Abstract
The mammalian order Lagomorpha has been the subject of many morphometric studies aimed at understanding the relationship between form and function as it relates to locomotion, primarily in postcranial morphology. The leporid cranial skeleton, however, may also reveal information about their ecology, particularly locomotion and vision. Here we investigate the relationship between cranial shape and the degree of facial tilt with locomotion (cursoriality, saltation, and burrowing) within crown leporids. Our results suggest that facial tilt is more pronounced in cursors and saltators compared to generalists, and that increasing facial tilt may be driven by a need for expanded visual fields. Our phylogenetically informed analyses indicate that burrowing behavior, facial tilt, and locomotor behavior do not predict cranial shape. However, we find that variables such as bullae size, size of the splenius capitus fossa, and overall rostral dimensions are important components for understanding the cranial variation in leporids.
Keywords: Leporidae, Cranial morphology, Locomotion, Lagomorpha
Introduction
The relationship between form and function as it relates to locomotion has been extensively studied in a wide range of vertebrate groups (Webb, 1984; Hildebrand, 1988; Rayner, 1988; Aerts et al., 2000). The mammalian order Lagomorpha has been the subject of many morphometric studies aimed at understanding this relationship in postcranial morphology (e.g., Reese, Lanier & Sargis, 2013; Fostowicz-Frelik, 2007; Seckel & Janis, 2008; Young et al., 2014), and the impetus of these is largely to understand the high-speed form of leaping observed in some leporids (rabbits and hares). Leporids are peerless cursors for their size; some hares have been shown to achieve speeds greater than 70 km/h (Garland, 1983). Indeed, the leporid postcranial skeleton exhibits many derived features that are strongly associated with saltation and cursoriality, including limb element elongation (Szalay, 1985; Fostowicz-Frelik, 2007; Seckel & Janis, 2008).
The cranial skeleton is more often overlooked in studies of form and locomotion, though there are biologically relevant associations between skull form and locomotor behavior, such as the role of the skull in active headfirst burrowing (e.g., Gans, 1974; Barros, Herrel & Kohlsdorf, 2011; Sherratt et al., 2014; Hopkins & Davis, 2009; and see Wake, 1993 for a review). In leporids, it has been suggested that morphological transformations of the skull may also be related to their ecology, particularly locomotion and vision (DuBrul, 1950; Bramble, 1989). The leporid skull is highly transformed, exhibiting a combination of features that clearly distinguish it from a more typical mammalian skull. A striking, yet often overlooked, characteristic is the broad dorsal arching of the cranium (Thompson, 1942), which is achieved via expansion and folding of the supraoccipital, and a distinct flexure near the basisphenoid/presphenoid suture (Fig. 1). A prominent ridge on the dorsal portion of the posterior cranial roof, which is superficially similar to an occipital crest, is actually a distinct flexure within the supraoccipital bone. Based on the position of the rabbit skull in resting position (De Beer, 1947: Fig. 9; Vidal, Graf & Berthoz, 1986: Fig. 4B, and see our Fig. 2), this flexure results in significant tilting of the facial region ventrally relative to the basicranium, which we here refer to as Facial Tilt (FT). DuBrul (1950) discusses this feature in detail within hares, and points out that the facial tilt of leporids is likely related to their unique mode of locomotor behavior. DuBrul (1950) also discusses the similarities in leporid skull transformations to those of our own lineage; in our hominin relatives, increased basicranial flexion is associated with the onset of bipedal locomotion (Strait & Ross, 1999).
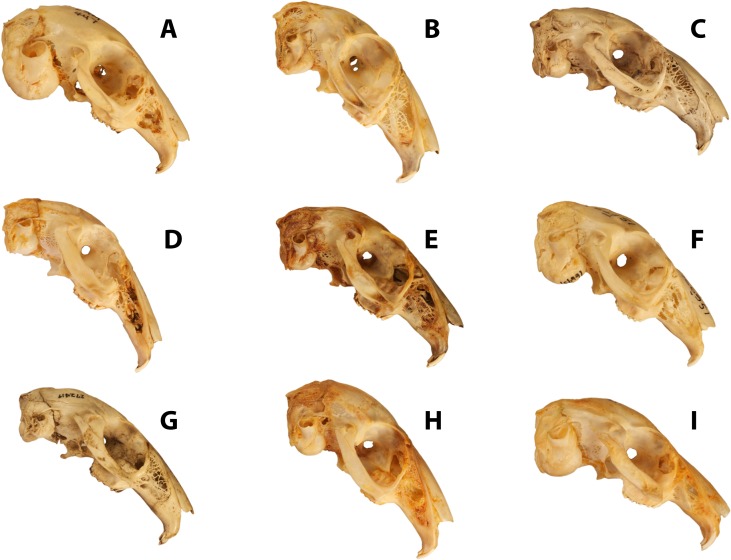
Figure 1. Disparity of leporid skulls.
