Abstract
Foraging honey bees (Apis mellifera L.) can routinely travel as far as several kilometers from their hive in the process of collecting nectar and pollen from floral patches within the surrounding landscape. Since the availability of floral resources at the landscape scale is a function of landscape composition, apiculturists have long recognized that landscape composition is a critical determinant of honey bee colony success. Nevertheless, very few studies present quantitative data relating colony success metrics to local landscape composition. We employed a beekeeper survey in conjunction with GIS-based landscape analysis to model colony success as a function of landscape composition in the State of Ohio, USA, a region characterized by intensive cropland, urban development, deciduous forest, and grassland. We found that colony food accumulation and wax production were positively related to cropland and negatively related to forest and grassland, a pattern that may be driven by the abundance of dandelion and clovers in agricultural areas compared to forest or mature grassland. Colony food accumulation was also negatively correlated with urban land cover in sites dominated by urban and agricultural land use, which does not support the popular opinion that the urban environment is more favorable to honey bees than cropland.
Keywords: Apiculture, Pollinator, Citizen-science, Urban beekeeping, Landscape ecology
Introduction
Honey bees (Apis mellifera, L.) exist in large, eusocial colonies that require massive and sustained inputs of floral nectar and pollen. They meet this demand by foraging at an extremely large spatial scale and with rapid responsiveness to changes in the surrounding floral community (Visscher & Seeley, 1982; Seeley, 1995). Depending on local floral availability, colonies may routinely forage over an area of more than 100 km2 (Seeley, 1995), and much larger ranges have been reported under extreme conditions (Eckert, 1931; Beekman & Ratnieks, 2001).
Because honey bee foraging is a decidedly landscape-scale process, one should expect landscape composition to interact meaningfully with colony nutrition and overall colony success. While the plausibility of such a relationship is widely acknowledged (Steffan-Dewenter & Kuhn, 2003; Naug, 2009; Van Engelsdorp & Meixner, 2010; Härtel & Steffan-Dewenter, 2014), and the importance of apiary location is axiomatic among practicing beekeepers, there are very few published studies that quantitatively measure colony success in response to local landscape variables. As rapid landscape conversion continues as a global phenomenon, and beekeepers in many regions continue to suffer unsustainable losses, the task of refining and expanding our knowledge of honey bee landscape ecology takes on obvious urgency.
Several studies have indirectly explored the relationship between landscape and colony success by analyzing the spatial information encoded in the honey bee dance language (von Frisch , 1967). Waddington et al. (1994) found that colonies located in two suburban landscapes tended to forage over a smaller area and with a less clumped distribution than a previously studied colony located in a temperate deciduous forest (Visscher & Seeley, 1982), suggesting that suburban landscapes might provide richer and more evenly distributed resource patches. Similarly, Garbuzov, Schürch & Ratnieks (2014) found that colonies in the city of Brighton, UK, concentrated most of their foraging within city limits rather than venturing into surrounding countryside that was well within their foraging range. Conversely, Beekman & Ratnieks (2001) observed remarkably long-distance foraging under conditions of apparently scarce local resources in a suburban landscape and highly rewarding resources in outlying seminatural heather moors. In agricultural landscapes, honey bee foraging patterns suggest that pollen sources can be scarcer and floral patches less spatially and temporally variable in highly simplified cropping systems compared to more structurally complex habitats (Steffan-Dewenter & Kuhn, 2003), while conservation management within farmlands can increase the availability of bee-attractive flora (Couvillon, Schürch & Ratnieks, 2014).
Landscape composition can also influence the type and quality of pollen foraged by honey bees. Donkersley et al. (2014) found that the protein content of “beebread” (processed pollen stored by honey bees) was negatively correlated with agricultural land cover and positively correlated with broad-leaf forest, improved grassland, and urban land cover.
Two recent studies have directly related colony success to local landscape variables (Sande et al., 2009; Odoux et al., 2014). In the dry coastal forest habitat of southeastern Kenya, Sande et al. (2009) found that a colony’s honey production was positively correlated with its proximity to forest patches. Odoux et al. (2014) similarly found that colony size was positively correlated with forest land cover in the intensively agricultural landscape of central-western France.
Among non-peer-reviewed sources, there is a widely circulated opinion that honey bee success is favored by urban/suburban landscapes, especially in comparison to cropland (Graham, 1992; Anonymous, 2008; Wilson-Rich, 2012). These claims remain unsubstantiated but plausible given the ostensibly positive effects of suburban land use suggested by Waddington et al. (1994) and the more direct evidence supporting the favorability of suburban land use for bumble bees (Hymenoptera: Bombus, Latreille) living in predominantly agricultural areas (Goulson et al., 2002; Goulson et al., 2010).
Here, we present a quantitative study of honey bee colony success in relation to landscape composition in the State of Ohio, USA, a region characterized by a mixture of intensive cropland, deciduous forest, grassland, and urban development. While there are many ways to measure colony success, we focus on four metrics that are highly relevant to beekeepers and easily assessed through simple hive inspection: honey and pollen accumulation, wax production, adult population, and brood population. Using a citizen-science survey, we investigate the relationship between colony success and the landscape as a whole, accounting for all major land cover types and also for the potential influence of hive management variables that vary between beekeepers. Then, we specifically evaluate the putative favorability of urban land use using a subset of sites dominated by urban development and/or cropland.
Materials and Methods
Survey design
In 2012 and 2013, we used a survey-based, citizen-science approach to measure the productivity of honey bee colonies in the state of Ohio, USA. All participants were beekeepers whose hives were registered with the Ohio Department of Agriculture and who volunteered to participate in our study. Volunteers were enlisted through a combination of email communications, public speaking engagements, and cooperation with local beekeeping organizations; our study was publicized as widely as possible, and we did not attempt to target any particular demographic. Our survey was conducted with written exemption from IRB review by the Ohio State University Office of Responsible Research Practices (Protocol # 2012E0136 and 2013E0012).
