiated cardiomyocytes during aging or after injury in adulthood is highly debated (Bolli et al, 2011; Jesty et al, 2012; Ellison et al, 2013; Molkentin & Houser, 2013, 2014; Torella et al, 2014). In mice, the ability of these cells to give rise to cardiomyocytes is elevated shortly after birth, but decreases significantly over time and is virtually negligible in adult animals (Zaruba et al, 2010). A recent study using genetic lineage tracing experiments with a Kit-Cre conditional reporter mouse line showed that the generation of new cardiomyocytes from endogenous c-kit+ cells is a rare event (0.027%), even after cardiac injury, whereas c-kit+ cells amply contribute to cardiac ECs (van Berlo et al, 2014). This is consistent with previous reports showing that c-kit+ cardiac cells transplanted into injured rodent hearts are not likely to be the predominant source of newly formed cardiomyocytes, but instead promote cardiac proliferation/regeneration by secreting paracrine cytokines and growth factors (Tang et al, 2010; Loffredo et al, 2011). The cardiac-resident c-kit+ CPCs originally described by Beltrami et al could originate from extra-cardiac sources, as shown by the fact that 74% of c-kit+ cells found in the heart after myocardial infarction (MI) appear to be bone marrow derived (Fazel et al, 2006). As c-kit is broadly expressed in various cell types, including hematopoietic lineage cells (Smith et al, 2014), the use of this single marker to isolate CPCs from adult mammalian hearts is challenging and susceptible to contamination from non-CPC populations.
Aside from c-kit+ CPCs, other types of CPCs, such as Sca1+ cardiac cells (Oh et al, 2003; Matsuura et al, 2004), cardiospheres and cardiosphere-derived cells (Messina et al, 2004), and cardiac side population cells (Martin et al, 2004), have also been reported. Similar to c-kit+ CPCs, they are heterogeneous, and whether they can be a reproducible source of newly generated cardiomyocytes after cardiac injury remains controversial. Furthermore, clear evidence that they can have clinically beneficial effects on global heart function and cardiac repair is still lacking (Garbern & Lee, 2013; Sanganalmath & Bolli, 2013).
Essential signaling pathways and molecular drivers of cardiogenesis
Extracellular signaling pathways and their interaction with transcriptional regulators are tightly regulated during embryonic cardiogenesis and control patterning of embryonic CPCs, including the FHF/SHF progenitors. The major signaling cascades involved in cardiac muscle creation include canonical Wnts, the transforming growth factor (TGF)β superfamily such as bone morphogenetic proteins (BMPs) and Activin/Nodal, fibroblast growth factors (FGFs), non-canonical Wnts, and Hedgehog and Notch pathways, all of which function sequentially and cooperatively (Fig2). A complex network of these signaling pathways and transcriptional regulators controls cardiac progenitor specification, proliferation, and differentiation into diverse cardiac cell lineages, ultimately giving rise to the entire heart, as further reviewed elsewhere (Vincent & Buckingham, 2010; Noseda et al, 2011).
Figure 2.
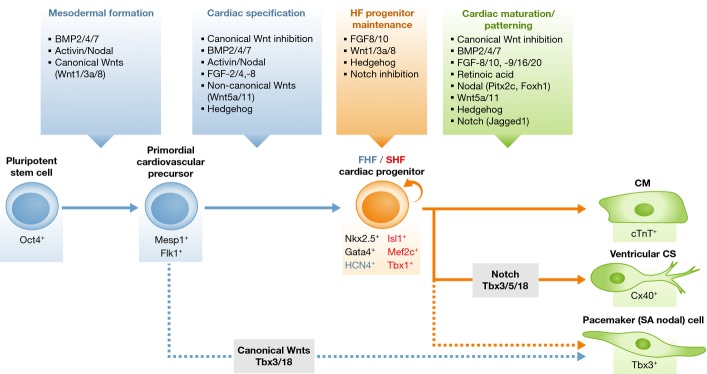
Signaling pathways programming a heart cell during embryogenesis
The flow chart shows the representative signaling pathways and/or modulators working in a stepwise manner at various stages of cardiac development/cardiac cell differentiation, including mesodermal formation, cardiac specification, maintenance of heart field (HF) progenitors, and cardiac maturation and patterning (see text for details). A circular arrow denotes self-renewal. First heart field (FHF) marker gene is in blue, and second heart field (SHF) marker genes in red. CM, cardiomyocyte; CS, conduction system; and SA, sino-atrial.
Mesodermal formation and cardiac specification
Bone morphogenetic protein signaling is necessary for gastrulation and primitive mesoderm formation in mammals. Germline deletion of Bmp4 or the BMP type I receptor (Bmpr1a) causes embryonic death before E9.5 (Mishina et al, 1995; Winnier et al, 1995). Abnormal cardiac morphogenesis occurs in mice upon conditional deletion of Bmp4 using Tnnt2-Cre or Nkx2-5-Cre lines (Jiao et al, 2003; Liu et al, 2004; Jayawardena et al, 2012). When conditional deletion of Bmpr1a was introduced with a Mesp1-Cre allele, the cardiac crescent (FHF) was not formed, and the FHF markers Hand1 and Tbx5 were also absent (Klaus et al, 2007). This indicated an indispensable role of BMP signaling for cardiac specification in the mammalian heart.
Activin and Nodal belong to the TGFβ superfamily (Kitisin et al, 2007). This signaling pathway is essential in early mouse embryos for positional patterning, gastrulation, primitive streak, and mesoderm/endoderm formation, and later for cardiac myogenesis (Conlon et al, 1994; Schier, 2003). Activin A, in concert with BMPs, has been shown to successfully induce cardiac myogenesis in mouse and human embryonic stem cells (ESCs) and in human inducible pluripotent stem cells (iPSCs) (Burridge et al, 2007; Takahashi et al, 2007; Flaim et al, 2008).
The “canonical” Wingless-Int (Wnt) ligands include Wnt1, -2a, -3a, and -8, which require β-catenin for signaling translation into nuclei (Clevers, 2006; Gordon & Nusse, 2006). Before gastrulation, canonical Wnt/β-catenin signals are involved in primitive streak formation and the induction of primitive mesoderm and endoderm (Rivera-Perez & Magnuson, 2005; Barrow et al, 2007). However, after gastrulation, these signals are inhibited by a secreted Frizzled-related protein (sFRP) and Dickkopf1 (Dkk1), which are produced in the adjacent endoderm and are essential for further cardiac specification in the mesoderm (Foley & Mercola, 2005; Mii & Taira, 2009). This biphasic effect of canonical Wnt/β-catenin signals has been recapitulated in cultured mouse ESCs and human ESCs/iPSCs, collectively referred to as pluripotent stem cells (PSCs). The Wnt/β-catenin pathway is necessary for mesoderm and endoderm formation from mouse/human PSCs, cultured with Wnt3A-conditioned medium or the inhibitor of glycogen synthase kinase (GSK) 3β that phosphorylates and degrades β-catenin, yet the same signaling pathway inhibits cardiac myogenesis once mesoderm has been created (Lindsley et al, 2006; Naito et al, 2006; Ueno et al, 2007; Yoshida & Yamanaka, 2011).
The FGF pathway involves approximately 20 ligands and 4 transmembrane receptor tyrosine kinases (FGFRs) (Itoh & Ornitz, 2004; Turner & Grose, 2010). Studies using hypomorphic alleles or conditional deletion of Fgf8 with a Tbx1-Cre allele demonstrated impaired outflow tract aligning and septation, indicating that mesodermal Fgf8 expression is crucial for SHF development (Frank et al, 2002; Ilagan et al, 2006). At the cellular level, Fgf8 regulates expression of the SHF marker genes Isl1 and its target Mef2c (Park et al, 2006), leading to proliferation of the SHF progenitor population. Fgf10 is also expressed in the SHF (Marguerie et al, 2006). In human ESCs, FGF2, in combination with Activin and BMP4, is known to specifically promote mesoderm-committed precursor formation (Evseenko et al, 2010).
HF progenitor maintenance and cardiac cell maturation/patterning
Canonical Wnt/β-catenin signaling also plays important roles at later stages of embryonic cardiogenesis, in proliferation/maintenance of SHF progenitors and prevention of their differentiation (Cohen et al, 2008). Wnt/β-catenin signaling is activated in the SHF, and β-catenin can directly enhance expression of the SHF transcription factors Isl1 and Fgf10 (Cohen et al, 2007; Lin et al, 2007). Conditional deletion in the SHF of the β-catenin gene by using an Isl1-Cre or Mef2c-Cre driver mouse line causes right ventricular and outflow tract hypoplasia, probably due to impaired SHF proliferation. Conversely, stable expression of β-catenin in the Isl1+ or Mef2c+ SHF progenitor population leads to right ventricular enlargement and hyperplasia (Ai et al, 2007; Kwon et al, 2007; Lin et al, 2007; Qyang et al, 2007). Of interest, canonical Wnt signaling blocks differentiation of SHF progenitors. Isl1+ cells in which β-catenin is specifically stabilized down-regulate the gene encoding Myocardin, which promotes myocardial and smooth muscle differentiation in concert with serum response factor (SRF) (Evans et al, 2010). Consequently, maintenance of Isl1+ cells in the outflow tract causes a delay in cardiac differentiation (Kwon et al, 2009).
Bone morphogenetic protein signaling promotes cardiac specification and myocardial differentiation (Tirosh-Finkel et al, 2006). Conditional deletion of Bmpr1a in Isl1+ SHF progenitors at late embryonic stages causes right ventricle and outflow tract hypoplasia with increased numbers of Isl1+ cells, indicating failure of the SHF progenitors to differentiate (Yang et al, 2006; Klaus et al, 2007). BMP signaling, mainly through BMP2, BMP4, and BMP7, is hence likely to affect myocardium maturation. FGF signaling also affects the myocardium maturation step. FGF9 and its relatives FGF16 and FGF20 are expressed in both endocardium and epicardium at mid-gestation and contribute to myocardial proliferation (Lavine et al, 2005). Conditional deletion of the Fgfr1 and Fgfr2 genes with the ventricle-specific driver Mlc2v-Cre causes severe ventricular defects (Lavine et al, 2005).
Patterning of the SHF along the anterior/posterior axis is regulated by retinoic acid (RA) signaling (Sirbu et al, 2008). RA, a biologically active derivative of vitamin A, is produced by retinaldehyde dehydrogenase (Raldh) 2, and in the Raldh2-mutant mouse embryos, the anterior SHF marker genes such as Tbx1 and Fgf8/10 show abnormal expression patterns, which expand posteriorly (Ryckebusch et al, 2008). In mice, RA and its receptors are essential for normal cardiac morphogenesis, with atrial development being more affected by loss of Raldh2 than ventricular development (Niederreither et al, 2001).
Hedgehog ligands bind to patched 12-span transporter-like receptors that inhibit the function of Smoothened (Smo) serpentine receptors in the absence of ligands (Wilson & Chuang, 2010). In zebrafish, Hedgehog signaling has been shown to promote cardiomyocyte formation (Thomas et al, 2008), whereas in mice, it is involved in the establishment of left/right asymmetry, coronary vasculature, atrial septation, and outflow tract morphogenesis (Kolesova et al, 2008; Lavine et al, 2008; Hoffmann et al, 2009). Hedgehog signals have been shown to be crucial for normal induction of Nkx2-5 or its equivalent in both zebrafish and mice (Zhang et al, 2001).
Non-canonical Wnt signaling (Wnt5a and Wnt11) is associated with cardiac specification and differentiation (Pandur et al, 2002; Palpant et al, 2007). Wnt5a- or Wnt11-null mice have pharyngeal artery patterning and outflow tract defects. However, expression of Isl1 and other SHF markers, such as Mef2c, is normal, suggesting that Wnt5a and Wnt11 control outflow tract maturation by affecting the cNCCs, but not the SHF (Schleiffarth et al, 2007; Zhou et al, 2007).
Notch signaling is associated with a wide range of developmental processes, including cell fate decisions in various cell types (Andersson et al, 2011). During embryonic cardiogenesis, Notch signaling affects both the SHF and the cNCCs, hence controlling right ventricle and outflow tract formation, vascular smooth muscle development, chamber specification, and trabeculation (McCright et al, 2001; High et al, 2007, 2008; Xin et al, 2007; Varadkar et al, 2008). SHF-specific deletion of Notch1 with an Isl1-Cre line promoted proliferation of Isl1+ progenitors and caused over-expression of β-catenin in the SHF, resulting in defects of the arterial pole including the right ventricle (Cohen et al, 2007). Thus, Notch signaling interferes with canonical Wnt/β-catenin signaling in the SHF, thereby inhibiting proliferation of SHF progenitors and promoting their differentiation.
The Epstein group recently showed that forced expression of Notch signaling in vitro can reprogram neonatal murine cardiomyocytes to display a conduction-like phenotype, including action potential characteristics (Rentschler et al, 2012). This suggests that Notch signaling, similar to T-box transcription factor Tbx3, Tbx5, and Tbx18 (Hoogaars et al, 2007; Bakker et al, 2008; Wiese et al, 2009), plays an important role in the specification of cardiac conductive cells (Fig2). Recent reports also showed that forced expression of Tbx18 or Tbx3 could reprogram mature ventricular cardiomyocytes to a pacemaker-like phenotype in vitro and in vivo (Bakker et al, 2012; Kapoor et al, 2013). The Mikawa group reported that the fate of pacemaker cells, derived from the “tertiary” heart field in the chick embryo, is controlled by canonical Wnt signaling at early stages (Fig2) (Bressan et al, 2013).
Finally, chromatin remodeling has also been shown to be a key event in establishing the cardiomyogenic program. The Bruneau group demonstrated that a cardiac-specific subunit of the chromatin remodeling complex Baf60c (BRG1-associated factor 60c), in combination with the cardiac transcription factors Gata4 and Tbx5, can ectopically trans-differentiate mouse mesoderm into beating cardiomyocytes (Takeuchi & Bruneau, 2009).