Disparity in facial tilt and cranial shape in selected leporids, including (A) Brachylagus idahoensis (LACM 447; SLD ∼50 mm), (B) Lepus capensis (LACM 40152; SLD ∼82 mm), (C) Poelagus marjorita (AMNH 51056; SLD ∼80 mm), (D) Pronolagus crassicaudatus (AMNH 89033; SLD ∼80 mm), (E) Lepus americanus (LACM 93737; SLD ∼75 mm), (F) Oryctolagus cuniculus (AMNH 166951; SLD ∼78 mm), (G) Nesolagus timminsi (AMNH 272419; SLD ∼78 mm), (H) Bunolagus monticularis (AMNH 146662; SLD ∼78 mm), and (I) Romerolagus diazi (AMNH 148181; SLD ∼60 mm). All skull images are scaled to approximately the same skull length, skull length measurements are an approximation based on our measured specimens.
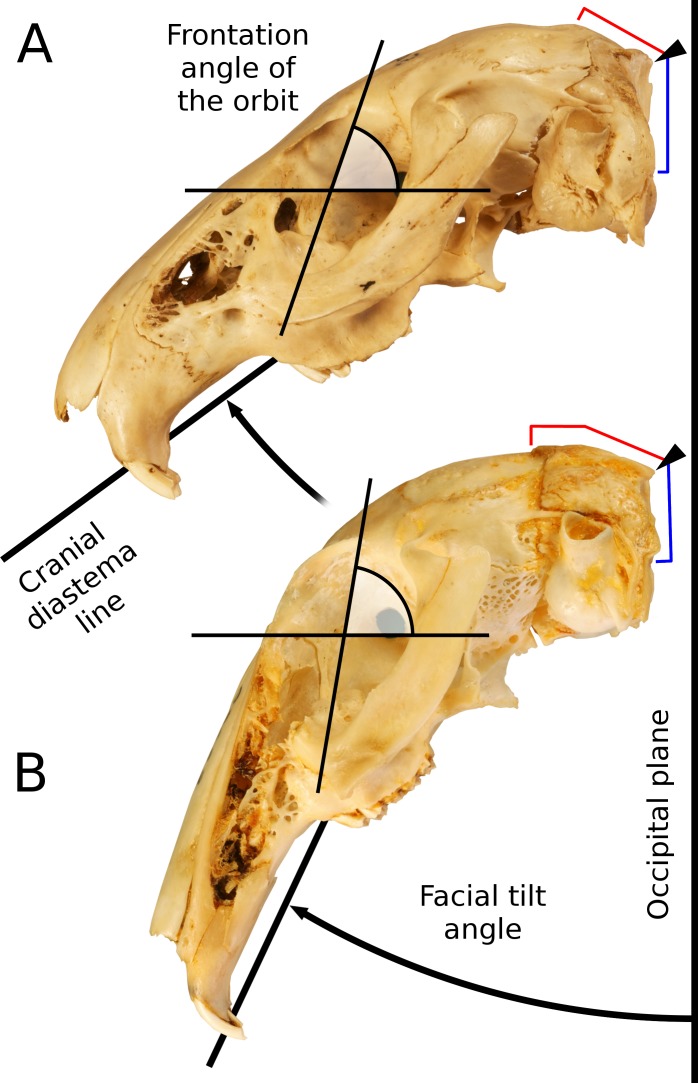
Figure 2. Facial tilt in leporids.
The crania of (A) Caprolagus hispidus (AMNH 54852, above) and (B) Pronolagus crassicaudatus (AMNH 89033, below) are shown in left lateral view. Facial tilt (FT) is defined herein as the angle between the upper diastema and the occipital plane, where increased values indicated a skull orientation closer the horizontal plane. The triangle indicates the position of the external occipital protuberance (EOP), and from that, both the dorsal (red) and occipital (blue) extent of the supraoccipital bones is outlined.
The goal of this study is to investigate the relationship between cranial shape and locomotion (cursoriality, saltation, and burrowing) within crown leporids. Our study is driven by hypotheses previously stated (DuBrul, 1950; Bramble, 1989) but never quantitatively tested. We use a large morphometric dataset spanning 16 phylogenetically constrained extant taxa (Table 1) to evaluate hypotheses about the relationship between skull shape and facial tilt with locomotor ecology.
Table 1. Leporid species studied.
See Appendix S1 for specific specimens measured, and the text for discussion regarding the assessment of ecological variables.