In order to standardize the initial strength of the colonies in our study (hereafter “study colonies”) and minimize the influence of parasites and pathogens, we restricted our study to colonies that had been started from artificial swarms, known as “package bees,” in the spring of each study year. Honey bee packages are created by combining a standard quantity of worker bees (usually 1.36 kg) with a newly mated queen. The initial strength of colonies started from package bees is, therefore, less variable than that of over-wintered colonies. Moreover, because they are sold without comb or brood, they tend to have reduced parasite and pathogen loads.
Data for each study colony were gathered using a two-part survey consisting of spring and fall components (hereafter “spring survey” and “fall survey”). The spring survey was made available beginning in early March, and participants were instructed to complete the survey immediately after installing their honey bee packages. In the spring survey, we gathered the geographic location of each study colony and the years of experience of each participating beekeeper (see Supplemental Information S1 for full spring survey questionnaire). The fall survey was made available in mid-September and completed by mid-October. To complete the fall survey, each participant performed a frame-by-frame hive inspection and reported the number of frames in the study hive belonging to the following categories: (1) more than half honey/nectar, (2) more than half pollen, (3) more than half brood, (4) more than half empty wax comb, (5) more than half bare foundation (no wax comb). Participants also reported the quantity of sugar syrup that had been given to their hives as supplemental feeding, a common beekeeping practice that could affect colony success. See Supplemental Information S2 for the full fall survey questionnaire.
Survey processing
Each beekeeper was instructed to submit data for only one study hive at one apiary site, and each beekeeper was included in only one of the two years of our study. The data quality of each survey was carefully vetted prior to analysis, and surveys missing critical data or having irreconcilable inconsistencies were discarded. Fall surveys reporting hives that had died since spring installation were also discarded. The final numbers of surveys included in analyses for 2012 and 2013 were 32 and 18, respectively; these were selected from a pre-processing total of 55 surveys in 2012 and 33 in 2013. The minimum distance between study hives, combining both years, was 2.65 km.
From our survey data, we derived four metrics to represent colony success: net food accumulation, net wax production, adult population, and brood population. For consistency, all metrics were recorded in units of standard deep frames.
Net food accumulation:
where H = honey/nectar frames in hive at time of inspection, Hharv = honey frames harvested prior to inspection, Hadd = honey frames added to the hive prior to inspection (beekeepers sometimes transfer honey frames between hives to increase food stores of weak colonies), and P = frames of pollen in hive at time of inspection. This variable will hereafter be abbreviated Food.
Net wax production:
where B = brood frames in hive at time of inspection, Brm = brood frames removed prior to inspection (brood frames may be transferred between colonies to modulate population size), D = drawn but mostly empty frames in hive at time of inspection, Badd = brood frames added to the hive prior to inspection, and Dadd = drawn but mostly empty frames (frames with wax comb constructed but no cell contents) added to hive prior to inspection. This variable will hereafter be abbreviated Wax.
Adult population (hereafter, AdultPop) was measured as the number of frames “more than half covered” with adult bees at time of inspection. Brood population (hereafter, BroodPop) was simply the number of “mostly brood” frames reported by the inspecting beekeeper.
We also measured two hive management variables: years of beekeeping experience of the participating beekeeper (experience) and quantity of sugar syrup fed to the study hive since its installation (syrup).
Landscape analysis
Geographic coordinates for each study hive were determined and mapped using QGIS v.2.1 (QGIS Development Team , 2014). To encompass a range of spatial scales at which landscape effects on colony success might be seen, we defined the landscape of each hive using six nested buffers having radii of 0.5, 1, 2, 3, 4, and 5 km, respectively. Land cover data for the State of Ohio were obtained from the 2006 dataset provided by the National Land Cover Database (NLCD 2006) (Fry et al., 2011). The NLCD 2006 land cover layer for Ohio is comprised primarily of seven land cover classes: cultivated crops, pasture/hay, deciduous forest, and four levels of urban development (open space, low intensity, medium intensity, high intensity). Minor classes, present only at very low abundance, include evergreen forest, mixed forest, woody wetland, herbaceous wetland, grassland/herbaceous, shrub/scrub, barren land, and open water. To simplify our analysis of landscape composition, we condensed the non-crop land cover classes (ignoring barren land and open water) into three aggregate classes: Forest (deciduous + evergreen + mixed + woody wetland + shrub/scrub), Grassland (pasture/hay + grassland/herbaceous + herbaceous wetland), and Urban (open space + low intensity + medium intensity + high intensity). The landscape composition of each study site, measured in terms of the total land cover of Crop (cultivated crop) and each aggregate class, was determined at each spatial scale using LECOS (Jung, 2013), a QGIS plugin for calculating patch-based landscape metrics. As a measure of overall landscape heterogeneity, we also calculated Simpson’s Diversity Index (D) based on the original, non-aggregated land cover classes.
Data analysis
We first reduced the dimensionality of our landscape data using principal components analysis (PCA) based on the covariance between the variables Crop, Forest, Grassland, and Urban. This step was repeated for each spatial scale. For all scales, the first two principal components (PC1 and PC2) explained >96% of total variance.
To model the relationship between landscape composition and colony success, accounting also for the management variables experience and syrup, we conducted model selection using Akaike’s Information Criterion corrected for small sample size (AICc) (Burnham & Anderson, 2002). Each success metric–Food, Wax, AdultPop, and BroodPop–was modeled separately. Fourteen candidate linear models were constructed for each success metric at each spatial scale; these included all combinations of the landscape variables (PC1, PC2, D) and the coupled management variables experience and syrup, a year-only model, and an intercept-only model. For each success metric, we present the candidate model having the lowest AICc score at each scale along with any competing models having an AICc difference of <2 (Table 1) (Burnham & Anderson, 2002). We then selected a single best model for each success metric by choosing the model with the lowest AICc score across all spatial scales.