Heart regeneration/replication capabilities
The long-standing concept that human heart cells exit the cell cycle after birth and are unable to renew themselves with aging or after injury has been drastically overturned by a growing body of contradictory evidence recently reported (Garbern & Lee, 2013), although the estimated rate of mammalian cardiomyocyte renewal is remarkably low, even in the injured heart (Bergmann et al, 2009; Kajstura et al, 2010; Mollova et al, 2013; Senyo et al, 2013). This indicates that the inherent capacity of the mammalian heart to replenish damaged myocardium is far from being sufficient to exploit in a clinical setting. In this section, we discuss recent knowledge and advances in cardiac regeneration/replication in various model organisms, including lower vertebrates (zebrafish and amphibians), and consider their relevance for cardiac regenerative medicine (Fig3).
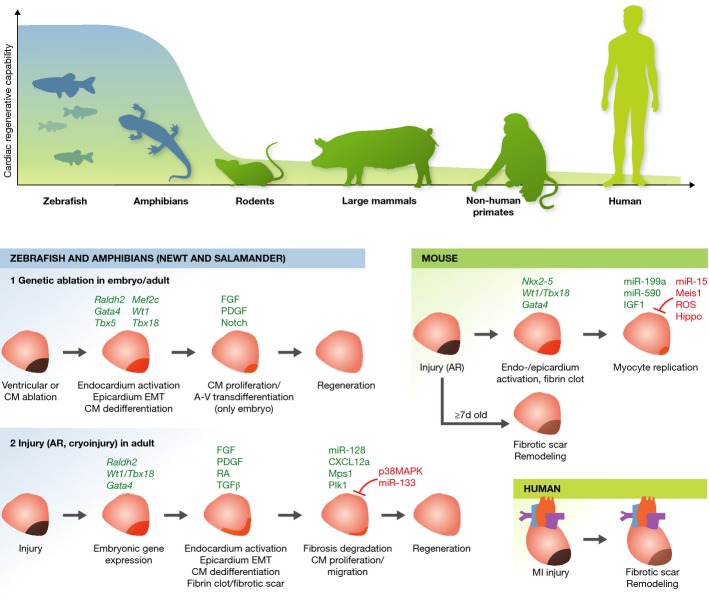
Figure 3.
Heart regeneration in various model organisms
(Top) Schematic representation indicates cardiac regenerative capabilities of various model organisms from lower vertebrates (zebrafish and amphibians) to human. (Bottom) Heart regenerative/replicative processes following injury in zebrafish and amphibians, neonatal/adult mice, and humans are shown. In zebrafish, various injury models (genetic ablation, apical resection (AR), and cryoinjury) are reported and exhibit different recovery processes, especially in terms of transient fibrotic scar formation. All ultimately lead to full regeneration of the ablated myocardium. Similar post-injury regenerative processes are seen in hearts of 1-day-old mice, but not after 7 days nor in humans, where fibrotic scar formation and pathological remodeling occur. Regeneration enhancers are indicated in green, inhibitors in red (see text for details). A-V, atrial-to-ventricular; CM, cardiomyocyte; EMT, epithelial-to-mesenchymal transition; MI, myocardial infarction.
Cardiac regeneration in zebrafish and amphibians
Urodele amphibians, such as salamanders and newts, have a remarkable capacity to regenerate injured tissues, including the heart (Brockes & Kumar, 2005; Roy & Gatien, 2008). Early studies showed that newts could survive after resection of a significant portion (up to 50%) of apical myocardium and that cardiomyocyte regeneration was evident 30 days after injury (Becker et al, 1974; Oberpriller & Oberpriller, 1974; Oberpriller et al, 1988). Furthermore, recent studies showed that newts are able to fully regenerate cardiac tissue within 60 days after amputation of 10–25% of apical myocardium (Witman et al, 2011; Mercer et al, 2013). Following the initial response to injury, which includes blood/fibrin clot formation, macrophage and lymphocyte infiltration, and deposition of extracellular matrix, DNA synthesis is detected within cardiomyocytes at the injury site at day 16 after injury. Although subject of debate, recent evidence suggests that urodele heart regeneration likely occurs via partial dedifferentiation of mature cardiomyocytes into progenitor-like cells (Laube et al, 2006). How urodele cardiomyocytes reenter the cell cycle and regenerate cardiac tissue following injury remains unclear. In this regard, FGFs, platelet-derived growth factors (PDGFs), thrombin, BMP signaling, and miRNAs such as miR-128 have all been proposed to be involved (Singh et al, 2010; Witman et al, 2013). Unlike mammalian cardiomyocytes, which are mostly multinucleated and/or polyploid (4n), 98% of uninjured urodele cardiomyocytes are mononucleated and diploid, and this may contribute to their inherent regenerative capacity (Neff et al, 1996).
Decades after the early urodele amphibian studies, similar observations were made in zebrafish. The adult zebrafish heart can regenerate completely within 60 days after resection of up to 20% of apical myocardium (Poss et al, 2002; Raya et al, 2003). Other types of injury were also employed, such as genetic ablation (Wang et al, 2011) and cardiac cryoinjury (Chablais et al, 2011; Gonzalez-Rosa et al, 2011), to evaluate whether the same regenerative responses appeared (Fig3). Genetic ablation is based on conditional expression of the cytotoxic diphtheria toxin A gene under the control of tamoxifen-inducible Cre recombinase driven by the promotor for the contractile gene cardiac myosin light chain-2 (cmlc2), which allows to specifically ablate up to 60% of cardiomyocytes (Wang et al, 2011). For cardiac cryoinjury, a nitrogen-cooled probe is used to damage 20–30% of the ventricular myocardium, together with endocardium and epicardium. As a first response, all of these injury models induce the reactivation of genes expressed during embryonic heart development, such as Gata4, Nkx2-5, Raldh2, Wt1, and Tbx18, followed by activation of endocardium and epicardium, including EMT of epicardial cells (Kikuchi & Poss, 2012). While genetic ablation of cardiomyocytes does not induce deposition of collagen matrix but only formation of a blood/fibrin clot after amputation, the cryoinjury model produces massive, but transient, scar-like fibrosis around the injured area. Scar formation is mediated by the TGF-β/Activin signaling pathway and might be necessary for heart regeneration in this model, as, unlike the fibrotic scar that forms in injured mammalian hearts, it is later degraded (Fig3) (Chablais & Jazwinska, 2012). Following scar formation and/or EMT of epicardial cells, which causes FGF- and PDGF-driven revascularization into the myocardium (Lepilina et al, 2006; Kim et al, 2010), proliferating cardiomyocytes appear around the injured sites. Several paracrine signals, including RA, synthesized by the epicardium and endocardium, and C-X-C motif chemokine 12a (CXCL12a, also referred to as stromal cell-derived factor 1 [SDF-1]), expressed in epicardial cells, have been suggested to promote cardiomyocyte proliferation (Kikuchi et al, 2011; Gonzalez-Rosa & Mercader, 2012; Itou et al, 2012b). In summary, although the injury models differ in the recovery process in regard to transient fibrotic tissue accumulation and time span required to obtain complete heart regeneration, ranging from 30 days in the genetic ablation model to 60 days in the apical resection model to 130 days in the cryoinjury model (Poss et al, 2002; Schnabel et al, 2011; Wang et al, 2011), all of them ultimately lead to full regeneration of the ablated myocardium (Fig3).
Importantly, zebrafish regenerative potential does not decrease with age (Itou et al, 2012a). The source of post-injury regenerated cardiomyocytes is a subject of debate. Genetic fate-mapping approaches, using a zebrafish strain in which tamoxifen-inducible Cre is driven by cmlc2, revealed that regenerated cardiomyocytes derive from preexisting mature cardiomyocytes that undergo partial dedifferentiation, as shown by disassembly of their sarcomeric structure and expression of Gata4, a regulator of embryonic heart development, and thereafter re-enter the cell cycle (Jopling et al, 2010; Kikuchi et al, 2010). Although the mechanisms through which cardiomyocytes dedifferentiate and then proliferate following injury have not been fully determined, recent studies suggest that mitotic checkpoint kinase Mps1 and polo-like kinase 1 (Plk1) positively regulate heart regeneration in zebrafish, whereas cardiomyocyte proliferation is inhibited by miR-133 and p38 mitogen-activated protein kinase (MAPK) (Jopling et al, 2010, 2012; Yin et al, 2012). Of note, a recent report showed that an in vivo cardiac reprogramming event, the atrial-to-ventricular cardiomyocyte trans-differentiation, contributes to heart regeneration in zebrafish embryos, but not in adults (Zhang et al, 2013). The authors used a ventricle-specific genetic ablation system, in which metronidazole was administered to ablate transgenic ventricular cardiomyocytes expressing nitroreductase driven by the ventricular myosin heavy chain (vmhc) promoter. Ventricle-specific ablation of cardiomyocytes was performed 3–4 days post-fertilization, when the zebrafish heart has completed cardiac looping and cardiac chamber cardiomyocytes have fully differentiated (de Pater et al, 2009). Re-expression of key cardiogenic transcription factors, such as Gata4, Nkx2-5, Hand2, Tbx5/20, and Mef2c, occurs in injured hearts, and atrial cardiomyocytes adjacent to the atrio-ventricular canal dedifferentiate into intermediate reprogramming stages and then trans-differentiate into ventricular cardiomyocytes, thereby contributing to ventricular regeneration. This trans-differentiation capacity was shown to be age dependent and partly mediated by Notch signaling activation in the atrial endocardium following ventricular ablation (Fig3) (Zhang et al, 2013).
Mammalian cardiomyocyte turnover
There is now agreement that cardiomyocyte turnover does occur throughout life in mammals, including humans, although this turnover capacity is considerably limited (Bergmann et al, 2009; Kajstura et al, 2010; Mollova et al, 2013; Senyo et al, 2013). In a groundbreaking study, the Frisén group determined the birth date of cardiomyocytes in humans by measuring nuclear carbon-14 (14C) content with accelerator mass spectrometry (Bergmann et al, 2009). They showed that new cardiomyocytes form in the human heart at a rate of around 1.5% per year at 25 years of age and that this turnover rate declines with age, and concluded that approximately 50% of human cardiomyocytes are replaced during an entire life span. Their data are consistent with those of newer studies in both mouse (Malliaras et al, 2013; Senyo et al, 2013) and human (Mollova et al, 2013). Using cardiomyocyte-specific fluorescent reporter mouse lines and multi-isotope imaging mass spectrometry, which monitors DNA synthesis at high resolution using the rare stable isotope of nitrogen (15N), the Lee group showed that in a healthy mouse during normal aging (≥10 weeks old), the annual birth rate of cardiomyocytes is 0.76%, whereas 8 weeks after MI, roughly 3.2% of the cardiomyocytes adjacent to the infarct undergo cell division (Senyo et al, 2013). Although there is some variation among different reports (Laflamme & Murry, 2011), the estimated rate of mammalian cardiomyocyte turnover is approximately 1% per year, which increases modestly in response to injury, but declines with aging.
Multiple sources have been proposed to explain the origin of newly generated cardiomyocytes during both normal homeostasis and repair, including preexisting mature cardiomyocytes and quiescent CPCs (Garbern & Lee, 2013). Recent studies suggest that at least during normal homeostasis, preexisting cardiomyocytes that undergo dedifferentiation followed by proliferation might be the predominant source of newly formed cardiomyocytes (Mollova et al, 2013). However, CPCs may also participate in cardiomyocyte generation following injury (Porrello et al, 2011; Senyo et al, 2013). Importantly though, these two scenarios are not mutually exclusive, and both constitute possible avenues for increasing de novo cardiomyocyte generation for cardiac regenerative medicine.
Mammalian heart regenerative/proliferative response to injury
In mammals, unlike zebrafish and amphibians, cardiac injury such as MI induces permanent cardiomyocyte cell death and the formation of an irreversible fibrotic scar. This leads to electrical uncoupling to the remaining myocardium, causing arrhythmias, unfavorable remodeling of ventricular walls, reduction of ventricular function, and finally heart failure (Fig3) (Hasenfuss, 1998). Challenging this dogma, recent evidence suggests that similar to zebrafish and amphibian hearts, the 1-day-old neonatal mouse heart can regenerate completely 21 days after resection of approximately 15% of apical ventricular tissue (Porrello et al, 2011; Strungs et al, 2013; Naqvi et al, 2014). This regenerative capacity of the mouse heart is rapidly lost by 7 days after birth, when the injured heart develops fibrotic scars instead, as seen in adult mice and humans (Porrello et al, 2011; Mahmoud et al, 2013). A newer study, however, showed that in 1-day-old neonatal mice undergoing apical resection, the regeneration process is incomplete and accompanied by fibrotic scar formation, thereby questioning the cardiac regenerative capacity of the neonatal mouse (Andersen et al, 2014). Nevertheless, these experiments clearly show the highly activated regenerative capacity of the 1-day-old neonatal mouse heart, the extent of which diminishes rapidly after the first few days of life, suggesting that key programs/mechanisms regulating inherent regeneration must exist in the first week of life in mammals (Kotlikoff et al, 2014). Of interest, another recent study showed that during preadolescence (at post-natal day 15 in mice), a transient burst of cardiomyocyte proliferation occurs, with an increase in cardiomyocyte numbers by around 40%. Proliferation seems to be driven by a surge in the levels of thyroid hormones, which appear to activate the IGF-1/IGF-1-R/Akt pathway, although the causal relationship between thyroid hormones, IGF signaling, and this proliferative burst was not fully clarified (Naqvi et al, 2014). This indicates that, to a certain extent, mammalian cardiomyocyte proliferative capacities may persist beyond the perinatal period.
Why can the adult mammalian heart not regenerate? To understand the rapid regenerative loss upon birth and identify the mechanisms involved, multiple hypotheses are currently being investigated (Fig3). miRNAs, Hippo signaling, oxidative stress, and the transcription factor Meis1 have recently attracted attention in this regard and are described in detail below (see, “Cell-free therapies” section). In addition, cardiac regeneration following injury in the neonatal mouse is preceded by stronger activation of Wt1 and Tbx18 expression than observed in the adult, indicating that the enhanced epicardial response might play an important role in heart regeneration (Smart et al, 2011). A role of the epicardium as source of paracrine signals, including vascular endothelial growth factor-A (VEGF-A), SDF-1, and FGFs, is supported by experiments showing the efficacy of transplanted EPDCs or administered EPDC supernatant in promoting regeneration in injured mouse hearts (Winter et al, 2007; Zhou et al, 2011).