| Species | Locomotion type | Burrowing | Abbreviation | n |
|---|---|---|---|---|
| Romerolagus | Saltatorial | Yes | Ro | 7 |
| Bunolagus | Saltatorial | Yes | Bu | 2 |
| Caprolagus | Generalized | Yes | Ca | 2 |
| Brachylagus | Generalized | Yes | Br | 10 |
| Sylvilagus floridanus | Saltatorial | No | Sfl | 10 |
| Sylvilagus palustris | Generalized | No | Spal | 10 |
| Sylvilagus audobonii | Saltatorial | Yes | Sau | 10 |
| Poelagus marjorita | Saltatorial | No | Po | 10 |
| Pronolagus crossicaudatus | Saltatorial | No | Pc | 10 |
| Oryctolagus cuninculus | Saltatorial | Yes | Oc | 10 |
| Nesolagus timminsi | Saltatorial | Yes | Nt | 2 |
| Lepus americanus | Saltatorial | No | Lam | 10 |
| Lepus timidus | Saltatorial | Yes | Lti | 10 |
| Lepus capensis | Cursorial | Yes | Lcap | 10 |
| Lepus californicus | Cursorial | No | Lcal | 12 |
| Lepus saxatilis | Cursorial | No | Lsax | 9 |
Study System and Hypotheses
The mammalian order Lagomorpha is composed of two families, Leporidae (rabbits and hares) and Ochotonidae (pikas). Ochotonids are represented by one living genus, Ochotona, which includes two North American and 28 Eurasian species (Alves & Hackländer, 2008). Leporids include 11 living genera with 62 species overall. The majority of species are found within two genera (Alves & Hackländer, 2008); Lepus (hares, 32 species) and Sylvilagus (a portion of rabbits, 17 species). Of the remaining nine genera, seven are monotypic, while two genera, Nesolagus and Pronolagus, only include two and four species, respectively. Overall, sixteen leporids species are currently considered endangered or critically endangered by the IUCN (Alves & Hackländer, 2008), and conservation issues are compounded by the lack of natural history data for many of these species. Leporids are found on every continent except Antarctica, from the high arctic to dry, hot deserts (Chapman & Flux, 1990; Chapman & Flux , 2008). Some leporids are nocturnal, some are social, and some live in dense cover as opposed to the open plains often associated with these animals (Stoner, Bininda-Emonds & Caro, 2003). In terms of size, our study includes (Appendix S1) the smallest leporid, Brachylagus (mean skull length ∼50 mm) to one of the largest, Lepus timidus (mean skull length ∼90 mm). Genera such as Pentalagus and Caprolagus have heavy, robust skulls, compared to the typically gracile skulls of most taxa. These cranial differences manifest themselves morphometrically via a wide range of snout lengths and marked differences in skull robustness and form (Fig. 1). While leaping abilities are common among most leporid lineages, they are also known to be facultatively semiaquatic, scansorial, fossorial, or exhibit a more generalized, non-hopping form of locomotion (Chapman & Flux, 1990). We distinguish here between the saltatory locomotion (i.e., hopping) most typical among leporids (Table 1 and Fig. 3), and its cursorial form observed in some hare lineages (Gambaryan & Hardin, 1974; Bramble, 1989). Generalists are recognized as those who don’t exhibit clear hopping, but rather move in a more scampering habit.
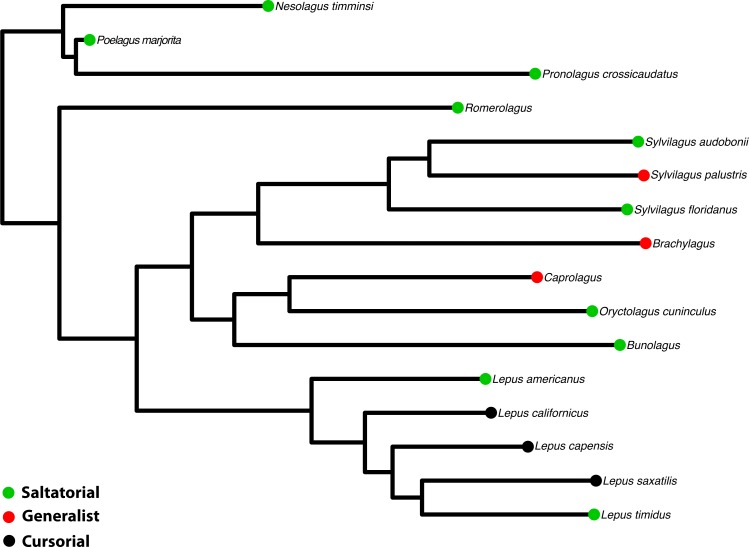
Figure 3. Phylogeny of Leporidae.
The phylogenetic hypothesis of the 16 taxa used in this study, pruned from the supermatrix maximum likelihood phylogeny in Matthee et al. (2004). Locomotor styles from Table 1.