Table 1. Summary of model selection statistics for each colony success metric.
Only models with AICc < 2 are presented as competing models. Models within each spatial scale are listed in order of increasing AICc value. The best model for each success metric is depicted in bold.
| Metric | Radius (km) | Model | Log-likelihood | Ki | AICc | ΔAICc | Wi | Adjusted r2 | Coefficients |
|---|---|---|---|---|---|---|---|---|---|
| Food | 0.5 | PC2 | −165.808 | 3 | 338.138 | 0.00 | 0.233 | 0.047 | −5.9142 |
| ” | 0.5 | PC1 + PC2 | −165.060 | 4 | 339.008 | 0.87 | 0.151 | 0.055 | PC2 = −5.9142, PC1 = 2.5032 |
| ” | 0.5 | Intercept | −167.515 | 2 | 339.286 | 1.15 | 0.563 | 0.131 | |
| ” | 1 | PC2 | −165.134 | 3 | 336.791 | 0.00 | 0.260 | 0.072 | −7.3139 |
| ” | 1 | PC1 + PC2 | −164.175 | 4 | 337.240 | 0.45 | 0.208 | 0.088 | PC2 = −7.3139, PC1 = 2.9608 |
| ” | 2 | PC2 | −165.686 | 3 | 337.894 | 0.00 | 0.197 | 0.051 | −6.541 |
| ” | 2 | PC1 + PC2 | −164.553 | 4 | 337.995 | 0.10 | 0.187 | 0.074 | PC2 = −6.5409, PC1 = 3.5536 |
| ” | 2 | intercept | −167.515 | 2 | 339.286 | 1.39 | 0.499 | 0.098 | |
| ” | 2 | PC1 + PC2 + D | −163.990 | 5 | 339.343 | 1.45 | 0.095 | 0.075 | PC2 = −7.529, PC1 = 5.195, D = 7.674 |
| ” | 2 | PC1 | −166.464 | 3 | 339.450 | 1.56 | 0.090 | 0.021 | 3.5536 |
| ” | 3 | PC2 | −165.871 | 3 | 338.265 | 0.00 | 0.183 | 0.044 | −6.0981 |
| ” | 3 | PC1 + PC2 | −164.733 | 4 | 338.355 | 0.09 | 0.175 | 0.067 | PC2 = −6.0981, PC1 = 3.7970 |
| ” | 3 | intercept | −167.515 | 2 | 339.286 | 1.02 | 0.600 | 0.110 | |
| ” | 3 | PC1 | −166.451 | 3 | 339.424 | 1.16 | 0.103 | 0.022 | 3.7970 |
| ” | 3 | PC1 + PC2 + D | −164.247 | 5 | 339.858 | 1.59 | 0.083 | 0.065 | PC2 = −6.554, PC1 = 5.729, D = 7.200 |
| ” | 4 | PC2 | −166.135 | 3 | 338.791 | 0.00 | 0.179 | 0.034 | −5.5831 |
| ” | 4 | intercept | −167.515 | 2 | 339.286 | 0.49 | 0.781 | 0.140 | |
| ” | 4 | PC1 + PC2 | −165.202 | 4 | 339.293 | 0.50 | 0.139 | 0.050 | PC2 = −5.5831, PC1 = 3.5906 |
| ” | 4 | PC1 | −166.634 | 3 | 339.789 | 1.00 | 0.109 | 0.015 | 3.5906 |
| ” | 5 | PC2 | −166.203 | 3 | 338.928 | 0.00 | 0.174 | 0.031 | −5.378 |
| ” | 5 | intercept | −167.515 | 2 | 339.286 | 0.36 | 0.836 | 0.145 | |
| ” | 5 | PC1 + PC2 | −165.269 | 4 | 339.428 | 0.50 | 0.135 | 0.047 | PC2 = −5.3783, PC1 = 3.6745 |
| ” | 5 | PC1 | −166.630 | 3 | 339.782 | 0.85 | 0.113 | 0.015 | 3.6745 |
| Wax | 0.5 | PC2 | −180.163 | 3 | 366.848 | 0.00 | 0.242 | 0.041 | −7.525 |
| ” | 0.5 | Year | −180.538 | 3 | 367.598 | 0.75 | 0.687 | 0.167 | 0.02648 |
| ” | 0.5 | intercept | −181.724 | 2 | 367.704 | 0.86 | 0.652 | 0.158 | |
| ” | 1 | PC2 | −179.240 | 3 | 365.001 | 0.00 | 0.299 | 0.076 | −9.917 |
| ” | 1 | PC2 + D | −178.958 | 4 | 366.804 | 1.80 | 0.122 | 0.067 | PC2 = −8.892, D = −6.540 |
| ” | 2 | PC2 | −178.695 | 3 | 363.911 | 0.00 | 0.341 | 0.096 | −11.053 |
| ” | 2 | PC2 + D | −178.388 | 4 | 365.665 | 1.75 | 0.142 | 0.088 | PC2 = −10.247, D = −6.265 |
| ” | 2 | PC2 + years + syrup | −177.249 | 5 | 365.862 | 1.95 | 0.129 | 0.109 | PC2 = −11.8583, years = 0.1252, syrup = 0.2578 |
| ” | 3 | PC2 | −179.076 | 3 | 364.673 | 0.00 | 0.278 | 0.082 | −10.183 |
| ” | 3 | PC2 + D | −178.374 | 4 | 365.636 | 0.96 | 0.172 | 0.088 | PC2 = −9.611, D = −9.020 |
| ” | 3 | PC2 + years + syrup | −177.453 | 5 | 366.270 | 1.60 | 0.125 | 0.102 | PC2 = −11.4033, years = 0.1346, syrup = 0.2765 |