Therapeutic strategies for human cardiac regeneration
It has been estimated that after MI, a patient loses on average around one billion cardiomyocytes (Laflamme & Murry, 2005)—a massive amount that the human body cannot replace on its own, given the extremely low cardiomyocyte turnover rate (as discussed in the previous section). There are multiple different approaches to promote cardiomyocyte regeneration/proliferation in human injured hearts, including transplantation of autologous non-cardiac/cardiac somatic stem cells, injection of in vitro-derived cardiomyocytes, direct lineage conversion (“reprogramming”) of cardiac fibroblasts into cardiomyocytes in vivo, stimulation of dedifferentiation/proliferation of preexisting cardiomyocytes, and activation of endogenous CPC populations (Fig4). These therapeutic strategies, classified as either cell-based or cell-free, are currently being investigated for their cardiac regenerative potential and feasibility of clinical application (Vunjak-Novakovic et al, 2011). Here, we review recent advances in both groups of therapies.
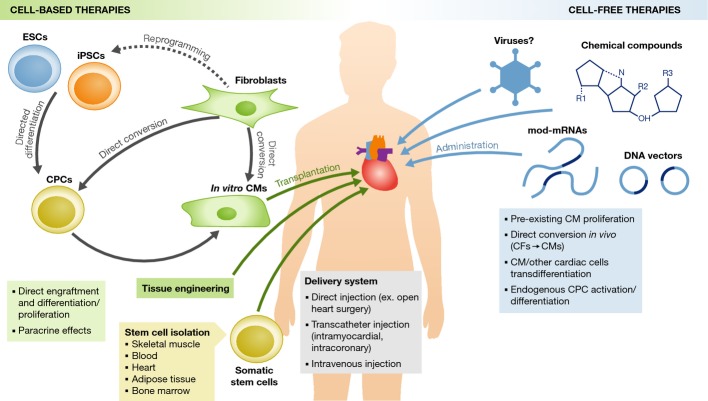
Figure 4.
Therapeutic strategies for cardiac regeneration
(Left) Cell-based therapies involve transplantation into the damaged heart of in vitro-derived cardiomyocytes (CMs) or somatic stem cells. CM differentiation can be induced from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) obtained by reprogramming differentiated somatic cells, such as fibroblasts. Alternatively, by forced expression of cardiac-specific factors, the pluripotent state can be bypassed and fibroblasts directly converted into CMs or cardiac progenitor cells (CPCs). Different types of cardiac and non-cardiac somatic stem cells can also be transplanted. Cell-based therapies can produce beneficial effects on heart function directly, by engrafting into the host tissue and differentiating/proliferating in vivo, or indirectly via paracrine effects that act on host cells. (Right) Cell-free therapies involve administration of chemical compounds or genes (via viral vectors, non-viral DNA vectors, or modified mRNAs) that act on host cells to stimulate cardiac regeneration. Mechanisms of action include the stimulation of proliferation of preexisting CMs, direct conversion of cardiac fibroblasts (CFs) into CMs, trans-differentiation of CMs or other cardiac cells from one cellular subtype to another, and activation and differentiation of endogenous CPCs. Cardiac delivery routes for both cell-based and cell-free therapeutic agents are also indicated.
Cell-based therapies
Cell-based therapies involve transplantation into the injured heart of cells that have the ability to repopulate the damaged myocardium and integrate functionally with preexisting tissue, ultimately restoring normal cardiac activity. Two types of cells can be employed: (i) in vitro-derived cardiomyocytes, obtained via PSC differentiation or via direct conversion of terminally differentiated somatic cells, or (ii) adult stem/progenitor cells, which can differentiate into cardiomyocytes in vivo (Fig4, left). In both cases, cells can be first expanded in vitro so that large amounts of starting material are readily available for manipulation and transplantation.
Directed cardiomyocyte differentiation from pluripotent stem cells
The first way to derive cardiomyocytes for transplantation purposes is through directed differentiation from PSCs, such as ESCs. Alternatively, cardiomyocytes can be obtained from terminally differentiated non-cardiac somatic cells, provided that they are first converted into iPSCs via reprogramming (Takahashi & Yamanaka, 2006). Compared to ESCs, iPSCs have a critically important advantage: They can be derived from the somatic cells of any patient, thus circumventing graft rejection problems often associated with non-autologous cell transplants. A multitude of cardiomyocyte differentiation protocols have been developed over the years. Since their aim is to recapitulate embryonic development in a dish, protocol optimization requires a detailed understanding of the key signaling pathways that orchestrate heart development in vivo (Fig2). Cardiomyogenic differentiation methods generally employ one of two alternative techniques, depending on whether the PSCs are cultured in three-dimensional aggregates, termed embryoid bodies (EBs), or in monolayer format.
In one of the first efforts to derive cardiomyocytes in vitro, spheres of human ESCs were generated in suspension cultures and allowed to spontaneously recapitulate early embryonic development (Kehat et al, 2001). The process, however, was extremely inefficient, with spontaneously contracting areas appearing in only 8% of EBs. It was clear that the timed addition of extracellular molecules acting on specific cardiogenic signaling pathways was going to be needed to improve the efficiency of differentiation. Key drivers of in vivo cardiogenesis have been described above and include Activin/Nodal-, BMP-, FGF-, and Wnt-mediated signaling cascades. The same pathways also play pivotal roles in promoting cardiomyogenic differentiation from PSCs. More than 30% cardiomyocytes can be obtained from human ESCs by exposure to Activin A and BMP4 (Laflamme et al, 2007). However, optimal levels of Activin, Nodal, and BMP signaling are required for cardiac lineage formation from different human ESC and iPSC lines (Kattman et al, 2011). Combinations of Activin A, BMP4, basic FGF (bFGF), VEGF-A, and Dkk1 have also been used to generate cardiovascular progenitors from human ESCs. This progenitor population, identified by low kinase insert domain receptor (KDR) expression and absence of c-kit, was able to give rise to more than 50% contracting cardiomyocytes (Yang et al, 2008). More recently, dual Nodal and BMP inhibition by antagonist ligand Cerberus-1 (Cer1) was shown to drive cardiomyocyte differentiation from both mouse and human ESCs (Cai et al, 2013).
Unlike EB differentiation, monolayer differentiation protocols involve culturing PSCs in standard two-dimensional format. Sequential treatment of high-density monolayer ESC cultures with Activin A and BMP4 was reported to yield more than 30% cardiomyocytes (Laflamme et al, 2007; Melkoumian et al, 2010), although not consistently. A different study in fact noted that the Activin A-/BMP4-directed differentiation protocol is not always successful and can sometimes yield less than 5–10% cardiomyocytes (Paige et al, 2010). The authors went on to show that efficient mesoderm induction and subsequent cardiac differentiation from human ESCs require fine-tuning of the cross talk between Activin A/BMP4 and Wnt/β-catenin signaling pathways (Paige et al, 2010). Recently, a robust cardiomyocyte differentiation protocol has been developed: By culturing pluripotent cells as monolayers and manipulating canonical Wnt signaling, around 80% cardiomyocytes can be reproducibly obtained from different human ESC lines (Lian et al, 2012, 2013).
Cardiomyocyte enrichment protocols have also been described that are able to yield, independently of the efficiency of differentiation, up to 99% pure PSC-derived cardiomyocytes. Different strategies have been used so far for such enrichment steps, including the use of mitochondria-specific fluorescent dyes that preferentially bind to cells with high mitochondrial content such as cardiomyocytes (Hattori et al, 2010), cell sorting with an antibody against the cardiomyocyte-specific marker SIRPA (signal-regulatory protein alpha) (Fujioka et al, 1996; Kharitonenkov et al, 1997; Dubois et al, 2011), and a biochemical purification method based on differences in sugar metabolism between cardiomyocytes and non-cardiomyocytes (Tohyama et al, 2013).
To date, there is no clinical test of human PSC-derived cardiomyocyte transplantation into human patients, but the first clinical-scale transplantation of in vitro-derived cardiomyocytes into a non-human primate (monkey) has been reported very recently (Chong et al, 2014b). One billion human ESC-derived cardiomyocytes were produced via the Activin A- and BMP4-mediated monolayer differentiation protocol (Laflamme et al, 2007) and delivered intra-myocardially into the infarcted heart of a non-human primate model. After transplantation, the authors observed remuscularization of the damaged monkey heart, with formation of new muscle grafts that were electromechanically coupled to the host cardiomyocytes and rapidly perfused by the host vasculature (Chong et al, 2014b). However, the transplanted cells appeared quite diverse in terms of atrial–ventricular electrophysiological properties and were only partially mature, bearing more resemblance to fetal rather than adult cardiomyocytes. As a consequence, non-lethal ventricular arrhythmias were observed in the recipient monkeys. Other concerns, regarding the small number of animals studied, insufficient analyses of cardiac mechanics, and failure to provide evidence that transplanted cardiomyocytes improve cardiac function, have been raised and are discussed elsewhere (Anderson et al, 2014; Murry et al, 2014). While this study sheds hope on the possibility of remuscularizing a damaged human heart with a similar approach (Lian et al, 2014), it also highlights some of the crucial issues we need to address before in vitro-derived cardiomyocyte transplantation therapies can truly move into the clinical setting, as discussed below.
Direct conversion of differentiated somatic cells into cardiomyocytes
In recent years, an alternative method to produce cardiomyocytes in vitro has been developed. It is often referred to as direct conversion, because it involves a cell fate switch from a fully differentiated cell type into another, without going through the pluripotent state (Sancho-Martinez et al, 2012). In analogy to conventional reprogramming, in which somatic cells are converted into pluripotent ones by overexpressing pluripotency-associated transcription factors (Takahashi & Yamanaka, 2006), direct conversion is achieved by forcing expression of key lineage-specific factors. The first experiment of this kind was performed almost 30 years ago, when overexpression of a single gene, the myogenic transcription factor MyoD, was shown to be sufficient to convert fibroblasts into skeletal muscle cells (Davis et al, 1987). Similarly, overexpression of the smooth muscle coactivator myocardin (Myocd) can force fibroblasts into adopting a smooth muscle cell fate (Wang et al, 2003). Successful direct lineage conversions have since been reported for a plethora of cell types, including the hematopoietic system (Xie et al, 2004; Laiosa et al, 2006; Szabo et al, 2010), pancreatic exocrine cells (Zhou et al, 2008b), the hepatic system (Huang et al, 2011; Sekiya & Suzuki, 2011), and neuronal lineages (Vierbuchen et al, 2010; Caiazzo et al, 2011). Unfortunately, no single “master regulator” of cardiomyocyte development has been identified to date, but lessons from both iPSC generation and direct conversions in other systems suggest that combinations of specific factors can alter the gene expression profile of the donor cell and induce its conversion into cardiac cell types.
The therapeutic implications of being able to produce cardiomyocytes via direct conversion, rather than via PSC differentiation, are multiple. Firstly, the ability to bypass the pluripotent state may reduce the potential risk of tumorigenesis after transplantation. Secondly, similar to iPSC differentiation, immunologically matched tissue can be produced from a patient's own cells to circumvent graft rejection. Finally, the ability to directly convert non-cardiomyocytes into cardiomyocytes in vitro offers the enticing possibility to do the same in vivo and reprogram resident cardiac fibroblasts by introducing defined factors directly into the patient's heart.
A variety of recipes designed to steer fibroblast cells into a cardiomyogenic fate have been published so far, each employing a unique combination of cardiac-specific transcription factors, miRNAs, and/or chemical molecules (Table1). The first study started out by testing fourteen different factors for their ability to induce a cardiomyocyte-like phenotype from mouse post-natal fibroblasts. Three were deemed sufficient for reprogramming: Gata4, Mef2c, and Tbx5, hereafter referred to as GMT factors (Ieda et al, 2010). The trans-differentiation process was found to be direct, with no transition through a multipotent cardiac progenitor-like state. Successful conversion into the cardiomyocyte lineage was judged by up-regulation of a cardiac-specific reporter gene, which occurred in up to 25% of transfected cells. Differentiation into cardiomyocyte-like cells was also observed when transduced fibroblasts were transplanted into immunocompromised mouse hearts 1 day after introduction of GMT factors. However, only 1% of induced cardiomyocytes (iCMs) displayed spontaneous contractions in vitro, suggesting overall inefficient conversion into fully mature, functional cardiomyocytes. Two additional studies later examined the utility of GMT factors for cardiac reprogramming (Chen et al, 2012; Inagawa et al, 2012). In one of them, expression of the three transcription factors via a single polycistronic vector, rather than three separate constructs, was found to enhance differentiation of iCMs obtained from mouse cardiac fibroblasts (Inagawa et al, 2012). When the GMT factors were delivered as separate viral vectors, most reprogrammed cells expressing cardiac markers remained smaller than endogenous ventricular cardiomyocytes and never displayed clear cross striations, even after 1 month from transduction. However, cardiomyocyte maturation was greatly enhanced upon GMT factor delivery as a single polycistronic vector – with cross striations appearing in 30% of transduced cells – highlighting the importance of choosing the appropriate delivery method and optimizing transcription factor dosage for optimal reprogramming. Interestingly, the second study that attempted to recapitulate findings by Ieda et al obtained markedly different results (Chen et al, 2012). GMT-mediated reprogramming efficiency was tested in fibroblasts derived from multiple transgenic mouse lines. In all of them, cardiac-specific gene expression was only marginally elevated as a result of GMT transduction. Importantly, the efficiency of reprogramming was extremely variable according to which reporter gene was chosen as read-out: 35% of GMT-transfected fibroblasts expressed cTnT, but none of these cells showed activation of two other cardiac-specific markers, αMHC and Nkx2.5. Unlike what reported by Ieda et al, GMT-overexpressing cells exhibited no spontaneous action potential in vitro and, when transplanted into injured mouse hearts, displayed poor survival and minimal activation of cardiac gene expression (Chen et al, 2012). While the reasons for such discrepancies may lie in the different experimental protocols and reagents used to achieve GMT overexpression, findings by Chen et al point out that the choice of reporters, cell types, and methods used to evaluate cardiac phenotypes have profound influences on the assessment of reprogramming efficiency and should therefore be standardized through further investigation.
Table 1.