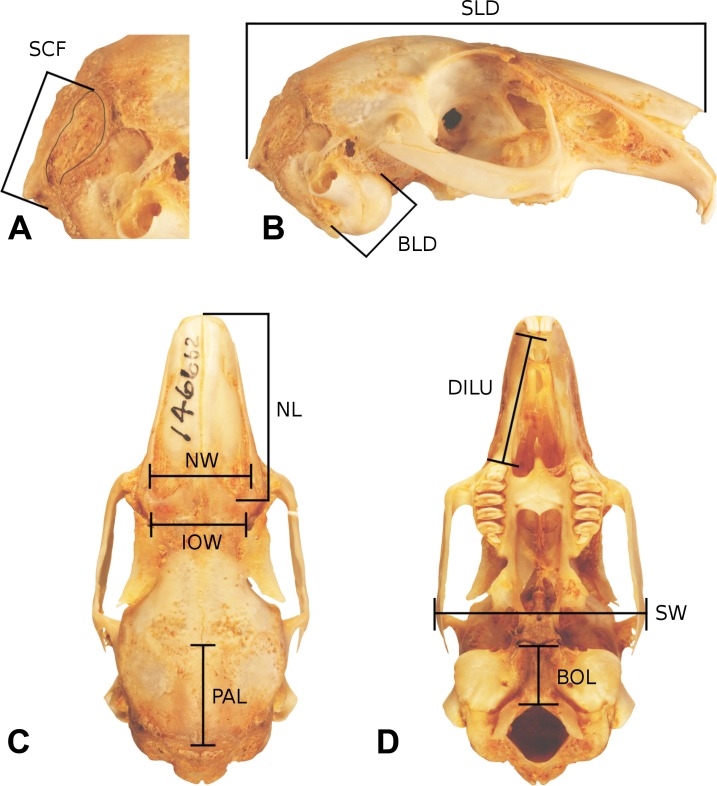
Figure 4. Skull measurements.
A representative leporid skull showing measurements used in this analysis. The cranium of Bunolagus monticularis (AMNH 146662) is shown in ((A) and (B)) right lateral (top), (C) dorsal (lower left), and (D) ventral (lower right) views. Abbreviations follow Table 1. See figure 3 for a description of Facial Tilt (FT).
Hypothesis 1–facial tilt
A high degree of facial tilting (e.g., ventral flexion of the facial region) should (a) be positively correlated with more active (e.g., saltatorial or cursorial) locomotor styles, and (b) show no correlation with burrowing habit
Variation in the degree of facial tilt among leporids has strong effects on orbital orientation (Fig. 3). There is substantial literature discussing the relationship between orbit orientation and ecology within vertebrates (Noble, Kowalski & Ravosa, 2000; Cox, 2008; Heesy, 2008; Iwaniuk et al., 2008; Jeffery & Cox, 2010), and Cartmill (1970) established the terms ‘orbital convergence’ and ‘frontation’ to understand these relationships. While orbit orientation is influenced by brain size and jaw mastication (Lieberman, Ross & Ravosa, 2000; Cox, 2008), within primates, orbital convergence is also strongly associated with increased binocular visual field overlap observed in nocturnal predatory species (Heesy, 2004; Heesy, 2008). Various groups exhibit a high degree of both orbital convergence and orbital frontation (Cox & Jeffery , 2008), with hominids serving as an exemplar; orbital frontation is strongly positively correlated with basicranial flexion (Ross, 1995). As DuBrul (1950) points out, facial tilt transformations among leporids are nearly identical to basicranial flexion observed within anthropoids; increased facial tilt and basicranial flexion both result in increased orbital frontation (see Fig. 2 for changes in frontation related to increased FT). Several workers have shown that increased frontation is positively correlated with arboreal taxa (Cartmill, 1970; Heesy, 2008); increased frontation changes the visual field to allow for better visualization of substrate. Jeffery & Cox (2010) show that leporids have relatively low degrees of convergence and frontation. As we discuss below, however, when facial tilt is taken into consideration, leporids actually demonstrate a relatively higher degree of frontation (as indicated by the orbital plane relative to the vertical plane). More importantly, regardless of the absolute measure of frontation within leporids, we expect that frontation will vary among leporids correlated with varying degrees of facial tilt. For this reason, we expect that facial tilt (as a proxy for frontation) should be strongly correlated to locomotor styles that would require enhanced substrate perception (saltatorial and cursorial), but we do not expect that facial tilt will be related to burrowing habit.
Hypothesis 2—Skull shape
We expect that there will be significant skull shape differences among (a) locomotor styles, and (b) burrowing habits
We have no a priori expectations about how overall skull shape might change with locomotor mode or burrowing habit. Instead we will investigate the more fundamental question of whether skull shape is related to locomotion and burrowing habit at all. Our interest in this question is therefore more a form of exploratory data analysis than a test of a specific hypothesis.
Materials and Methods
We collected morphometric data (Table 2 and Appendix S1) from 140 leporid skulls spanning 16 taxa (Table 1) housed in the departments of Mammalogy at the American Museum of Natural History (AMNH) and the Los Angeles County Museum of Natural History (LACM). Care was made to use only adult specimens, characterized by fully fused occipital sutures (Hoffmeister & Zimmerman, 1967). Ten linear measurements (Table 3 and Fig. 3) were recorded per specimen using digital calipers by three authors (BPK, MW, and NB), and a repeatability analysis (consisting of 10 specimens measured 3 times, results not presented) was performed to ensure there was no intercollector bias introduced. The ten cranial measurements were analyzed using the log-shape ratios approach (Mosimann, 1970; Mosimann & James, 1979). For each specimen, size was computed as the geometric mean of all measurements, and then each measurement was divided by size to obtain the shape ratios. We then used the log of this quantity as raw data for the subsequent analyses.
Table 2. Skull measurements used.