| ” | 4 | PC2* | −179.411 | 3 | 365.344 | 0.00 | 0.260 | 0.069 | −9.514 |
| ” | 4 | PC2 + D | −178.721 | 4 | 366.331 | 0.99 | 0.159 | 0.075 | PC2 = −9.281, D = −8.998 |
| ” | 4 | PC2 + years + syrup | −177.827 | 5 | 367.017 | 1.67 | 0.113 | 0.089 | PC2 = −10.7781, years = 0.1244, syrup = 0.2762 |
| ” | 5 | PC2* | −179.465 | 3 | 365.451 | 0.00 | 0.255 | 0.067 | −9.290 |
| ” | 5 | PC2 + D | −178.750 | 4 | 366.389 | 0.94 | 0.159 | 0.074 | PC2 = −9.253, D = −9.112 |
| ” | 5 | PC2 + years + syrup | −177.865 | 5 | 367.095 | 1.64 | 0.112 | 0.087 | PC2 = −10.5842, years = 0.1317, syrup = 0.2776 |
| AdultPop | 2 | PC2 + years + syrup | −160.864 | 5 | 333.092 | 1.47 | 0.205 | 0.172 | PC2 = −3.0878, years = 0.4904, syrup = 0.2896 |
| ” | 3 | PC2 + years + syrup | −160.590 | 5 | 332.544 | 0.92 | 0.247 | 0.181 | PC2: −3.7837, years: 0.4939, syrup: 0.2991 |
| ” | 4 | PC2 + years + syrup | −160.652 | 5 | 332.668 | 1.05 | 0.235 | 0.179 | PC2 = −3.6243, years = 0.4906, syrup = 0.2993 |
| ” | 4 | D + years + syrup | −161.090 | 5 | 333.544 | 1.92 | 0.151 | 0.164 | D: 4.2943, years: 0.5219, syrup: 0.3059 |
| ” | 5 | PC2 + years + syrup | −160.634 | 5 | 332.631 | 1.01 | 0.234 | 0.180 | PC2 = −3.6267, years = 0.4931, syrup = 0.3002 |
| ” | 5 | D + years + syrup | −161.002 | 5 | 333.367 | 1.75 | 0.162 | 0.167 | D = 4.9270, years = 0.5308, syrup = 0.3094 |
| ” | NA | years+syrup | −161.365 | 4 | 331.620 | 0.00 | 0.475 | 0.173 | years = 0.4887, syrup = 0.2774 |
To evaluate the prediction that urban land cover favors honey bee success relative to agricultural land cover, we first extracted the subset of our sites (n = 30–33, varying with spatial scale) for which Urban + Crop was greater than 50% of total landcover, a threshold chosen a priori to identify sites that were strongly characterized by urban and/or agricultural land use. Then, we then set up separate linear regression models for Food and Wax with Urban as the explanatory variable. Only Food and Wax were analyzed because the results of the PCA described above indicated that only these two success metrics should be expected to respond to landscape variables. We did not use experience and syrup as covariates because previous analysis showed they were not predictive of Food or Wax. Regression analysis was repeated for each spatial scale.
All analyses were performed in R statistical software (R Core Team, 2013). AICc model selection used the package AICcmodavg (Mazerolle, 2014). Model assumptions were verified by visual assessment using the plot (lm) function in R.
Results
Landscape analysis
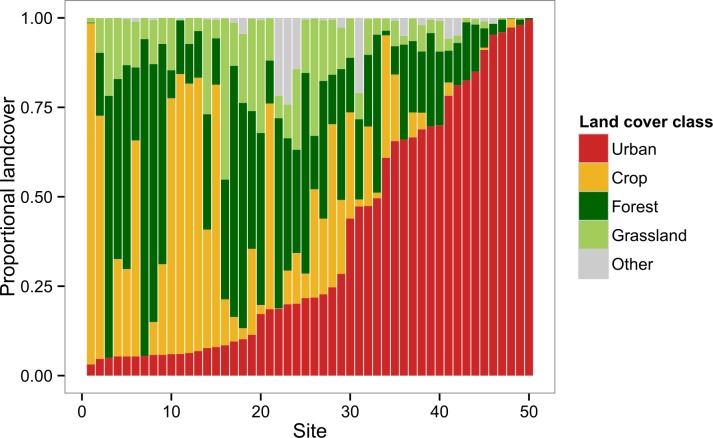
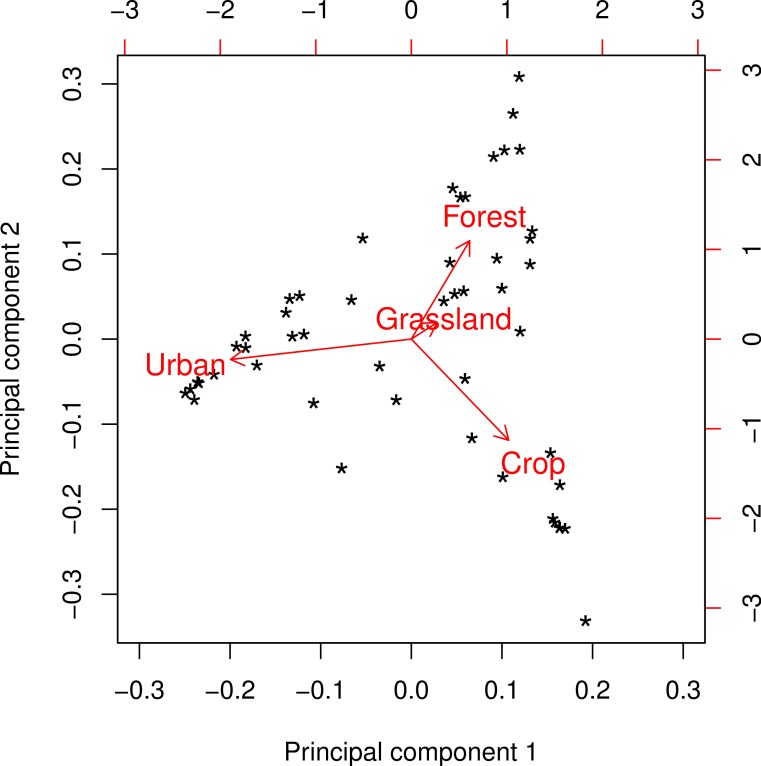
The landscapes surrounding the colonies in our survey represented a broad range of landscape composition in terms of the major land cover classes: Crop, Forest, Grassland, and Urban (Fig. 1). Principal components analysis of these four variables yielded two readily interpretable axes that explained greater than 96% of total variance (Fig. 2). PC1 was essentially an urban-rural axis, with sites dominated by Urban scoring low and sites dominated by combinations of Crop, Forest, and/or Grassland scoring high. PC2 partitioned non-urban landscapes into those characterized by Crop and those characterized by Forest and, to a lesser extent, Grassland.