Cardiac reprogramming studies
| (a) Fibroblasts → Cardiomyocytes | |||||||
|---|---|---|---|---|---|---|---|
| References | Species | Recipient cell type/Organism | Genes/Molecules | Gene delivery method | Reprogramming read-out | Reprogramming efficiency | In vivo |
| Ieda et al (2010) | M | Post-natal CFs and TTFs | Gata4, Mef2c, Tbx5 | Retrovirus | αMHC reporter/cTnT | 5–25% | − |
| Chen et al (2012) | M | CFs and TTFs | Gata4, Mef2c, Tbx5 | Lentivirus | αMHC/Nkx2.5/cTnT reporters | 0–35% | − |
| Inagawa et al (2012) | M | Adult CFs | Gata4, Mef2c, Tbx5 | Retrovirus | αMHC reporter | 3–7% | − |
| MI model | αMHC reporter and α-actinin | 1% | + | ||||
| Qian et al (2012) | M | MI model | Gata4, Mef2c, Tbx5 | Retrovirus | Postn/Fsp1 lineage tracing, α-actinin & sarcomeric structure | 10–15% | + |
| Song et al (2012) | M | Adult CFs and TTFs | Gata4, Mef2c, Tbx5, Hand2 | Retrovirus | αMHC reporter and cTnT | 5–20% | − |
| MI model | Fsp1/Tcf2l lineage tracing and cTnT | 1–8% | + | ||||
| Protze et al (2012) | M | Embryonic fibroblasts and neonatal CFs | Mef2c, Tbx5, Myocd | Lentivirus | αMHC reporter and cTnT | 1–2% | − |
| Jayawardena et al (2012) | M | Neonatal CFs, adult CFs, and TTFs | miR-1, -133, -208, -499, +JI1 | Lipid carrier | αMHC reporter | 13–28% | − |
| MI model | miR-1, -133, -208, -499 | Lentivirus | Fsp1 lineage tracing and αMHC reporter | 1% | + | ||
| Efe et al (2011) | M | Embryonic fibroblasts | Oct4, Sox2, Klf4, +JI1, +BMP4 | Retrovirus | cTnT | 40% | − |
| Wang et al (2014) | M | Embryonic fibroblasts and TTFs | Oct4, small-molecule cocktail | Retrovirus | Beating cell clusters | 50–116 beating clusters/10,000 plated fibroblasts | − |
| Islas et al (2012) | H | DFs | ETS2, MESP1, +ActivinA, +BMP2 | Lentivirus/Recombinant proteins | NKX2.5 reporter/αMHC reporter and Ca2+ transients | 2–9% | − |
| Nam et al (2013) | H | Neonatal FFs, adult CFs, and DFs | GATA4, HAND2, TBX5, MYOCD, miR-1, miR-133 | Retrovirus | cTnT/tropomyosin | 9–45% | − |
| Fu et al (2013) | H | ESC-derived fibroblasts, fetal CFs, and neonatal DFs | GATA4, MEF2C, TBX5, ESSRG, MESP1, MYOCD, ZFPM2 (+SIS3) | Retrovirus | αMHC reporter and cTnT | 1–22% | − |
| Wada et al (2013) | H | CFs and DFs | GATA4, MEF2C, TBX5, MESP1, MYOCD | Retrovirus | α-actinin/cTnT | 5% | − |
| (b) Cardiomyocytes → Conduction system cells | |||||||
|---|---|---|---|---|---|---|---|
| References | Species | Genes | Gene delivery/Expression method | Cell type obtained | Reprogramming readout | Reprogramming efficiency | In vivo |
| Bakker et al (2012) | M | Tbx3 | Lentivirus | Pacemaker (SAN-like?) cells | Gene expression, electrophysiological parameters | N.D. | − |
| Inducible transgene | + | ||||||
| Rentschler et al (2012) | M | NICD | Retrovirus | Purkinje-like conduction cells | Electrophysiological parameters | N.D. | − |
| Notch activation | Inducible system | + | |||||
| Kapoor et al (2013) | M | Tbx18 | Adenovirus | SAN cells | Electrophysiological parameters | N.D. | − |
| GP | + | ||||||
GP, guinea pig; H, human; M, mouse; CFs, cardiac fibroblasts; DFs, dermal fibroblasts; MI, myocardial infarction; TTFs, tail tip fibroblasts; SAN, sino-atrial node; N.D., not determined.
Lipid-based transient transfection of four cardiac-specific miRNAs (miR-1, -133, -208, -499) was reported to convert mouse fibroblasts into cardiomyocytes in vitro, and the conversion efficiency enhanced up to almost 30% by inhibiting the JAK1 kinase (Jayawardena et al, 2012). However, no cardiac marker gene was activated when the same miRNAs were virally transduced into mouse fibroblasts by another group (Nam et al, 2013). Another example of experimental variables leading to markedly different reprogramming efficiencies is a study by the Olson laboratory (Song et al, 2012). In an effort to identify a better combination of cardiac reprogramming factors, they also transduced mouse fibroblasts with virally encoded GMT factors, followed by evaluation of the number of cells expressing both αMHC and cTnT reporter genes. Unlike Ieda et al, who reported a reprogramming efficiency of more than 20%, they found that GMT factors could only induce cardiac reprogramming, as assessed by expression of their two reporter genes, in around 6% of transfected cells. Adding one more cardiac transcription factor – Hand2 – to the reprogramming cocktail, then referred to as GMTH, did however induce up to 20% of cells to become positive for both αMHC and cTnT expressions (Song et al, 2012). The importance of verifying the expression of multiple, lineage-specific genes when assessing reprogramming efficiency was also pointed out by another study, in which all triplet combinations of ten candidate factors were screened for their ability to induce a variety of cardiac-specific genes, whose expression reflects multiple heart functions (Protze et al, 2012). Interestingly, the broadest spectrum of cardiac genes was up-regulated not upon transfection of the GMT factors, but when Gata4 was substituted with Myocd, suggesting that the GMT factors do not achieve full reprogramming of fibroblasts into cardiomyocytes. In conclusion, the ability to quantify reprogramming events necessarily relies on a reporter gene expressed in the desired final cell type, but such a reporter must accurately reflect the fully reprogrammed state. Overexpressing certain transcription factors might cause induction of the reporter (such as the αMHC-GFP reporter analyzed by Ieda et al), but not of the fully differentiated cell program. To avoid selecting for factor combinations that only achieve partial reprogramming, it is therefore essential to screen for other hallmarks of a differentiated cardiomyocyte, such as global cardiac gene expression, sarcomeric structure, and action potentials (Protze et al, 2012).
Cardiac reprogramming of fibroblast cells can be achieved by direct conversion, with no appearance of an intermediate cardiac precursor-like state, as reported by some of the studies above (Ieda et al, 2010; Song et al, 2012), or by an indirect switch in cell fate, which transitions through a cardiac progenitor state. By adapting the conventional iPSC reprogramming protocol, Efe et al achieved partial dedifferentiation of mouse embryonic fibroblasts, followed by differentiation into cardiomyocytes through a mitotically active intermediate expressing early (Mesp1, Flk1) and then mid-stage (Nkx2.5, Gata4, Isl1) cardiac progenitor markers (Efe et al, 2011). After initial overexpression via inducible viral vectors of the pluripotency factors Oct4, Sox2, and Klf4, cells were exposed to the small-molecule JAK inhibitor JI1, followed by culturing in a chemically defined medium containing the cardiomyogenic growth factor BMP4. With this protocol, the conventional reprogramming route toward pluripotency was shortcut and directed toward cardiac cytogenesis instead, with a conversion efficiency of 40% (Table1) (Efe et al, 2011). In a similar recent study, mouse fibroblasts were initially transduced with Oct4 alone and then exposed to a cocktail of lineage-specific soluble signals, including an anaplastic lymphoma kinase (ALK) inhibitor and a GSK3β inhibitor, to achieve trans-differentiation into the cardiac lineage (Wang et al, 2014). Despite initial Oct4 overexpression from an inducible viral construct, converted cells never enter the pluripotent state but transition through a cardiac progenitor-like stage (as determined by expression of Flk1, Mesp1, Isl1, and Gata4) before turning into differentiated cardiomyocytes. Finally, viral-mediated co-expression of Mesp1 and Ets2 transcription factors or cell treatment with MESP1 and ETS2 recombinant proteins was reported to reprogram human dermal fibroblasts into cardiac progenitors, marked by expression of core cardiac transcription factors (Nkx2-5, Isl1, Tbx5, Mef2c, Gata4) (Islas et al, 2012).
Given the increased complexity of gene regulatory pathways in human cells, cardiomyocyte generation from human fibroblasts requires more factors than those needed to reprogram mouse fibroblasts. This observation is in line with what is reported for the generation of human iPSCs or neuronal cells, which also require different culture conditions and/or additional transcription factors compared to mouse cells. Species-specific requirements likely reflect differences in gene expression and regulation between mouse and human fibroblasts and different susceptibility of lineage-specific genes to activation in different cell types. For example, both GMT and GMTH factor combinations are insufficient for cardiac reprogramming of human fibroblasts (Islas et al, 2012; Nam et al, 2013; Wada et al, 2013). By employing a combination of four transcription factors (Gata4, Hand2, Tbx5, Myocd) and two miRNAs (miR-1, miR-133), human neonatal and adult fibroblasts were successfully converted into cardiomyocyte-like cells characterized by cardiac gene activation and sarcomeric-like structures (Nam et al, 2013). Despite initial high reprogramming efficiency (up to 45% depending on readout and fibroblast origin), human iCMs required longer maturation time compared to their murine counterparts and displayed low-amplitude calcium transients in response to electrical stimulation and extremely rare spontaneous contractions. Moreover, human iCMs appeared to be heterogeneous, with each cell expressing different levels of cardiac and non-cardiac genes (Nam et al, 2013). This heterogeneity may partly be ascribed to the mixed age and genetic background of the human fibroblasts tested, which will influence cell-to-cell variability in epigenetic landscapes and therefore in susceptibility to the reprogramming process. Importantly, utilizing different combinations of reprogramming factors does not seem to improve the outcome considerably. Induced cardiomyocytes generated by overexpressing GMT factors plus Mesp1 and Myocd in human cardiac or dermal fibroblasts are functionally immature, as indicated by cell morphology, expression of embryonic cardiomyocyte marker genes, and slow calcium oscillations (Wada et al, 2013). Similar findings were reported when fibroblasts were transduced with GMT plus Esrrg (estrogen-related receptor gamma), Mesp1, Myocd, and Zfpm2 (zinc finger protein, FOG family member 2) (Fu et al, 2013). Despite the appearance of a cardiomyocyte-like phenotype, with cardiac-specific gene up-regulation and sarcomere assembly, only a few of the reprogrammed cells fired action potentials upon electrical stimulation and none of them displayed any spontaneous contractions, even after a long time in culture.
Immediately after the first successful attempts at in vitro cardiomyocyte reprogramming of fibroblast cells were reported, several groups began to try the same in vivo, by injecting cardiac reprogramming factors directly into mouse hearts (Table1). Intramyocardial delivery of retroviral vectors expressing the GMT factors in a mouse model of MI was shown to reprogram resident fibroblasts into cardiomyocyte-like cells (Qian et al, 2012). Interestingly, the initial reprogramming efficiency achieved in vivo (10–15%) was similar to that observed by the same group during in vitro conversion experiments, but in vivo-derived iCMs appeared more fully reprogrammed and more similar to endogenous cardiomyocytes than in vitro-derived ones (Ieda et al, 2010; Qian et al, 2012) (Table1). This would suggest that factors within the native milieu that are absent in a cell culture dish – such as extracellular matrix and secreted proteins – enhance the cell fate switch. Importantly, in vivo cardiac reprogramming was accompanied by an improvement in cardiac function and a reduction in scar size following injury, but the functional improvement of GMT-injected hearts seemed greater than what one might expect from the relatively inefficient reprogramming of adult cardiac fibroblasts in vitro. This could mean that the observed benefits may arise from a combination of new muscle formation and other non-cell-autonomous effects, such as growth factor secretion. A similar conclusion was made when the GMTH factors were employed, as they only reprogrammed 1–8% of transduced cardiac fibroblasts but caused a much more pronounced improvement in heart function (Song et al, 2012). In another study, injection of GMT-expressing retroviral constructs in vivo only caused 1% of transduced fibroblasts to convert into cardiomyocyte-like cells, a strikingly lower reprogramming efficiency than the 10–15% claimed by Qian et al (Inagawa et al, 2012). As pointed out above for the in vitro studies, these differences may be due to a number of experimental variables that affect the efficiency of conversion in vivo, such as the mouse strain employed or the transgene expression levels achieved. Moreover, the study by Inagawa et al used immunosuppressed nude mice, because the number of retrovirus-infected cells was dramatically reduced 2 weeks after MI in immunocompetent mice (Inagawa et al, 2012), whereas no loss of reprogrammed cells was seen in the immunocompetent mice used by Qian et al, even four weeks post-infection (Qian et al, 2012). It is clear that additional experiments are needed to redeem these controversies before any conclusion concerning the utility of in vivo reprogramming therapies can be made.
Somatic stem cell transplantation therapies
Since the realization that adult somatic stem cells can be isolated from many different tissue sources and can spontaneously differentiate in vivo in response to endogenous cues, the idea to use these cells to repair cardiac injury has been all the rage and translational efforts have proceeded at the speed of light (Hansson & Lendahl, 2013; Matar & Chong, 2014). Here, we briefly discuss some of the somatic stem cell transplantation therapies for heart diseases that have undergone clinical testing. A detailed review is obtained elsewhere (Rosen et al, 2014).
The first somatic stem cells to be tested were skeletal myoblasts (Taylor et al, 1998; Menasche et al, 2001). Upon muscle injury, skeletal muscle progenitor cells proliferate and promote regeneration by differentiating into new muscle fibers, making them attractive candidates for cardiac regeneration tools. The MAGIC (myoblast autologous grafting in ischemic cardiomyopathy) clinical trial examined the effects of intra-myocardial injection of skeletal myoblasts in 97 patients with severe ischemic heart failure (HF) (Menasche et al, 2008) (Table2). Smaller clinical trials had previously yielded encouraging results, but MAGIC did not corroborate these findings. Although the occurrence of malignant arrhythmias between the myoblast-treated patients and controls was the same, at the end of the 6-month-long observation period, myoblast treatment did not cause any significant improvement in cardiac function or clinical status. Due to the overall discouraging results of the MAGIC trial, the risk of arrhythmias associated with skeletal myoblast transplantation as shown by the MARVEL (myoblast implantation into myocardium post myocardial infarction) trial (Povsic et al, 2011) (Table2), and the availability of more attractive cell sources, interest in skeletal myoblasts has waned in recent years.