Variables used in this study and description; see Figs. 2 and 4 for illustrations of the measurement conventions.
| Abbr. | Variable | Measurement convention |
|---|---|---|
| BLD | Bulla diameter | Maximum diameter (in any direction) of right bulla |
| BOL | Basioccipital length | Maximum midsagittal length from anterior basioccipital to foramen magnum |
| DIL | Diastema length | Maximum distance between right I2 and M1 |
| IOW | Interorbital width | Minimum transverse width between dorsal rims of orbits |
| NL | Nasal length | Maximum parasagittal length of nasal bones (i.e., orthogonal antero-posterior but not along midline) |
| NW | Nasal width | Maximum transverse width across posterior nasal bones |
| PAL | Parietal length | Maximum midsagittal length of parietal bones |
| SCF | Splenius capitis fossa | Maximum parasagittal length from anterior margin of M. splenius capitis insertion fossa to opisthocranion |
| SLD | Skull length dorsal | Maximum midsagittal length from anterior nasal bones to Opisthocranion (just dorsal to incisors) to opisthocranion |
| SW | Skull width | Maximum transverse width across zygomatic processes |
Table 3. PCA loadings.
The first four principal component (PC) axes contribute to 90.2% of the total variation of the ten log normal variables. For each PC, the proportion of total variance (%) and the loadings on these are given. The variables with the highest loading are shown in bold and are discussed within the text.
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Proportion of variance | 43.5 | 24.4 | 13.2 | 9.1 |
| BLD | 0.85046873 | 0.02545804 | 0.016857617 | 0.272291215 |
| BOL | 0.159862063 | 0.161106564 | 0.012761305 | −0.070830236 |
| DIL | −0.246528468 | −0.026035317 | −0.415136231 | 0.156169866 |
| IOW | −0.249260494 | 0.331660879 | 0.681589555 | 0.259415969 |
| NL | −0.175108112 | 0.062932441 | −0.505939657 | 0.130829626 |
| NW | −0.296951523 | 0.01926985 | 0.131337875 | 0.224903873 |
| PAL | 0.027064391 | 0.140782388 | 0.078947604 | −0.866213361 |
| SCF | −0.050436754 | −0.905402173 | 0.218089373 | −0.039704014 |
| SLD | −0.064043373 | 0.114113325 | −0.185926838 | 0.027064132 |
| SW | 0.044933539 | 0.076114004 | −0.032580602 | −0.093927069 |
Facial tilt was measured by photographing each skull in lateral view using a Nikon D80 digital camera (Nikon, Tokyo, Japan). The skulls were placed in a sandbox to ensure that the sagittal plane was orthogonal to the focal direction. Facial tilt angle was acquired from the digital photos within Adobe Photoshop©, measured as the angular difference between the ‘occipital plane’ and a line parallel to the cranial diastema (Fig. 3). Variation among individuals for the cranial variables weas explored using principal components analysis on the covariance matrix of the log-shape ratios shape variables within the statistical software R v3.1.1 (R Core Team, 2014, http://cran.r-project.org/).
Phylogenetically informed analyses
To examine facial tilt angle and cranial shape in a phylogenetic context, we used the phylogenetic relationships among species of Leporidae recently published by Matthee et al. (2004). The original tree was constructed using seven genes (five nuclear and 2 mt) for 25 ingroup taxa. We pruned the tree using Mesquite© (Maddison & Maddison, 2015) to include only the 16 taxa studied here (Fig. 3), and retained the information on branch lengths (details of which are in (Matthee et al., 2004)).
We first examined the amount of phylogenetic signal in the morphometric variables, calculating the K statistic (Blomberg, Garland & Ives, 2003) for facial tilt angle, and the multivariate equivalent Kmult (Adams, 2014a) for all log-shape ratios. The K statistics provide a measure of the strength of phylogenetic signal for univariate and multivariate traits respectively, and in each case provides a single statistic. A value of less than one implies that taxa resemble each other phenotypically less than expected under Brownian motion, while values of more than 1 implies that close relatives are more similar to one another phenotypically than expected under Brownian motion. Significance testing was performed using a permutation procedure whereby the variables are randomized relative to the tree, and 1000 permutations were performed for each test (Blomberg, Garland & Ives, 2003).
Log-shape ratios and facial tilt angle were compared to several key ecological indicators, including locomotor type and burrowing habit (Table 1). Ecological data were obtained from Chapman & Flux (1990) and Stoner, Bininda-Emonds & Caro (2003). We divided leporids into three locomotor categories: generalized or ‘scramble’ locomotors, which tend to be the slowest-moving; saltatory or hopping locomotors; and fast-moving taxa that practice cursorial (leaping and bounding) locomotion, which is essentially a specialized form of saltation. Regarding burrowing habits, some leporids dig their own burrows (e.g., Oryctolagus and Romerolagus), whereas others simply occupy preexisting burrows excavated by other animals. For the purposes of this study, we refer to leporids as burrowers if they occupy burrows consistently, regardless of whether they dig the burrows.