Figure 1. Landscape composition of study sites at 2 km radius.
Sites are depicted in order of increasing urban (red) land cover. Other major land cover classes include crop (gold), forest (dark green), and grassland (light green). Remaining land cover (grey) consisted of barren land and open water.
Figure 2. Principal components biplot of major land cover classes at a radius of 2 km.
Principal component 1 (PC1) comprises an urban-rural axis, with lower scores corresponding to higher urbanness. Principal component 2 (PC2) forms an axis that separates sites characterized by forest/grassland from those characterized by cropland. This pattern was consistent at all spatial scales with only minor variation.
Modeling colony success metrics by landscape principal components
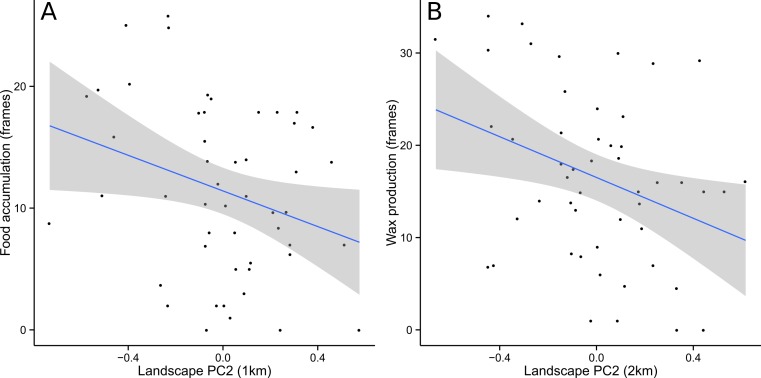
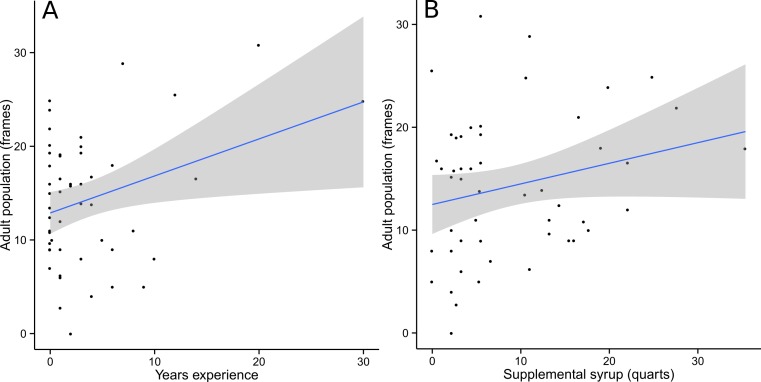
Food and Wax were best modeled with PC2 as the only explanatory variable. Almost all competing models (ΔAICc <2) included PC2 alongside other explanatory variables, further supporting the conclusion that PC2 was the single most important predictor (Table 1). For Food, the optimal spatial scale was a 1 km radius, while Wax was best predicted at a 2 km radius. In both cases, the relationship was negative and the linear regression models were statistically significant (Food: F = 4.796, df = 48, p = 0.033; Wax: F = 6.184, df = 48, p = 0.016) (Fig. 3). AdultPop was best modeled with the coupled management variables experience and syrup as the only explanatory variables. The relationship was positive and the linear regression model was significant (F = 6.128, df = 47, p = 0.004), with significant contributions from both experience (t = 2.98, df = 47, p = 0.005) and syrup (t = 2.474, df = 47, p = 0.017) (Fig. 4). BroodPop was best predicted by the intercept-only model, indicating that none of our measured explanatory variables were good predictors of this success metric.
Figure 3. Food accumulation and wax production were negatively correlated with PC2.
This indicates that productivity in terms of food and wax increased in the direction of cropland and decreased in the direction of forest/grassland. A 95% confidence band is shaded in gray.
Figure 4. Adult population was positively correlated with beekeeper years of experience (A) and supplemental syrup feeding (B).
A 95% confidence band is shaded in gray.