Table 2.
Selected clinical trials of stem cell therapy for cardiac regeneration
| Trial name/References | Classification | Cell type | Delivery method | Patient number | Follow-up period | Outcome | Side effects |
|---|---|---|---|---|---|---|---|
| MAGIC (Menasche et al, 2008) | Phase I/II | SKMs | Intramyocardial injection during CABG | 97 | 6 m | Unchanged LVEF and regional wall motion/decreased LVEDV in patients receiving high dose | Ventricular arrhythmias in 9 of 63 patients, and 9 of 63 patients died |
| MARVEL (Povsic et al, 2011) | Phase I/II | SKMs | Transcatheter intramyocardial | 20 | 3, 6 m | Improved 6-minute walk distance | Ventricular tachycardia in 7 of 14 patients |
| Repair-AMI (Assmus et al, 2014) (Schachinger et al, 2009) | Phase III | BMMNCs | Intracoronary | 204 | 4, 12 m; 2,5 y | Reduced LV remodeling/Improved ventricular function | 5 of 101 patients hospitalized for heart failure, and 7 of 101 patients died |
| BAMI (NCT01569178) | Phase III | BMMNCs | Intracoronary | 3000 | 3 y | Currently recruiting | |
| RENEW (Povsic et al, 2013) | Phase III | BM-derived CD34+ stem cells | Intramyocardial | 444 | 12 m | Currently ongoing | |
| PERFECT (Donndorf et al, 2012) | Phase III | BM-derived CD133+ stem cells | Intramyocardial injection during CABG | 142 | 6 m | Currently recruiting | |
| POSEIDON (Hare et al, 2012) (Suncion et al, 2014) | Phase I/II | BM-derived MSCs | Intramyocardial (transendocardial) | 30 | 13 m | Unchanged LVEF/decreased LVEDV and scar mass/improved physical performance | 3 patients hospitalized for heart failure |
| PROMETHEUS (Karantalis et al, 2014) | Phase I/II | BM-derived MSCs | Intramyocardial injection during CABG | 6 | 3, 6, 18 m | Increased LVEF/reduced scar mass | No major complications reported |
| TAC-HFT (Heldman et al, 2014) | Phase I/II | BMMNCs vs. BM-derived MSCs | Intramyocardial (transendocardial) | 65 | 3, 6, 12 m | Unchanged LVEF and LV volumes/improved regional LV function and decreased infarct size (only with MSCs) | No major complications reported |
| ADVANCE (NCT01216995) | Phase II | Adipose-derived MSCs | Intracoronary | 216 | 6, 12 m; 3 y | Currently ongoing | |
| SCIPIO (Chugh et al, 2012) | Phase I | c-kit+ CPCs | Intracoronary | 33 | 4, 12 m | Increased LVEF/reduced infarct size/increased viable mass | No major complications reported |
| ALCADIA (NCT00981006) | Phase I | CPCs | Intramyocardial injection during CABG | 6 | 12 m | Currently ongoing | |
| CADUCEUS (Malliaras et al, 2014) | Phase I | Cardiosphere-derived CPCs | Intracoronary | 25 | 6, 12 m | Unchanged LVEF and LV volumes/reduced scar mass/increased viable mass | Serious adverse events in 6 of 17 patients, and 1 of 17 patients died |
| ALLSTAR (NCT01458405) | Phase I/II | Cardiosphere-derived CPCs | Intracoronary | 274 | 12 m | Currently recruiting | |
For ongoing trials with no published results, the ClinicalTrials.gov Identifier (NCT…) has been indicated.
BM, bone marrow; BMMNCs, bone marrow mononuclear cells; CPCs, cardiac progenitor cells; MSCs, mesenchymal stem cells; SKMs, skeletal myoblasts; CABG, coronary artery bypass graft; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; m, months; y, years.
The majority of preclinical and clinical studies of somatic stem cell therapy for HF patients employed bone marrow-derived stem cells, including hematopoietic and non-hematopoietic stem cell populations, which can differentiate into diverse cellular phenotypes. Cells used in clinical trials include unfractionated bone marrow mononuclear cells (BMMNCs), CD34+ and/or CD133+ hematopoietic and endothelial stem/progenitor cells, and mesenchymal stem cells (MSCs). BMMNCs have been investigated multiple times in animal models of acute MI with encouraging results (Balsam et al, 2004; Murry et al, 2004). In the clinical setting, however, conflicting results have been obtained in patients with ischemic/non-ischemic HF treated with BMMNCs. The REPAIR-AMI (intracoronary progenitor cells in acute myocardial infarction) trial aimed to investigate the effects of intracoronary injection of autologous bone marrow cells in patients within 7 days after the onset of acute MI and successful reperfusion therapy. Published results reported improved ventricular function and event-free survival in cell-treated patients compared to the placebo group, up to 5 years post-transplantation (Schachinger et al, 2009; Assmus et al, 2014). However, other trials have failed to confirm the beneficial effects of intracoronary delivery of BMMNCs in ischemic HF (Ang et al, 2008; Yao et al, 2008). To definitively examine whether BMMNCs can reduce mortality after MI, a large multicenter European clinical trial called BAMI (the effect of intracoronary reinfusion of BMMNCs on all cause mortality in acute myocardial infarction) has been initiated (Table2).
BMMNCs contain a low percentage (0.5–2.5%) of hematopoietic stem cells and endothelial progenitor cells, which are marked by cell surface markers CD34 and/or CD133 (Mackie & Losordo, 2011). Autologous CD34+ cells have been transplanted into patients affected by either ischemic (Patel et al, 2005) or non-ischemic (Vrtovec et al, 2013) cardiomyopathy, and found to improve cardiac function. While transplanted CD34+ cells are unlikely to trans-differentiate into cardiomyocytes (Murry et al, 2004), they might be inducing cardiac repair by promoting neovascularization via both direct engraftment and indirect paracrine effects (Mackie & Losordo, 2011). The RENEW study is planning to assess efficacy and safety of intramyocardial autologous CD34+ cell transplantation in patients with refractory angina (Table2) (Povsic et al, 2013). Effects of bone marrow-derived CD133+ cells were examined in patients with ischemic HF (Stamm et al, 2007). Preliminary results indicate that intra-myocardial CD133+ cell transplantation improves perfusion and contractile function of the infarcted myocardium, presumably because of increased neovascularization. A larger study, termed PERFECT (intramyocardial transplantation of bone marrow stem cells in addition to coronary artery bypass graft surgery), is now planning to enroll 142 patients to determine the potential of bone marrow-derived CD133+ cells to promote cardiac regeneration (Donndorf et al, 2012) (Table2).
Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent stem cells marked by expression of the cell surface markers CD105, CD73, CD90, and CD146 and by absence of the hematopoietic markers CD45, CD34, and CD14/CD11b (Barry & Murphy, 2004). MSCs can differentiate into adipocytes, osteoblasts, chondrocytes, and skeletal muscle cells and have also been shown to differentiate into endothelial cells and possibly cardiomyocytes (Pittenger et al, 1999; Reyes et al, 2002; Toma et al, 2002; Reinecke et al, 2008). Encouraged by animal studies reporting that transplanted MSCs could increase vascularization of the damaged heart and improve cardiac function (Silva et al, 2005), scientists initiated the PROMETHEUS (prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery) trial, in which autologous bone marrow-derived MSCs were administered to patients with chronic ischemic HF (Table2). Recently published results show that intra-myocardial MSC injection resulted in scar size reduction, improvement in contractile function, and increased perfusion (Karantalis et al, 2014). However, it must be noted that the study was only conducted on a very limited sample population of six patients. The effects of bone marrow-derived MSCs on chronic ischemic HF were also examined by the POSEIDON trial (percutaneous stem cell injection delivery effects on neomyogenesis). Importantly, although initial results reported that MSCs exert favorable effects on ventricular remodeling, functional capacity, and patient quality of life (Hare et al, 2012), the POSEIDON trial failed to show an improvement in global ventricular function (Suncion et al, 2014).
Much controversy surrounds clinical trials employing putative CPCs, such as those marked by c-kit expression. Despite the heated debate around the origin and cardiomyogenic potential of c-kit+ cells, SCIPIO (stem cell infusion in patients with ischemic cardiomyopathy)—the first CPC clinical trial—was initiated (Bolli et al, 2011) (Table2). Autologous c-kit+ CPCs were isolated from cardiac tissue obtained during surgery, expanded ex vivo, and delivered via intracoronary infusion in patients affected by ischemic HF. Although the planned two-year follow-up period still awaits completion, interim observations indicate that infusion of c-kit+ cells is associated with LVEF improvement and infarct size reduction that persist after one year (Bolli et al, 2011; Chugh et al, 2012). It must be noted though that concerns regarding patient randomization and the integrity of certain data generated during the SCIPIO trial have recently been raised (Nowbar et al, 2014; The Lancet Editors, 2014).
Another prominent clinical trial of autologous human CPC transplantation involved the use of cardiosphere-derived cardiac stem cells. Cardiospheres are multipotent cardiac-derived cells that can grow as self-adherent clusters and differentiate into all three major cardiac lineages in vitro (Messina et al, 2004). Cardiospheres isolated from human endomyocardial biopsies can be cultured in vitro to generate cardiosphere-derived cells (CDCs) (Smith et al, 2007). CDCs retain their cardiomyogenic differentiation potential in vitro and are able to promote cardiac regeneration and improve heart function in vivo in both murine and porcine MI models (Smith et al, 2007; Johnston et al, 2009). The regenerative potential of CDCs in human beings has been addressed by the CADUCEUS (cardiosphere-derived autologous stem cells to reverse ventricular dysfunction) trial (Table2). Autologous CDCs derived from endomyocardial biopsies were expanded in vitro, followed by intracoronary infusion into patients with a recent episode of MI. CDC administration led to a reduction in scar size, an increase in the amount of viable myocardium but only regional improvement in left ventricular function (Makkar et al, 2012; Malliaras et al, 2014). Data from the CADUCEUS trial do not definitively prove CDC-mediated cardiac regeneration, and the clinical effects of CDCs thus remain unclear. It should also be noted that both cardiospheres and CDCs are heterogeneous mixtures containing different types of cells that express endothelial and mesenchymal antigens (Messina et al, 2004). Which of these cell types is responsible for the observed effects on heart remodeling and function is at this point unknown. A larger clinical trial, termed ALLSTAR (allogeneic heart stem cells to achieve myocardial regeneration), is now planning to investigate the effects of allogeneic CDC therapy in approximately 300 patients (Table2).
In summary, although a multitude of clinical trials have been performed to date, their results remain ambiguous and no single-cell-based therapy for heart disease has been conclusively proven effective so far, particularly in improving life expectancy in patients. Trial outcomes are frequently affected by noise, poor trial design (especially the absence of blinding), and normal human tendencies toward optimism and denial, making it difficult to justify and conclude them clearly (Rosen et al, 2014). Rigorous long-term studies with adequate patient population size will have to be conducted in the future to solve the many controversies.
Cell-free therapies
Lessons from cell-based therapies indicate that, rather than transplanting cells that directly engraft and differentiate/proliferate in the host tissue, delivering paracrine factors alone to the damaged heart may be sufficient to activate repair mechanisms. This hypothesis has opened new avenues for cell-free therapies in cardiac regenerative medicine (Fig4, right). Factors of choice can be delivered to the heart in one of multiple forms, including viral and non-viral DNA vectors, modified mRNAs, small-molecule chemical compounds, and recombinant proteins. Studies of mammalian and non-mammalian heart injury models have taught us that there are multiple mechanisms involved in cardiac regeneration/replication (Fig3) and these pathways/modulators can be exploited to develop novel, non-cell-based therapies for heart disease. Current efforts are thus trying to identify clinically useful factors or molecules that can regenerate the damaged heart by (i) inducing proliferation of preexisting cardiomyocytes, (ii) reprogramming cardiac fibroblasts into cardiomyocytes by direct conversion in vivo (as described above) (iii) trans-differentiating one type of cardiomyocyte or other resident cardiac cell into another, or (iv) activating endogenous CPCs toward their differentiation. These mechanisms, together with other indirect ones acting on non-cardiomyocytes, such as the induction of angiogenesis and new vessel formation and the reduction of fibrotic scars, are likely to promote cardiac repair in a cooperative fashion (Fig4).
Preexisting cardiomyocyte proliferation
As detailed above (Fig3), the neonatal mouse heart possesses a robust but transient regenerative capacity that rapidly disappears after the first few days of life. Manipulating the signaling pathways/drivers that control post-natal loss of cardiomyocyte proliferation represents an attractive strategy to reactivate proliferation mechanisms in the injured adult mammalian heart. One such target is the p38 MAPK signaling pathway, as cardiomyocytes seem to control cell cycle progression by modulating p38 MAPK activity. Overexpression of p38 was shown to block proliferation of rat fetal cardiomyocytes, whereas its inhibition in adult cardiomyocytes could promote cell division (Engel et al, 2005). However, p38 MAPK inhibition alone was not able to rescue heart function in adult rodent models of myocardial injury (Engel et al, 2006). An improvement in cardiac function was only observed when animals were also treated with FGF1, which may promote survival of newly generated cardiomyocytes by improving angiogenesis. A p38 MAPK inhibitor and FGF1 could therefore be employed together as therapeutic agents for cardiac regeneration.
High-throughput functional screening for human miRNAs has identified a subset of miRNAs, represented by miR-590 and miR-199a, as being able to induce both DNA synthesis and cytokinesis in neonatal mouse and rat cardiomyocytes (Eulalio et al, 2012). Following MI in mice, these miRNAs stimulated marked cardiac regeneration and significantly improved cardiac function. In contrast, miR-15 was shown to be up-regulated after the first post-natal week in mice, coinciding with the rapid loss of regenerative capacity (Porrello et al, 2013). miR-15 inhibition in mice improves cardiac function after injury, suggesting its potential as a therapeutic target.
The homeodomain transcription factor Meis1 has been identified as a critical transcriptional regulator of cardiomyocyte cell cycle and proliferation through activation of the cyclin-dependent kinase (CDK) inhibitors p15, p16, and p21 (Mahmoud et al, 2013). Meis1 represses neonatal cardiomyocyte proliferation and notably, Meis1 deletion was sufficient to extend the post-natal proliferative window of cardiomyocytes in mice (Mahmoud et al, 2013), indicating that inhibition of this pathway may have therapeutic potential for cardiac regeneration/proliferation.