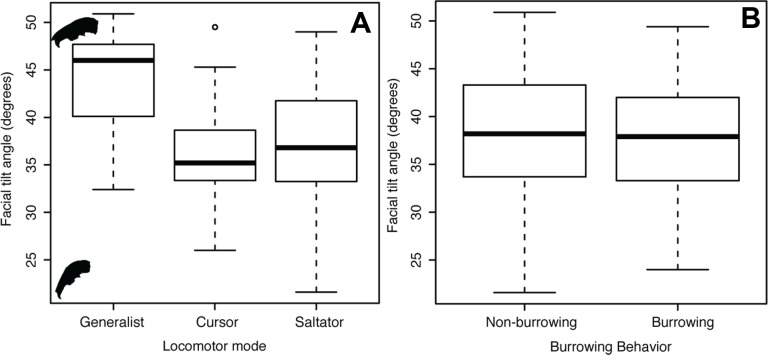
To test whether or not the degree of facial tilt differs among the three locomotor categories, we performed a one-way Analysis of Variance (ANOVA) in an evolutionary context, under a Brownian motion model of evolution. This was done by using species means of the FT angle in a distance-based phylogenetic generalized least squares analysis (D-PGLS; Adams, 2014b). A distance-based approach provides numerically identical estimates of evolutionary patterns to those obtained from standard implementations of PGLS on univariate datasets, and was used here for consistency with analyses below on the log-shape ratios. The statistical significance of each term in the D-PGLS was assessed using 1000 permutations whereby the species means are shuffled among the tips of the phylogeny. We performed a second ANOVA as above to test whether facial tilt differs between taxa that utilize burrows (“burrowing”) and those that do not (“non-burrowing”). Box and whisker plots were used to visualize the individual variation in facial tilt angle among groups.
To test whether or not cranial shape, as represented by ten morphometric variables, differs among the three locomotor types, we performed a multivariate analysis of variance in an evolutionary context under a Brownian motion model of evolution. This was done as a D-PGLS with the species means of the ten log-shape ratios. The D-PGLS performs better than a regular PGLS when the number of variables begins to approach the number of species (Adams, 2014b). The statistical significance of each term in the D-PGLS was assessed using 1,000 permutations of the species means. Similarly, we tested whether or not cranial shape differs between burrowing and non-burrowing taxa using a D-PGLS as above.
Finally, to test whether or not facial tilt is a significant predictor of cranial shape, we performed a multivariate regression in an evolutionary context, under a Brownian motion model of evolution, again using the D-PGLS approach. The statistical significance was assessed using 1000 permutations of the species means of the log-shape ratios. All of the phylogenetically informed analyses were done using the geomorph package (Adams et al., 2014) in the statistical software R v3.1.1 (R Core Team, 2014). The ANOVAs on FT, the MANOVAs on cranial log-shape rations, and the multivariate regression were done using the procD.pgls function, and phylogenetic signal was calculated with the physignal function.
Results
Facial tilt
Facial tilt (FT) summarizes the broad dorsal arching of the skull roof that is prominent among living leporids (Fig. 3). Across the species in this study, the measure of facial tilt angle has a very low value for K, implying that the taxa resemble each other morphologically less than expected under Brownian motion, and the test is not significant (K = 0.62, P = 0.53). Overall, there is a nearly 30° range of variation in FT among specimens of all species in this sample (Appendix S1). We found a significant difference among locomotor types for facial tilt angle (D-PGLS, F = 7.02, P = 0.016; Fig. 5A). The mean FT angle for generalized locomotors (mean, μ = 44.0, standard deviation, σ = 5.48) is substantially higher than that of cursorial (μ = 36.3, σ = 5.46) and saltatorial taxa (μ = 37.2, σ = 5.91) (Fig. 5A). This indicates that taxa that are either saltatorial or cursorial tend to have facial regions that are more ventrally deflected. By contrast, we found no significant difference in FT angle between burrowing and non-burrowing taxa (Fig. 5B; D-PGLS, F = 0.0037, P = 0.973; Fig. 5B).
Figure 5. Facial tilt ANOVA.
Box and whisker plot summarizing facial tilt angle for all specimens, showing how the angle differs between locomotor types (A) and burrowing behavior (B).