Modeling colony success metrics by urban landcover
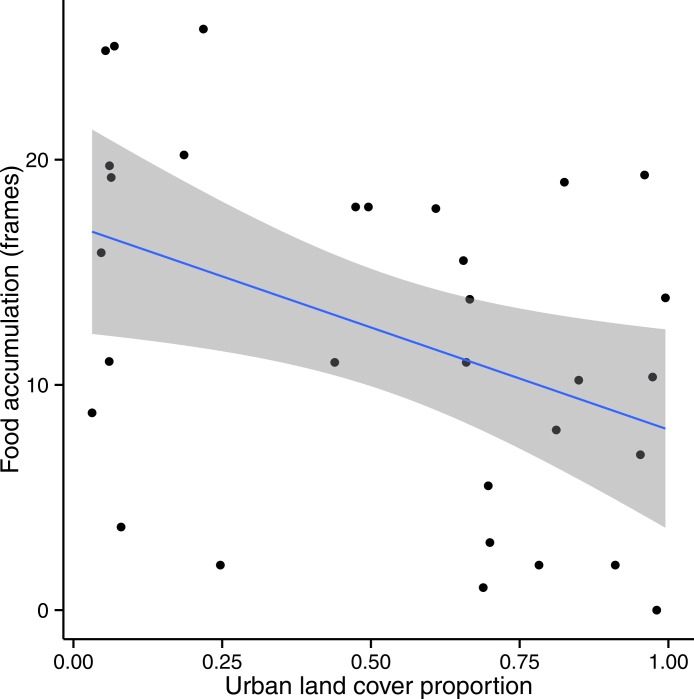
In the subset of sites for which Urban + Crop was greater than 50% of total land cover, we found a significant (p < 0.05) negative relationship between Food and Urban (Fig. 5) at all spatial scales except for the two extremes of 0.5 km and 5 km; the relationship was strongest at the 2 km scale (F = 6.041, df = 29, p = 0.02). Wax was not significantly related to Urban (p > 0.05).
Figure 5. Colony food accumulation decreased significantly with increasing urban land cover in sites where Urban + Crop > 50%.
This pattern was strongest at a 1 km radius (shown above). A 95% confidence band is shaded in gray.
Discussion
The negative responses of Food and Wax to PC2 indicate that food accumulation and wax production increase with surrounding cropland and decrease with forest/grassland. This finding seems to contradict the conventional wisdom that agricultural land conversion threatens honey bee nutrition through the depauperation of floral resources relative to semi-natural environments (De La Rúa et al., 2009), but is consistent with studies that have found honey bees to be notably resilient to natural habitat loss compared to other bee taxa (Ricketts et al., 2008; Winfree et al., 2009). The productivity of honey bees does not depend so much on the presence of undisturbed natural floral communities as it does on the availability of rich resources that can be exploited efficiently by cooperative foraging (Visscher & Seeley, 1982), and agricultural environments can offer honey bees surprisingly rich floral resources in the form of “weeds” (Odoux et al., 2012; Requier et al., in press). In Ohio, the largest honey yield is believed to come from non-native clovers (Trifolium spp. L.) (Pellett, 1920; Bailey, 1955; Goltz, 1975); these plants grow abundantly along roadsides, in field margins, and in grassy yards, but they are scarce in habitats shaded by forest canopy or dominated by the dense herbaceous vegetation of unmowed grassland. In addition to the clovers, Erickson (1984) observed that, under some conditions, honey bees will forage very productively on soybean (Glycine max (L.) Merr.), and corn/soybean rotations comprise the vast majority of Ohio cropland. Dandelion (Taraxacum officinale FH Wigg.), one of the most important spring flora for honey bees in the Midwest (Jaycox, 1976) during the period of peak wax production, is distributed in much the same pattern as the clovers, thus favoring wax production in cropland over seminatural forest and grassland.
Interestingly, our finding that colony productivity is favored by cropland relative to forest/grassland is strikingly consistent with an anecdotal description of regional honey production in Ohio published nearly forty years ago (Goltz, 1975). In Goltz’ account, the areas of “primary” and “secondary” importance for honey production are in the heavily cultivated glacial plains that comprise most of the state, while the forest-dominated Appalachian Plateaus in the southeast are described as only “marginally” productive.
The positive response of AdultPop to the management variables experience and syrup is difficult to interpret. In early spring, when new colonies are very small and limited in their foraging ability, it is standard practice to supplement colony nutrition with sugar syrup. All workers produced during the period of spring build-up, though, died long before colonies were inspected in the fall, so any positive effect of springtime management on adult population at time of inspection would have to be mediated by factors that allow colonies to increase reproduction later in the year. An alternative interpretation is plausible if we allow that significant feeding may have occurred later in the year. While supplemental feeding is normally concentrated in early spring, some Ohio beekeepers also feed their colonies in mid-late summer, a period of perceived dearth in natural forage. Feeding during the summer dearth period might trigger a population increase that would persist until fall inspection. Our survey did not distinguish between feeding at different times during the season. The effect of beekeeper experience on adult population is difficult to parse, as all aspects of hive management would be expected to improve with increasing experience. Somewhat ironically, a positive relationship between colony success and beekeeper experience might be explained by the tendency of more experienced beekeepers to perform less colony management; the enthusiasm of new beekeepers can lead to unnecessary interventions that do more to disturb natural colony function than to ameliorate ills (J Tew, pers. comm., 2014).
By late September and early October, when beekeepers were inspecting their colonies for the fall survey, the bees had likely already begun to reduce brood rearing in preparation for winter (Graham, 1992). This would explain the failure of both landscape and management variables in predicting BroodPop.
The negative relationship observed between Food and Urban in the subset of our sites strongly characterized by urban and/or agricultural land use does not support the popular opinion that urban landscapes favor honey bee success relative to agricultural landscapes. At least in Ohio, the relationship appears to be the opposite, and the fact that Food was the only success metric to respond to Urban ratio suggests a likely mechanism. The last major nectar and pollen flow in Ohio is usually from goldenrods (Solidago spp. L.) (Morse, 1972; DB Sponsler, 2014, unpublished data), which bloom prolifically from late summer into fall, roughly the same period during which beekeepers in our study were conducting fall hive inspections and filling out the fall survey. At this time of year, honey bees rarely produce additional wax (Lee & Winston, 1985), and brood rearing has begun to slow down in preparation for winter (Graham, 1992), so incoming food is stored rather than being invested in brood or wax production. Goldenrods occur abundantly in uncultivated fields and conservation strips throughout agricultural landscapes, but they are relatively scarce in developed areas where vegetation is more often subject to mowing and weed control. This is consistent with the anecdotal observation of Burgett, Caron & Ambrose (1978) that urban hives tend to have poor late-season honey production, which the authors attribute to scarcity of late-blooming “weeds,” including goldenrods.