The oxygen-rich post-natal environment has been shown to induce cardiomyocyte cell cycle arrest through DNA damage response and to be associated with the post-natal loss of regenerative capacity (Puente et al, 2014). After birth, mammalian cardiomyocytes switch from a glycolytic to an oxidative metabolism. These post-natal metabolic changes are associated with marked increases of reactive oxygen species (ROS), oxidative DNA damage, and DNA damage response markers in the heart. Of interest, interfering with these processes by reducing oxidative stress was shown to successfully increase cardiomyocyte proliferation beyond its normally permissive time frame, suggesting that the reduction of oxidative stress may be a novel mechanism to stimulate mammalian cardiomyocyte proliferation (Puente et al, 2014).
Hippo signaling, an ancient organ size control pathway, inhibits developing cardiomyocyte proliferation (von Gise et al, 2012) and a recent study showed that inactivation of this pathway prompts adult mouse cardiomyocytes to re-enter the cell cycle and undergo cytokinesis, promoting cardiac regeneration following injury in both adult and neonatal mice (at post-natal day 8) (Heallen et al, 2013). In concert with this finding, Yes-associated protein (YAP), the terminal effector of the Hippo signaling pathway, was shown to be crucial for regulating mouse cardiomyocyte proliferation (Lin et al, 2014) and thus might also become a novel therapeutic target for cardiac repair.
Positive regulators of cardiomyocyte proliferation include members of the neuregulin-1 (NRG1)/ERRB4 and periostin (POSTN) signaling cascades. Increasing signaling through the NRG1/ERBB4 pathway may represent a novel therapeutic strategy to promote cardiac regeneration, as shown by the beneficial effects of NRG1 injection in adult mice, which induced cardiomyocyte proliferation and improved heart function after MI (Bersell et al, 2009). Similarly, POSTN ligand can stimulate differentiated cardiomyocytes to re-enter the cell cycle by binding the cell surface integrin receptors αV, β1, β3, and β5 and inducing downstream PI3K activation, which is sufficient to promote cardiomyocyte renewal in the absence of POSTN (Kuhn et al, 2007). POSTN/PI3K-mediated cardiomyocyte proliferation was shown to have beneficial effects on heart morphology and function after MI (Kuhn et al, 2007).
Trans-differentiation of cardiomyocytes or other cardiac cells
Direct cardiac reprogramming efforts have mainly focused on converting resident cardiac fibroblasts into cardiomyocytes, but there is no reason why the technology should be limited to these cell types. Successful trans-differentiation of cardiomyocytes into conduction system cells has been reported both in vitro and in vivo (Table1) and may revolutionize current clinical treatment of cardiac conduction system disorders, which generally involves the implantation of costly electronic pacemakers. Ectopic activation of the pacemaker cell-related transcription factor Tbx3 causes cardiomyocytes to change their gene expression profile from that of working myocardium to that of pacemaker myocardium (Bakker et al, 2012). Unfortunately, although induced sinoatrial-like cells display pacemaker characteristics in vitro, no biological pacemaker activity was created in vivo. Tbx18 transduction also converts cardiomyocytes into sinoatrial-like cells and appears to be superior to Tbx3 in generating both automaticity in vitro and biological pacemaker activity in vivo (Kapoor et al, 2013). Finally, a third study recently reported in vitro and in vivo cardiomyocyte reprogramming into Purkinje-like cells by activating the Notch signaling cascade (Rentschler et al, 2012).
As described above (Fig3), a recent study in zebrafish embryos showed that differentiated atrial cardiomyocytes can trans-differentiate into ventricular cardiomyocytes and contribute to ventricular regeneration upon injury, and that this process depends on Notch signaling activation (Zhang et al, 2013). Although it remains to be elucidated whether mammalian atrial cardiomyocytes have similar lineage plasticity, recent studies have shown that a cardiomyocyte progenitor population in mammalian fetal hearts is indeed enriched in the atrial chambers (Laugwitz et al, 2005; Bu et al, 2009; Genead et al, 2010). Together, these observations suggest that the trans-differentiating atrial cardiomyocytes found in zebrafish may be analogous to the atrial-resident multipotent CPCs found in mammals.
Finally, it has been recently shown that cardiac fibroblasts can trans-differentiate into endothelial-like cells after cardiac injury (Ubil et al, 2014). After acute ischemic heart injury, some cardiac fibroblasts rapidly adopt an endothelial-cell-like phenotype in response to p53 activation and contribute to neovascularization, which overall improves cardiac function. This native fibroblast-to-endothelial cell conversion could thus represent a novel therapeutic target to enhance cardiac repair.
Endogenous cardiac progenitor cell activation and differentiation
Recent studies suggest that the predominant source of post-injury formed cardiomyocytes is preexisting cardiomyocytes (Garbern & Lee, 2013; Mollova et al, 2013). However, there is still the possibility for an alternative cardiac progenitor source to contribute to new generation of cardiomyocytes after injury (Senyo et al, 2013). Thus, activation of endogenous CPCs toward differentiation into cardiac cells is another important therapeutic strategy for cardiac repair.
The mammalian heart develops from multipotent cardiovascular progenitors, including the FHF and SHF CPCs, and EPDCs (Fig1). The clinical utility of FHF- and SHF-derived progenitors is limited by their absence in the adult heart, making it unlikely that they could serve as a source of new cardiac cells after myocardial damage (Weinberger et al, 2012). Conversely, a number of resident cardiac progenitor populations have been identified in the adult epicardium, including EPDCs marked by expression of Wt1 (Zhou et al, 2008a; Smart et al, 2011; Zangi et al, 2013), Tbx18 (Cai et al, 2008; Zhou et al, 2011), or Tcf21 (Braitsch et al, 2013). All of these EPDCs play a role in forming the fibrotic scar that arises after MI, and it remains unclear how to drive these cells away from the cardiac fibroblast fate and into a more useful cardiomyogenic cell fate. Wt1+ EPDCs, for instance, are present at very low numbers in the normal adult heart and can readily differentiate into cardiac fibroblasts or smooth muscle cells in response to appropriate stimuli. However, they have limited potential to differentiate into endothelial cells and little, if any, capacity to form cardiomyocytes in both normal and pathological conditions (Smart et al, 2011; Zhou et al, 2011; Zangi et al, 2013). Priming, or pretreatment, of adult mouse hearts with the peptide thymosin β4 (Tβ4) was shown to drive endogenous EPDCs into a vascular cell fate that could promote neovascularization (Smart et al, 2007). In a follow-up study, the same group described Tβ4 priming before injury as a way to mobilize Wt1+ progenitor cells and stimulate their differentiation into novel cardiomyocytes that can integrate structurally and functionally with the surrounding muscle after MI (Smart et al, 2011). Hence, Tβ4 has been heralded as a means to enhance response of the adult mammalian heart to injury, via initiation of a novel cardiomyogenic program. Unfortunately, the process is relatively inefficient and the Tβ4 peptide does not reprogram EPDCs into cardiomyocytes when administered after injury, making its use as a novel therapeutic agent extremely unlikely (Zhou et al, 2012).
Recently, direct injection after MI of a chemically modified mRNA (modRNA) encoding a single paracrine factor, VEGF-A, was shown to induce reactivation of the quiescent adult endogenous Wt1+ EPDCs. The transient pulse of VEGF-A overexpression was sufficient to promote EPDC proliferation and to drive them away from the fibroblast, scar-forming fate, and toward an endothelial/smooth muscle cell fate that could ultimately improve cardiac function (Zangi et al, 2013). VEGF-A also appeared to stimulate adult EPDCs to differentiate toward the cardiomyocyte lineage, although the number of generated cardiomyocytes was extremely low (Zangi et al, 2013). It is likely that a number of other paracrine factors, aside from VEGF-A, will be able to activate endogenous progenitor cells and promote their cardiomyocyte differentiation once delivered into the infarcted mammalian heart via modRNA injection. It is therefore worth screening paracrine factor libraries to identify the best candidate for this novel, cell-free therapeutic approach (Chien et al, 2014; Lui et al, 2014).
Unresolved issues in human cardiac regenerative therapeutics
As described above, various cell-based and cell-free therapeutic strategies for cardiac regeneration achieved encouraging results in animal experiments, often leading to their rapid promotion to clinical testing. However, the benefits of such treatments, if any, remain controversial and these therapies are still far from widespread clinical application (Garbern & Lee, 2013; Hansson & Lendahl, 2013; Sanganalmath & Bolli, 2013; Matar & Chong, 2014; Nowbar et al, 2014). Before proceeding these strategies toward realistic clinical translation, many critical issues will have to be addressed.
Cell type and scalability
Many different types of cells, including various CPCs, non-cardiac somatic stem cells, human PSC (hPSC)-derived cardiomyocytes, and more recently, directly reprogrammed cardiomyocyte-like cells have been considered for exogenous delivery. However, there is no consensus on the ideal cell type to adopt for cell transplantation in the setting of cardiac regenerative therapeutics for heart disease. Very few studies compared different cell types in regard to their therapeutic efficacy (Mathieu et al, 2009; Shintani et al, 2009; Mazo et al, 2010). In such studies, it is often hard to definitively conclude which cell type would be superior to others, given the difficulty to define the dose–response relationship for each cell type before the comparison is initiated. Conceptually, the ideal cell type should tolerate autologous transplantation, expand rapidly in vitro, differentiate specifically into cardiomyocytes, and couple electrically with the host cardiomyocytes. In addition, previous preclinical studies suggest that combinations of different cell types may be more efficient in promoting cardiac regeneration and function than a single-cell therapy, due to the complementary or even synergistic actions of different cell types (Bonaros et al, 2006; Williams et al, 2013), although further studies are needed.
The dose–response relationship and appropriate frequency of administration for each cell therapy are other undetermined issues that very few clinical studies tried to address so far (Menasche et al, 2008; Hare et al, 2012). Producing scalable cultures of hPSC-derived cells is challenging, but recent advances allow for large-scale production of hPSC-derived cardiomyocytes (Zhang et al, 2012; Lian et al, 2013) and/or endothelial progenitors (Sahara et al, 2014). Such technological advances encourage studies to determine the relationship between the number/frequency of cells administered and their effects on cardiac regeneration and function.
Mechanism by which cell therapy promotes cardiac regeneration
The mechanisms of action by which cell therapy contributes to the generation of new cardiomyocytes and/or to an improvement in cardiac function remain unclear (Fig4). It is possible that injected cells do so by engrafting directly into the damaged heart, proliferating, and differentiating into mature cardiac cell types, although previous studies suggest that these events are relatively rare (Tang et al, 2010; Loffredo et al, 2011). When c-kit+ CPCs were transplanted into rats 1 month after coronary occlusion followed by reperfusion, the rats exhibited more viable myocardium in the injured region, less fibrosis, and improved ventricular function 5 weeks after transplantation. However, the number of transplanted c-kit+ cell derivatives contributing to the newly formed myocardium was too small to account for the observed beneficial effects (transplanted c-kit+ cell-derived cardiomyocytes only accounted for 2.6% of the total cardiomyocyte population in the injured region and 1.1% in the non-infarcted region) (Tang et al, 2010). Similar findings were also reported in a porcine ischemic model (Bolli et al, 2013). Collectively, these observations suggest that, rather than by direct engraftment and differentiation into cardiac lineages, transplanted cells may exert their salutary effects indirectly, by secreting paracrine signals that act on surrounding cells (Gnecchi et al, 2008). Paracrine signals such as cytokines and growth factors may promote cardiac repair through a variety of mechanisms, including preexisting cardiomyocyte dedifferentiation/proliferation, recruitment/activation of endogenous CPCs, induction of angiogenesis and new vessel formation, reduction of fibrotic scars, and inhibition of apoptosis, ultimately resulting in enhanced cardiac function and myocardial repair. To develop future cell-based and/or cell-free therapies for cardiac regeneration, it is important to define the extent to which direct cell engraftment followed by differentiation/proliferation or rather the paracrine effects of transplanted cells account for observed beneficial effects.
Subtype of generated cardiomyocytes
Recent advanced differentiation protocols from hPSCs allow the generation of cardiomyocytes with unprecedentedly high efficiency (approximately 80%) (Zhang et al, 2012; Lian et al, 2013). However, the resultant cardiomyocyte populations are a heterogeneous and frequently uncharacterized mixture of different subtypes, such as atrial, ventricular, and conductive cells, which can be distinguished by their gene expression profile and electrophysiological properties (Xu et al, 2012; Weng et al, 2014). To obtain better effects on cardiac function and prevent arrhythmias after transplantation to the ventricle, it may be essential to selectively produce the ventricular subtype of cardiomyocytes through directed differentiation of hPSCs. In this regard, recent studies have reported that down-regulation of retinoic acid signaling (Zhang et al, 2011), or hypoxic conditions plus sequential addition of BMP4/Activin and a Wnt inhibitor (Weng et al, 2014) successfully generate ventricular cardiomyocytes, but not other subtypes, from hPSCs. Directed differentiation approaches need to be further optimized in this way to purify each subtype of cardiomyocytes for therapeutic transplantation purposes. hPSC-derived cardiomyocytes, as well as directly reprogrammed cardiomyocyte-like cells, exhibit structural and functional features of neonatal, rather than adult, cardiomyocytes (Ieda et al, 2010; Song et al, 2012; Lundy et al, 2013), which could lead to increased risk of arrhythmias, lower long-term stability, and poor integration into the host myocardium after transplantation. Structural and functional maturation to adult-like cells can be achieved by longer in vitro culture times (Lundy et al, 2013), but the extent to which cells need to be matured in vitro before transplantation to achieve post-transplantation efficacy and safety remains an important unanswered question.
Electrical coupling
One major caveat associated with cardiac cell therapy is the risk of arrhythmias, due to incomplete electrical coupling of transplanted cells with host cardiomyocytes. Ideally, transplanted cells have to align, engraft, and couple with host cardiomyocytes in an ordered fashion, although how this process is orchestrated is unclear. A recent study has shown that human ESC-derived cardiomyocytes can engraft into injured guinea pig hearts with 1:1 host–graft coupling, and this results in reduced risk of arrhythmias and improved cardiac function, indicating human ESCs as a potentially safe tool for cardiac regeneration (Shiba et al, 2012). As described above, human ESC-derived cardiomyocytes also seem to generate new muscle grafts when transplanted into the infarcted monkey heart (Chong et al, 2014b). However, non-fatal ventricular arrhythmias were observed in engrafted primates and further investigations are required to understand why electromechanical coupling appears to be incomplete (Anderson et al, 2014; Murry et al, 2014).