Cranial shape analyses
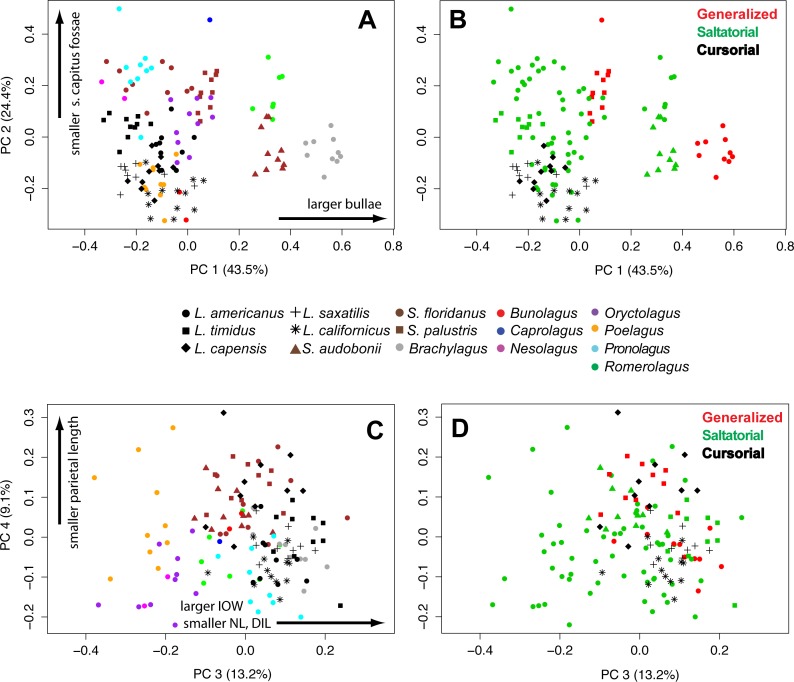
In a principal components analysis of the ten log-shape ratios among individuals, the first four PC axes account for 90.2% of total variance. PC1 accounts for 43.5% of cranial shape differences, PC2 accounts for 24.4%, PC3 accounts for 13.2%, and PC4 accounts for 9.0% of variance. (Table 3 and Fig. 6). The remaining PCs each contribute less than 10% of the total variation. A PCA of the species means (not shown) produced equivalent results (PC1 = 42.6%, PC2 = 33.4%, PC3 = 11.3%, PC4 = 5.9%), and with the same variables contributing highly on each axis, and thus we present only one analysis for brevity.
Figure 6. Multivariate PCAs.
Principal components analysis of 10 log-shape ratios measurements describing cranial shape for all specimens. Biplots show PC1 vs PC2 (top) and PC3 vs PC4 (bottom). (A) Colored symbols by species. (B) colored by LOC (with species symbols). Details of the loadings of each variable in the PCA are presented in Table 3.
The loadings of the PCA (Table 3) show that bullae diameter (BD; 0.85) has the strongest influence on PC1, substantially more than other variables. PC1 strongly separates Brachylagus, Romerolagus, and Bunolagus (all with larger bullae diameters) from all other leporid species (Fig. 6A). In terms of locomotor styles, cursorial species are isolated towards the negative portion of the PC1 axis. Similarly, PC2 strongly shows the effects of size of the splenius capitus fossa (SCF; −0.91). While this measure does not separate among saltators (Fig. 6B), there is some separation between generalists and cursorial species. Loadings for PC3 indicate that three variables are strongly affecting the variance along that axis: interorbital width (IOW; 0.68), nasal length (NL; −0.51), and diastema length (DILU; −0.42). PC3 shows separation of species (Fig. 6C), but no clear broader groupings. It also does not clearly distinguish locomotor modes, but saltators do occupy the negative portion of the axes where no generalists or cursors are found (Fig. 6D). Parietal length (PAL; −0.87) loads strongly along PC4. This axes does help to distinguish species (Fig. 6C), but shows little ability to distinguish among locomotor modes (Fig. 6D).
There is significant phylogenetic signal is cranial shape described by the ten log-shape ratios (Kmult = 0.91, P = 0.035). The value of kappa is substantially higher than that for facial tilt, but still below 1, implying that close relatives are moderately less similar to one another phenotypically than expected under Brownian motion. Phylogenetically informed analysis of variance (D-PGLS) indicates that there is no significant effect of locomotor habit on cranial shape (F = 1.3712, P = 0.28). Likewise, there is no significant effect on cranial shape by burrowing behavior (F = 1.2831, P = 0.56). Finally, a phylogenetically informed multivariate regression suggests that facial tilt angle is not a significant predictor of cranial shape (R2 = 0.097, P = 0.413)
Discussion
Given a clear correlation between the degree of facial tilt (FT) and locomotor style, and the lack of significant phylogenetic signal in FT angle, it is evident that this aspect of cranial morphology is strongly influenced by ecological factors within Leporidae. While the relationship between shape and function is established, the specific aspects of cranial shape that inform ecological function are only partially resolved from our multivariate analyses. Generalized locomotors exhibit less facial tilt, an anatomical condition that could properly be thought to be primitive for the mammalian skull, and given their fossil record, lagomorphs as well (Dice, 1933; Asher et al., 2005). Facial tilt within leporids is allowed via the expansion of the supraoccipital bone on the dorsal skull (Fig. 2), and along the ventral skull, there is a pronounced flexure near the basisphenoid/presphenoid juncture.
The complex architecture of the supraoccipital in leporids is the most marked change related to the dorsal arching the skull roof, but there are additional effects on the orientation of the orbit (Fig. 2). There is a vast literature on orbital orientation as it relates to locomotion, visual acuity, brain size, and masticatory anatomy (Noble, Kowalski & Ravosa, 2000; Heesy, 2005; Heesy, Ross & Demes, 2007; Iwaniuk et al., 2008; Heesy, 2008; Cox, 2008; Jeffery & Cox, 2010), and perhaps most clearly, changes in orbit orientation have direct affects on the range of visual fields. Both orbital convergence and frontation are commonly measured orbital variables that seem to be functionally predictive (Cox, 2008); increased convergence is thought to increase binocular field overlap within primates (Ross & Martin, 2007; Heesy, Ross & Demes, 2007), and orbital frontation allows for better substrate visualization (Cartmill, 1970; Heesy, 2008).