We conclude that both landscape composition and colony management contribute to the success of nascent honey bee colonies in our study region. Due to complexities not explored in this study, the prediction of colony success was partitioned such that landscape predicted food accumulation and wax production, while colony management predicted only adult worker population. We find no support for the opinion that honey bees in urban landscapes are more successful than those in cropland. To the contrary, we find that colony food accumulation responds negatively to urban land cover in landscapes dominated by urban or agricultural land use, a pattern that we attribute to the influence of late-season floral availability, particularly goldenrods.
It is important to note that while model selection identified landscape composition as the best predictor of colony food and wax accumulation, the amount of unexplained variation in our models was high, indicating that factors other than the ones we measured are also at play in the determination of colony success. Such factors may include (1) fine-scale landscape variables that were not measurable using the NLCD dataset, (2) hive management variables not accounted for in our beekeeper survey, and (3) “in-hive” determinants of colony success like queen fertility, disease prevalence, and parasite load. We also suggest caution in generalizing our results beyond our study region. While the landscape of Ohio is broadly similar to much of the American Midwest, it would be premature to extend our findings to other ecoregions that may differ strongly both in natural flora and in agricultural practices.
Supplemental Information
This spreadsheet contains the data compiled from our beekeeper surveys, including the variables experience, syrup, Food, Wax, AdultPop, and BroodPop.
This spreadsheet contains the land cover data for each study site, derived from the National Land Cover Database 2006 land cover layer.
This spreadsheet shows Simpson’s Diversity Index (DIVSI) calculated for each study site.
Full spring survey questionnaire filled out by beekeepers in our study.
Full fall survey questionnaire filled out by beekeepers in our study.
Acknowledgments
We sincerely thank the many Ohio beekeepers whose participation made this project possible. C Hoy and M Gardiner provided helpful statistical consultation, and JO Quijia-Pillajo assisted in GIS analysis.
Funding Statement
This study was funded by the Ohio State Beekeepers’ Association. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
DB Sponsler conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables.
RM Johnson conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
1. The Ohio State University Office of Responsible Research Practices.
2. Approval numbers: Protocol # 2012E0136, Protocol # 2013E0012.
References
- Anonymous (2008).Anonymous. 2008. French bees find a haven in Paris. New York Times. Available at: http://www.nytimes.com/2008/10/01/health/01iht-parisbees.16613547.html?_r=1&pagewanted=print .
- Bailey (1955).Bailey SE. Beekeeping in Ohio. Gleanings in Bee Culture. 1955;83:723–725. [Google Scholar]
- Beekman & Ratnieks (2001).Beekman M, Ratnieks F. Long-range foraging by the honey-bee, Apis mellifera L. Functional Ecology. 2001;14:490–496. doi: 10.1046/j.1365-2435.2000.00443.x. [DOI] [Google Scholar]
- Burgett, Caron & Ambrose (1978).Burgett M, Caron DM, Ambrose JT. Urban apiculture. In: Frankie GW, Kohler CS, editors. Perspectives in urban entomology. New York: Academic Press; 1978. pp. 187–219. [Google Scholar]
- Burnham & Anderson (2002).Burnham KP, Anderson DR. Model selection and multimodel inference. New York: Springer; 2002. [Google Scholar]
- Couvillon, Schürch & Ratnieks (2014).Couvillon MJ, Schürch R, Ratnieks FLW. Dancing bees communicate a foraging preference for rural lands in high-level agri-environment schemes. Current Biology. 2014;24:1–4. doi: 10.1016/j.cub.2014.03.072. [DOI] [PubMed] [Google Scholar]
- De La Rúa et al. (2009).De La Rúa P, Jaffé R, Dall’Olio R, Muñoz I, Serrano J. Biodiversity, conservation and current threats to European honeybees. Apidologie. 2009;40:263–284. doi: 10.1051/apido/2009027. [DOI] [Google Scholar]
- Donkersley et al. (2014).Donkersley P, Rhodes G, Pickup RW, Jones KC, Wilson K. Honeybee nutrition is linked to landscape composition. Ecology and Evolution. 2014;4:4195–4206. doi: 10.1002/ece3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert (1931).Eckert JE. The flight range of the honeybee. Journal of Agricultural Research. 1931;47:257–285. [Google Scholar]
- Erickson (1984).Erickson EH. Soybean pollination and honey production–a research progress report. American Bee Journal. 1984;124:775–779. [Google Scholar]
- Fry et al. (2011).Fry JA, Xian G, Jin S, Dewitz JA, Homer CG, Limin Y, Barnes CA, Herold ND, Wickham JD. Completion of the 2006 national land cover database for the conterminous United States. Photogrammetric Engineering and Remote Sensing. 2011;77:858–864. [Google Scholar]
- Garbuzov, Schürch & Ratnieks (2014).Garbuzov M, Schürch R, Ratnieks FLW. Eating locally: dance decoding demonstrates that urban honey bees in Brighton, UK, forage mainly in the surrounding urban area. Urban Ecosystems. 2014:1–8. [Google Scholar]
- Goltz (1975).Goltz L. Beekeeping in Ohio. Gleanings in Bee Culture. 1975;103:22,25. [Google Scholar]
- Goulson et al. (2002).Goulson D, Hughes W, Derwent L, Stout J. Colony growth of the bumblebee, Bombus terrestris, in improved and conventional agricultural and suburban habitats. Oecologia. 2002;130:267–273. doi: 10.1007/s004420100803. [DOI] [PubMed] [Google Scholar]