Teratoma formation
Another concern upon transplantation of hPSC-derived cells is the risk of teratoma formation (Lensch et al, 2007), which is obviously harmful to cardiac function and electrical homeostasis. When unpurified human ESC-derived cardiomyocytes were transplanted into non-human primates following cardiac injury, microteratomas formed in the scar region (Blin et al, 2010). However, when cells were committed to the cardiac lineage, as identified by expression of stage-specific embryonic antigen-1 (SSEA-1), there was no evidence of teratoma formation at 2 months after transplantation (Bel et al, 2010; Blin et al, 2010), suggesting that adequate purification of hPSC-derived cardiac lineages before transplantation might be sufficient to prevent tumor formation.
Delivery systems
In many preclinical and clinical tests, molecules and/or cells are often directly injected into the myocardium during open-heart surgery (Fig4). A less invasive delivery system, such as transcatheter injection, is however desirable in the clinical setting. There are two kinds of transcatheter approaches, involving either intramyocardial (transendocardial) or intracoronary injection, both of which have advantages and disadvantages associated with cell engraftment efficacy and side effects (tissue disruption, microvascular embolism, etc.) (Beeres et al, 2007; Ang et al, 2008; Li et al, 2011). Further investigations are needed to determine which delivery system is the most appropriate for maximal efficacy and safety (Sanganalmath & Bolli, 2013).
Virus issues
For direct fibroblast conversion into cardiomyocyte-like cells in vitro and in vivo, viral transduction of reprogramming factors is currently the main approach. Some viral vectors, however, integrate into the host genome, thereby carrying a potential risk of tumorigenicity. Before any clinical trial involving transplantation of induced cardiomyocytes can be designed, it is therefore imperative to develop non-integrative gene transfer approaches that can achieve efficient cell fate conversion without compromising on safety. Such non-integrative approaches include plasmid vectors, modRNAs, miRNAs, and chemical molecules (Fig4) and are currently under investigation (Kim et al, 2009; Warren et al, 2010; Jayawardena et al, 2012).
Long-term engraftment and tissue engineering
Another major issue in cell therapy is how to improve engraftment, because most cells transplanted into the heart do not survive long term. Independently of cell type and delivery system, more than 90% of injected cells disappear in the first few days and only 1–2% can still be detected 4 weeks after transplantation (Zeng et al, 2007; Hong et al, 2013). Massive loss of transplanted cells is likely to limit the efficacy of any type of cell therapy and may be one of the reasons behind the modest success and/or controversial results in the past trials of cardiac cell therapy (Sanganalmath & Bolli, 2013; Matar & Chong, 2014). To improve long-term cell engraftment in the ischemic heart, several strategies are under investigation, including pretreatment of transplanted cells by genetic modification or pharmacological preconditioning (Penn & Mangi, 2008), regression of fibrotic scar tissue (Chablais & Jazwinska, 2012), and cardiac tissue engineering (Hirt et al, 2014). Genetic modification strategies involve overexpression of anti-apoptotic genes, such as heme oxygenase-1 (HO-1) or proto-oncogene serine/threonine-protein kinase (Pim-1), which have been shown to improve the survival of mesenchymal or cardiac stem cells transplanted into ischemic hearts (Tang et al, 2005; Fischer et al, 2009). In addition, reestablishment of adequate vascularization might be essential for long-term engraftment of transplanted cardiac cells (Terrovitis et al, 2010).
Cardiac tissue engineering, which involves three-dimensional heart muscle constructs, has undergone remarkable progress in the past decades. Combining cells with natural or synthetic biomaterials, such as collagen, matrigel, fibrin, alginate, gelatin sponges, and polyglycolic acid, improves local cell retention and engraftment (Segers & Lee, 2011; Ye et al, 2011). More recently, biodegradable scaffolds/decellularized heart tissue (Schmidt et al, 2007; Ott et al, 2008) and cardiac patches/cell sheets containing cardiac cells (Wei et al, 2008; Kubo et al, 2013) have received much attention. Further details have been reviewed elsewhere (Cimetta et al, 2013; Hirt et al, 2014). Although cardiac tissue engineering is still in development, these approaches will enhance cell engraftment and likely result in better outcomes of cardiac cell therapy.
Future perspectives
Recent discoveries in the fields of stem cell and regenerative biology hold great promise for cardiac regenerative medicine. Some of the most recently developed therapeutic strategies, such as cardiac/somatic stem cell transplantation, have been or are currently being clinically tested. Despite encouraging results in experimental/preclinical settings, the clinical benefits of many of these therapies remain controversial. Moreover, there is no consensus on the strategy to use, including which cell type to transplant, which delivery system to adopt, and/or which biomaterials to co-administer, to improve efficacy and safety. Similarly, standardized protocols to produce hPSC-derived or directly reprogrammed cardiomyocytes exhibiting “appropriate” maturation levels and engraftment are lacking. These controversies might be attributable, at least in part, to our incomplete understanding of how the human heart develops and can regenerate, and of which intrinsic factor(s) account for the ultimate differences in regenerative capacities between lower vertebrate or neonatal mammalian hearts and adult mammalian hearts. The key to successful human cardiac regeneration in the near future lies in our continuing efforts to explore these core issues and to unravel the essential pathways and factors that govern the programming and reprogramming of a human heart cell. Latest experimental and therapeutic tools, involving modRNA, cellular reprogramming, cardiac tissue engineering, and next-generation sequencing of genomes and transcriptomes at the single cardiac cell levels, as well as different model organisms such as lower vertebrates and non-human primates, will help advance our understanding of human cardiogenesis and heart repair, opening novel paths toward an ultimate goal of establishing cardiac regenerative therapeutics.
Acknowledgments
We would like to thank Nevin Witman, Emil M. Hansson, and Jonathan Clarke for critical reading of the manuscript and Mattias Karlén for help with the artwork. Work in our laboratory is supported by the Knut and Alice Wallenberg Foundation, Vetenskapsrådet (Swedish Research Council), AstraZeneca Pharmaceuticals, and Karolinska Institute. FS is supported by an EMBO long-term fellowship (ALTF 620-2014).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci USA. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen DC, Ganesalingam S, Jensen CH, Sheikh SP. Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Reports. 2014;2:406–413. doi: 10.1016/j.stemcr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME, Goldhaber J, Houser SR, Puceat M, Sussman MA. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ Res. 2014;115:335–338. doi: 10.1161/CIRCRESAHA.114.304616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT, Galinanes M. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- Assmus B, Leistner DM, Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Sedding D, Yu J, Corti R, Mathey DG, Barth C, Mayer-Wehrstein C, Burck I, Sueselbeck T, Dill T, Hamm CW, Tonn T, Dimmeler S, Zeiher AM Group R-AS. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35:1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- Bakker ML, Boukens BJ, Mommersteeg MT, Brons JF, Wakker V, Moorman AF, Christoffels VM. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ Res. 2008;102:1340–1349. doi: 10.1161/CIRCRESAHA.107.169565. [DOI] [PubMed] [Google Scholar]
- Bakker ML, Boink GJ, Boukens BJ, Verkerk AO, van denBoogaard M, den Haan AD, Hoogaars WM, Buermans HP, de Bakker JM, Seppen J, Tan HL, Moorman AF, t Hoen PA, Christoffels VM. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc Res. 2012;94:439–449. doi: 10.1093/cvr/cvs120. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Howell WD, Rule M, Hayashi S, Thomas KR, Capecchi MR, McMahon AP. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RO, Chapin S, Sherry R. Regeneration of the ventricular myocardium in amphibians. Nature. 1974;248:145–147. doi: 10.1038/248145a0. [DOI] [PubMed] [Google Scholar]
- Beeres SL, Bax JJ, Dibbets-Schneider P, Stokkel MP, Fibbe WE, van der Wall EE, Schalij MJ, Atsma DE. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. Am J Cardiol. 2007;100:1094–1098. doi: 10.1016/j.amjcard.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, Bellabas L, Brinon B, Vanneaux V, Pradeau P, Peyrard S, Larghero J, Pouly J, Binder P, Garcia S, Shimizu T, Sawa Y, Okano T, Bruneval P, Desnos M, et al. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122:S118–S123. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rucker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagege A, Desnos M, Renaud JF, Menasche P, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaros N, Rauf R, Wolf D, Margreiter E, Tzankov A, Schlechta B, Kocher A, Ott H, Schachner T, Hering S, Bonatti J, Laufer G. Combined transplantation of skeletal myoblasts and angiopoietic progenitor cells reduces infarct size and apoptosis and improves cardiac function in chronic ischemic heart failure. J Thorac Cardiovasc Surg. 2006;132:1321–1328. doi: 10.1016/j.jtcvs.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Brade T, Pane LS, Moretti A, Chien KR, Laugwitz KL. Embryonic heart progenitors and cardiogenesis. Cold Spring Harb Perspect Med. 2013;3:a013847. doi: 10.1101/cshperspect.a013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan M, Liu G, Mikawa T. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science. 2013;340:744–748. doi: 10.1126/science.1232877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Burridge PW, Anderson D, Priddle H, Barbadillo Munoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Albini S, Wei K, Willems E, Guzzo RM, Tsuda M, Giordani L, Spiering S, Kurian L, Yeo GW, Puri PL, Mercola M. Coordinate Nodal and BMP inhibition directs Baf60c-dependent cardiomyocyte commitment. Genes Dev. 2013;27:2332–2344. doi: 10.1101/gad.225144.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Chablais F, Veit J, Rainer G, Jazwinska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chablais F, Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development. 2012;139:1921–1930. doi: 10.1242/dev.078543. [DOI] [PubMed] [Google Scholar]
- Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Zangi L, Lui KO. Synthetic Chemically Modified mRNA (modRNA): toward a New Technology Platform for Cardiovascular Biology and Medicine. Cold Spring Harb Perspect Med. 2014;5:a014035. doi: 10.1101/cshperspect.a014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JJ, Forte E, Harvey RP. Developmental origins and lineage descendants of endogenous adult cardiac progenitor cells. Stem Cell Research. 2014a;13:592–614. doi: 10.1016/j.scr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014b;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8-9. doi: 10.1038/nature07916. discussion E9-10. [DOI] [PubMed] [Google Scholar]
- Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimetta E, Godier-Furnemont A, Vunjak-Novakovic G. Bioengineering heart tissue for in vitro testing. Curr Opin Biotechnol. 2013;24:926–932. doi: 10.1016/j.copbio.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donndorf P, Kaminski A, Tiedemann G, Kundt G, Steinhoff G. Validating intramyocardial bone marrow stem cell therapy in combination with coronary artery bypass grafting, the PERFECT Phase III randomized multicenter trial: study protocol for a randomized controlled trial. Trials. 2012;13:99. doi: 10.1186/1745-6215-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, Scholes J, Dravid G, Li X, MacLellan WR, Crooks GM. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2010;107:13742–13747. doi: 10.1073/pnas.1002077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genead R, Danielsson C, Wardell E, Kjaeldgaard A, Westgren M, Sundstrom E, Franco-Cereceda A, Sylven C, Grinnemo KH. Early first trimester human embryonic cardiac Islet-1 progenitor cells and cardiomyocytes: immunohistochemical and electrophysiological characterization. Stem Cell Research. 2010;4:69–76. doi: 10.1016/j.scr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138:1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosa JM, Mercader N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat Protoc. 2012;7:782–788. doi: 10.1038/nprot.2012.025. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Hansson EM, Lendahl U. Regenerative medicine for the treatment of heart disease. J Intern Med. 2013;273:235–245. doi: 10.1111/joim.12033. [DOI] [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, Shimoji K, Ohno Y, Egashira T, Kaneda R, Murata M, Hidaka K, Morisaki T, Sasaki E, Suzuki T, Sano M, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. Jama. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117:353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci USA. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth V, Webb S, Bradshaw L, Brown NA, Anderson RH, Henderson DJ. Cells migrating from the neural crest contribute to the innervation of the venous pole of the heart. J Anat. 2008;212:1–11. doi: 10.1111/j.1469-7580.2007.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. 2014;114:354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. doi: 10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, Kurihara C, Obata Y, Miyake K, Fukuda K, Ieda M. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147–1156. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, Mercola M, Oshima RG, Willerson JT, Potaman VN, Schwartz RJ. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109:13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Itou J, Kawakami H, Burgoyne T, Kawakami Y. Life-long preservation of the regenerative capacity in the fin and heart in zebrafish. Biol Open. 2012a;1:739–746. doi: 10.1242/bio.20121057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itou J, Oishi I, Kawakami H, Glass TJ, Richter J, Johnson A, Lund TC, Kawakami Y. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development. 2012b;139:4133–4142. doi: 10.1242/dev.079756. [DOI] [PubMed] [Google Scholar]
- Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci USA. 2012;109:13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 1077 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Sune G, Morera C, Izpisua Belmonte JC. p38alpha MAPK regulates myocardial regeneration in zebrafish. Cell Cycle. 2012;11:1195–1201. doi: 10.4161/cc.11.6.19637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D'Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31:54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell. 2012;22:639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–741. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wu Q, Zhang Y, Wiens KM, Huang Y, Rubin N, Shimada H, Handin RI, Chao MY, Tuan TL, Starnes VA, Lien CL. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci USA. 2010;107:17206–17210. doi: 10.1073/pnas.0915016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, Kim C, Tang Y, Shetty K, Mishra B, Mishra L. Tgf-Beta signaling in development. Sci STKE. 2007;2007:cm1. doi: 10.1126/stke.3992007cm1. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesova H, Roelink H, Grim M. Sonic hedgehog is required for the assembly and remodeling of branchial arch blood vessels. Dev Dyn. 2008;237:1923–1934. doi: 10.1002/dvdy.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff MI, Hesse M, Fleischmann BK. Comment on “Do neonatal mouse hearts regenerate following heart apex resection”? Stem Cell Reports. 2014;3:2. doi: 10.1016/j.stemcr.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Shioyama T, Oura M, Suzuki A, Ogawa T, Makino H, Takeda S, Kino-oka M, Shimizu T, Okano T, Yamamori S. Development of automated 3-dimensional tissue fabrication system Tissue Factory – Automated cell isolation from tissue for regenerative medicine. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:358–361. doi: 10.1109/EMBC.2013.6609511. [DOI] [PubMed] [Google Scholar]
- Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci USA. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci. 2006;119:4719–4729. doi: 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest. 2008;118:2404–2414. doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensch MW, Schlaeger TM, Zon LI, Daley GQ. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–258. doi: 10.1016/j.stem.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O'Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–864. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Xu J, Li J, Chien KR. Next-Generation Models of Human Cardiogenesis via Genome Editing. Cold Spring Harb Perspect Med. 2014;4:a013920. doi: 10.1101/cshperspect.a013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wang G, Lin L, Lowe J, Zhang Q, Bu L, Chen Y, Chen J, Sun Y, Evans SM. HCN4 dynamically marks the first heart field and conduction system precursors. Circ Res. 2013;113:399–407. doi: 10.1161/CIRCRESAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Lui KO, Zangi L, Chien KR. Cardiovascular regenerative therapeutics via synthetic paracrine factor modified mRNA. Stem Cell Research. 2014;13:693–704. doi: 10.1016/j.scr.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marban E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- Marguerie A, Bajolle F, Zaffran S, Brown NA, Dickson C, Buckingham ME, Kelly RG. Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice. Cardiovasc Res. 2006;71:50–60. doi: 10.1016/j.cardiores.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Matar AA, Chong JJ. Stem cell therapy for cardiac dysfunction. SpringerPlus. 2014;3:440. doi: 10.1186/2193-1801-3-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, Metens T, da Costa AM, Mahmoudabady M, Egrise D, Blocklet D, Mazouz N, Naeije R, Heyndrickx G, McEntee K. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg. 2009;138:646–653. doi: 10.1016/j.jtcvs.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Mazo M, Gavira JJ, Abizanda G, Moreno C, Ecay M, Soriano M, Aranda P, Collantes M, Alegria E, Merino J, Penuelas I, Garcia Verdugo JM, Pelacho B, Prosper F. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19:313–328. doi: 10.3727/096368909X480323. [DOI] [PubMed] [Google Scholar]
- McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, Yang J, Qiu L, Priest CA, Shogbon C, Martin AW, Nelson J, West P, Beltzer JP, Pal S, Brandenberger R. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Mercer SE, Odelberg SJ, Simon HG. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev Biol. 2013;382:457–469. doi: 10.1016/j.ydbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Houser SR. Are resident c-Kit+ cardiac stem cells really all that are needed to mend a broken heart? Circ Res. 2013;113:1037–1039. doi: 10.1161/CIRCRESAHA.113.302564. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Houser SR. Response to Torella et al. Circ Res. 2014;114:e27. doi: 10.1161/CIRCRESAHA.114.303361. [DOI] [PubMed] [Google Scholar]
- Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Murry CE, Chong JJ, Laflamme MA. Letter by murry et al. regarding article, “embryonic stem cell-derived cardiac myocytes are not ready for human trials”. Circ Res. 2014;115:e28–e29. doi: 10.1161/CIRCRESAHA.114.305042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musunuru K, Domian IJ, Chien KR. Stem cell models of cardiac development and disease. Annu Rev Cell Dev Biol. 2010;26:667–687. doi: 10.1146/annurev-cellbio-100109-103948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Nakano H, Chien KR. Multipotent islet-1 cardiovascular progenitors in development and disease. Cold Spring Harb Symp Quant Biol. 2008;73:297–306. doi: 10.1101/sqb.2008.73.055. [DOI] [PubMed] [Google Scholar]
- Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110:5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM, Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff AW, Dent AE, Armstrong JB. Heart development and regeneration in urodeles. Int J Dev Biol. 1996;40:719–725. [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- Noseda M, Peterkin T, Simoes FC, Patient R, Schneider MD. Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circ Res. 2011;108:129–152. doi: 10.1161/CIRCRESAHA.110.223792. [DOI] [PubMed] [Google Scholar]
- Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP DAMASCENE Writing Group. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- Oberpriller JO, Oberpriller JC, Arefyeva AM, Mitashov VI, Carlson BM. Nuclear characteristics of cardiac myocytes following the proliferative response to mincing of the myocardium in the adult newt, Notophthalmus viridescens. Cell Tissue Res. 1988;253:619–624. doi: 10.1007/BF00219752. [DOI] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpant NJ, Yasuda S, MacDougald O, Metzger JM. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol. 2007;43:362–370. doi: 10.1016/j.yjmcc.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr, Kormos R, Benetti F. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg. 2005;130:1631–1638. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102:1471–1482. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, O'Connor CM, Henry T, Taussig A, Kereiakes DJ, Fortuin FD, Niederman A, Schatz R, Spencer RT, Owens D, Banks M, Joseph D, Roberts R, Alexander JH, Sherman W. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J. 2011;162:654–662. doi: 10.1016/j.ahj.2011.07.020. e651. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, Junge C, Nada A, Schatz RA, Harrington RA, Davidson CJ, Fortuin FD, Kereiakes DJ, Mendelsohn FO, Sherman W, Schaer GL, White CJ, Stewart D, Story K, Losordo DW, Henry TD. A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J. 2013;165:854–861. doi: 10.1016/j.ahj.2013.03.003. e852. [DOI] [PubMed] [Google Scholar]
- Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53:323–332. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933–942. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, Sternik G, Tsai HJ, Rodriguez-Esteban C, Izpisua-Belmonte JC. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11889–11895. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler S, Yen AH, Lu J, Petrenko NB, Lu MM, Manderfield LJ, Patel VV, Fishman GI, Epstein JA. Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation. 2012;126:1058–1066. doi: 10.1161/CIRCULATIONAHA.112.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Rosen MR, Myerburg RJ, Francis DP, Cole GD, Marban E. Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. J Am Coll Cardiol. 2014;64:922–937. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Gatien S. Regeneration in axolotls: a model to aim for! Exp Gerontol. 2008;43:968–973. doi: 10.1016/j.exger.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ruiz-Villalba A, Ziogas A, Ehrbar M, Perez-Pomares JM. Characterization of epicardial-derived cardiac interstitial cells: differentiation and mobilization of heart fibroblast progenitors. PLoS ONE. 2013;8:e53694. doi: 10.1371/journal.pone.0053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara M, Hansson EM, Wernet O, Lui KO, Spater D, Chien KR. Manipulation of a VEGF-Notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2014;24:820–841. doi: 10.1038/cr.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012;14:892–899. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Yu J, Corti R, Mathey DG, Hamm CW, Tonn T, Dimmeler S, Zeiher AM, investigators R-A. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11:973–979. doi: 10.1093/eurjhf/hfp113. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, Lohr JL, Cornfield DN, Ekker SC, Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- Schlueter J, Brand T. Epicardial progenitor cells in cardiac development and regeneration. Cardiovasc Transl Res. 2012;5:641–653. doi: 10.1007/s12265-012-9377-4. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, Zund G, Hoerstrup SP. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation. 2007;116:I64–I70. doi: 10.1161/CIRCULATIONAHA.106.681494. [DOI] [PubMed] [Google Scholar]
- Schnabel K, Wu CC, Kurth T, Weidinger G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS ONE. 2011;6:e18503. doi: 10.1371/journal.pone.0018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukushima S, Varela-Carver A, Lee J, Coppen SR, Takahashi K, Brouilette SW, Yashiro K, Terracciano CM, Yacoub MH, Suzuki K. Donor cell-type specific paracrine effects of cell transplantation for post-infarction heart failure. J Mol Cell Cardiol. 2009;47:288–295. doi: 10.1016/j.yjmcc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Singh BN, Koyano-Nakagawa N, Garry JP, Weaver CV. Heart of newt: a recipe for regeneration. Cardiovasc Transl Res. 2010;3:397–409. doi: 10.1007/s12265-010-9191-9. [DOI] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Lewis FC, Aquila I, Waring CD, Nocera A, Agosti V, Nadal-Ginard B, Torella D, Ellison GM. Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc. 2014;9:1662–1681. doi: 10.1038/nprot.2014.113. [DOI] [PubMed] [Google Scholar]
- Soh BS, Buac K, Xu H, Li E, Ng SY, Wu H, Chmielowiec J, Jiang X, Bu L, Li RA, Cowan C, Chien KR. N-cadherin prevents the premature differentiation of anterior heart field progenitors in the pharyngeal mesodermal microenvironment. Cell Res. 2014;24:1420–1432. doi: 10.1038/cr.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spater D, Abramczuk MK, Buac K, Zangi L, Stachel MW, Clarke J, Sahara M, Ludwig A, Chien KR. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat Cell Biol. 2013;15:1098–1106. doi: 10.1038/ncb2824. [DOI] [PubMed] [Google Scholar]
- Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Strungs EG, Ongstad EL, O'Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol Biol. 2013;1037:343–353. doi: 10.1007/978-1-62703-505-7_20. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, Nelson KH, Gerstenblith G, DiFede Velazquez DL, Breton E, Sitammagari K, Schulman IH, Taldone SN, Williams AR, Sanina C, Johnston PV, Brinker J, Altman P, Mushtaq M, Trachtenberg B, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: an analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114:1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Editors. Expression of concern: the SCIPIO trial. Lancet. 2014;383:1279. doi: 10.1016/S0140-6736(14)60608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NA, Koudijs M, van Eeden FJ, Joyner AL, Yelon D. Hedgehog signaling plays a cell-autonomous role in maximizing cardiac developmental potential. Development. 2008;135:3789–3799. doi: 10.1242/dev.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133:1943–1953. doi: 10.1242/dev.02365. [DOI] [PubMed] [Google Scholar]
- Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Torella D, Ellison GM, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells fulfill Koch's postulates as causal agents for cardiac regeneration. Circ Res. 2014;114:e24–e26. doi: 10.1161/CIRCRESAHA.113.303313. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn. 2008;237:1144–1152. doi: 10.1002/dvdy.21502. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng. 2011;13:245–267. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013;110:12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138:3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao N, Spencer CI, Nie B, Ma T, Xu T, Zhang Y, Wang X, Srivastava D, Ding S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014;6:951–960. doi: 10.1016/j.celrep.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HJ, Chen CH, Lee WY, Chiu I, Hwang SM, Lin WW, Huang CC, Yeh YC, Chang Y, Sung HW. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Weinberger F, Mehrkens D, Friedrich FW, Stubbendorff M, Hua X, Muller JC, Schrepfer S, Evans SM, Carrier L, Eschenhagen T. Localization of Islet-1-positive cells in the healthy and infarcted adult murine heart. Circ Res. 2012;110:1303–1310. doi: 10.1161/CIRCRESAHA.111.259630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Z, Kong CW, Ren L, Karakikes I, Geng L, He J, Chow MZ, Mok CF, Keung W, Chow H, Leung AY, Hajjar RJ, Li RA, Chan CW. A simple, cost-effective but highly efficient system for deriving ventricular cardiomyocytes from human pluripotent stem cells. Stem Cells Dev. 2014;23:1704–1716. doi: 10.1089/scd.2013.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Grieskamp T, Airik R, Mommersteeg MT, Gardiwal A, de Gier-deVries C, Schuster-Gossler K, Moorman AF, Kispert A, Christoffels VM. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ Res. 2009;104:388–397. doi: 10.1161/CIRCRESAHA.108.187062. [DOI] [PubMed] [Google Scholar]
- Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Chuang PT. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development. 2010;137:2079–2094. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Winter EM, Gittenberger-de Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703. doi: 10.1007/s00018-007-6522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter EM, Grauss RW, Hogers B, van Tuyn J, van der Geest R, Lie-Venema H, Steijn RV, Maas S, DeRuiter MC, deVries AA, Steendijk P, Doevendans PA, van der Laarse A, Poelmann RE, Schalij MJ, Atsma DE, Gittenberger-de Groot AC. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007;116:917–927. doi: 10.1161/CIRCULATIONAHA.106.668178. [DOI] [PubMed] [Google Scholar]
- Witman N, Murtuza B, Davis B, Arner A, Morrison JI. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol. 2011;354:67–76. doi: 10.1016/j.ydbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Witman N, Heigwer J, Thaler B, Lui WO, Morrison JI. miR-128 regulates non-myocyte hyperplasia, deposition of extracellular matrix and Islet1 expression during newt cardiac regeneration. Dev Biol. 2013;383:253–263. doi: 10.1016/j.ydbio.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Xin M, Small EM, van Rooij E, Qi X, Richardson JA, Srivastava D, Nakagawa O, Olson EN. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci USA. 2007;104:7975–7980. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yi BA, Wu H, Bock C, Gu H, Lui KO, Park JH, Shao Y, Riley AK, Domian IJ, Hu E, Willette R, Lepore J, Meissner A, Wang Z, Chien KR. Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature. Cell Res. 2012;22:142–154. doi: 10.1038/cr.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Yao K, Huang R, Qian J, Cui J, Ge L, Li Y, Zhang F, Shi H, Huang D, Zhang S, Sun A, Zou Y, Ge J. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–1153. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhou Y, Cai H, Tan W. Myocardial regeneration: roles of stem cells and hydrogels. Adv Drug Deliv Rev. 2011;63:688–697. doi: 10.1016/j.addr.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Yin VP, Lepilina A, Smith A, Poss KD. Regulation of zebrafish heart regeneration by miR-133. Dev Biol. 2012;365:319–327. doi: 10.1016/j.ydbio.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Yamanaka S. iPS cells: a source of cardiac regeneration. J Mol Cell Cardiol. 2011;50:327–332. doi: 10.1016/j.yjmcc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Spater D, Xu H, Tabebordbar M, Gorbatov R, Sena B, Nahrendorf M, Briscoe DM, Li RA, Wagers AJ, Rossi DJ, Pu WT, Chien KR. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–792. [PubMed] [Google Scholar]
- Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Han P, Yang H, Ouyang K, Lee D, Lin YF, Ocorr K, Kang G, Chen J, Stainier DY, Yelon D, Chi NC. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498:497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008a;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008b;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, Ma Q, Oh JH, Lin RZ, Melero-Martin JM, von Gise A, Zhou P, Hu T, He L, Wu KH, Zhang H, Zhang Y, Pu WT. Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J Mol Cell Cardiol. 2012;52:43–47. doi: 10.1016/j.yjmcc.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]