- Goulson et al. (2010).Goulson D, Lepais O, O’Connor S, Osborne JL, Sanderson RA, Cussans J, Goffe L, Darvill B. Effects of land use at a landscape scale on bumblebee nest density and survival. Journal of Applied Ecology. 2010;47:1207–1215. doi: 10.1111/j.1365-2664.2010.01872.x. [DOI] [Google Scholar]
- Graham (1992).Graham JM. The hive and the honey bee. Hamilton: Dadant & Sons; 1992. [Google Scholar]
- Härtel & Steffan-Dewenter (2014).Härtel S, Steffan-Dewenter I. Ecology: honey bee foraging in human-modified landscapes. Current Biology. 2014;24:R524–R526. doi: 10.1016/j.cub.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Jaycox (1976).Jaycox ER. Beekeeping in the Midwest. Circular 1125. Champaign: University of Illinois Press; 1976. [Google Scholar]
- Lee & Winston (1985).Lee PC, Winston ML. The effect of swarm size and date of issue on comb construction in newly founded colonies of honeybees (Apis mellifera L.) Canadian Journal of Zoology. 1985;63:524–527. doi: 10.1139/z85-077. [DOI] [Google Scholar]
- Morse (1972).Morse RA. The complete guide to beekeeping. New York: E. P. Dutton & Co., Inc; 1972. [Google Scholar]
- Naug (2009).Naug D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biological Conservation. 2009;142:2369–2372. doi: 10.1016/j.biocon.2009.04.007. [DOI] [Google Scholar]
- Odoux et al. (2014).Odoux J-F, Aupinel P, Gateff S, Requier F, Henry M, Bretagnolle V. ECOBEE: a tool for long-term honey bee colony monitoring at the landscape scale in West European intensive agroecosystems. Journal of Apicultural Research. 2014;53:57–66. doi: 10.3896/IBRA.1.53.1.05. [DOI] [Google Scholar]
- Odoux et al. (2012).Odoux J-F, Feuillet D, Aupinel P, Loublier Y, Tasei J-N, Mateescu C. Territorial biodiversity and consequences on physico-chemical characteristics of pollen collected by honey bee colonies. Apidologie. 2012;43:561–575. doi: 10.1007/s13592-012-0125-1. [DOI] [Google Scholar]
- Pellett (1920).Pellett FC. American honey plants: together with those which are of special value to the beekeeper as sources of pollen. Hamilton: Dadant and Sons; 1920. Available at https://archive.org/details/americanhoney00pell . [Google Scholar]
- QGIS Development Team (2014).QGIS Development Team 2014. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at http://qgis.osgeo.org .
- R Core Team (2013).R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. Available at http://www.R-project.org/ [Google Scholar]
- Requier et al. (in press).Requier F, Odoux J-F, Tamic T, Moreau N, Henry M, Decourtye A, Bretagnolle V. Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecological Applications In press. [DOI] [PubMed]
- Ricketts et al. (2008).Ricketts TH, Regetz J, Steffan-Dewenter I, Cunningham SA, Kremen C, Bogdanski A, Gemmill-Herren B, Greenleaf SS, Klein AM, Mayfield MM, Morandin LA, Ochieng A, Viana BF. Landscape effects on crop pollination services: are there general patterns? Ecology Letters. 2008;11:499–515. doi: 10.1111/j.1461-0248.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- Sande et al. (2009).Sande SO, Crewe RM, Raina SK, Nicolson SW, Gordon I. Proximity to a forest leads to higher honey yield: Another reason to conserve. Biological Conservation. 2009;142:2703–2709. doi: 10.1016/j.biocon.2009.06.023. [DOI] [Google Scholar]
- Seeley (1995).Seeley TD. The wisdom of the hive. Cambridge: Harvard University Press; 1995. [Google Scholar]
- Steffan-Dewenter & Kuhn (2003).Steffan-Dewenter I, Kuhn A. Honeybee foraging in differentially structured landscapes. Proceedings of the Royal Society B: Biological Sciences. 2003;270:569–575. doi: 10.1098/rspb.2002.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Engelsdorp & Meixner (2010).Van Engelsdorp D, Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology. 2010;103:S80–S95. doi: 10.1016/j.jip.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Visscher & Seeley (1982).Visscher PK, Seeley TD. Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology. 1982;63:1790–1801. doi: 10.2307/1940121. [DOI] [Google Scholar]
- von Frisch (1967).von Frisch K. The dance language and orientation of bees. Cambridge: The Belknap Press of Harvard University; 1967. [Google Scholar]
- Waddington et al. (1994).Waddington KD, Herbert TJ, Visscher PK, Richter MR. Comparisons of forager distributions from matched honey bee colonies in suburban environments. Behavioral Ecology and Sociobiology. 1994;35:423–429. doi: 10.1007/BF00165845. [DOI] [Google Scholar]
- Wilson-Rich (2012).Wilson-Rich N. 2012. Every city needs healthy honey bees. Recorded presentation. Available at: http://www.ted.com/talks/noah_wilson_rich_every_city_needs_healthy_honey_bees?language=en .
- Winfree et al. (2009).Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology. 2009;90:2068–2076. doi: 10.1890/08-1245.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This spreadsheet contains the data compiled from our beekeeper surveys, including the variables experience, syrup, Food, Wax, AdultPop, and BroodPop.
This spreadsheet contains the land cover data for each study site, derived from the National Land Cover Database 2006 land cover layer.
This spreadsheet shows Simpson’s Diversity Index (DIVSI) calculated for each study site.
Full spring survey questionnaire filled out by beekeepers in our study.
Full fall survey questionnaire filled out by beekeepers in our study.