While Jeffery & Cox (2010) demonstrated that the rabbit shows low degrees convergence and frontation, frontation in the rabbit is complicated by skull transformations associated with facial tilt. Traditionally, frontation was considered as the degree to which the orbital plane is aligned vertically (Cartmill, 1970; Ross, 1995); whereas, Jeffery & Cox (2010) used angular differences between the lateral semicircular canal and the medial and lateral orbital rectus muscles as a proxy for frontation. While these later measures are distinct for rabbits (Cox, 2008; Jeffery & Cox, 2010) as compared to other mammals, due to the way rabbits hold their heads (De Beer, 1947; Vidal, Graf & Berthoz, 1986), angular differences between the lateral semi-circular canal and horizontal rectus muscles may not be a perfect summary of the degree to which the orbital plane approaches vertical. Interestingly, as Jeffery & Cox (2010) show, humans and rabbits are outliers with regard to this metric, as they both demonstrate strong misalignment of semicircular canal and rectus muscle orientations. This may be driven by the fact that both of these species exhibit skull shapes in which the basicranium is highly transformed relative to the facial region (DuBrul, 1950). Most importantly, and regardless of the absolute degree of frontation, facial tilting within leporids would have the effect of changing the orientation of the orbit and increasing frontation. Our data show that variation in facial tilt among leporids (∼30°) is explained by mode of locomotion. Presumably, pronounced facial tilt and the associated increase in frontation improve substrate visibility in fast-moving taxa.
In contrast to FT angle, overall cranial morphology as described by ten log-shape ratio measurements is not significantly different among locomotor modes or between burrowers and non-burrowers. Instead, the PCA of individual variation in our cranial variables clearly shows that among-species variation is a strong driver of morphospace organization (Fig. 6). The phylogenetic structure evident in the cranial variables shown in our PCA is supported by a high measure of phylogenetic signal. However, there is some separation of the three locomotor modes in morphospace (Fig. 6). Saltatorial species have a wide-variety of cranial morphologies, while the generalized locomotors are clustered in morphospace (in the negative quadrant of PC1 and PC2), likely due to their close ancestry.
Bulla length contributes the most to the first PCA axis, separating out a group of three species (larger bullae; Romerolagus, Bunolagus, and Brachylagus) from all other leporids, and thus this morphological trait is a candidate feature of adaptive differences between the different locomotors styles. The external bulla is a complicated structure, which receives contributions from different bones across Mammalia (Novacek, 1977). The external auditory bulla has been shown to be of significant systematic importance within carnivorans (Hunt, 1974; Ivanoff, 2001), but the function of bulla size is unclear for leporids. Pavlinov & Rogovin (2000) showed that bulla size is negatively correlated with pinna size in specialized desert rodents. They specifically remark that faster, more agile rodents within these groups tend toward smaller bulla and larger pinnae. While our data do not explicitly test this, our cursorial species appear toward the negative PC1 axes, which are represented by smaller bullae. We also note that while Romerolagus and Brachylagus exhibit large bullae and relatively small pinnae, Bunolagus does not fit this pattern as it has both large bullae and large pinnae. Liao, Zhang & Liu (2007) shows that bulla size is negatively correlated with altitude in the Daurian pika, Ochotona dauurica (Lagomorpha, Ochotonidae). This patterns does not match our observations, as one of our large bullae species, Romerolagus, is found at high elevations (Cervantes, Lorenzo & Hoffmann, 1990). We think that our PC1 axis may also reflect the relative size of the basicranium to the facial region within leporids, in addition to bullae size; we discuss this topic further below.
The second variable of interest, which loads strongly on PC2, is the splenius capitus fossae. Lateral to the external occipital protuberance (EOP; i.e., Inion) are two large fossae that extend to the parietal/occipital suture and allow for attachment of the splenius capitus mm. (Barone, Pavaux & Blin, 1973), which are involved in head extension and lateral rotation. The fossae can be clearly identified via the prominent superior nuchal line that extends rostrally from the EOP. The longissimus capitus m. inserts with the splenius capitus m. in the lateral, mastoid area, of the occipital region. A final long extensor muscle, the semispinalus capitus m., attaches to the lateral portions of the EOP. Together, these three long erectors serve to extend, stabilize, and laterally rotate the head (Igarashi et al., 2000). Upon comparison of leporid skulls (Fig. 1), it is apparent that those with significant facial tilt are expanding the rostral portions of the supraoccipital bone relative to the caudal portion, and indeed, this seems to be reflected in PC2 as the variance along that axis helps to separate out cursors (larger splenius capitus fossa) and generalists (smaller splenius capitus fossa). The expansion of the splenius capitus fossa should serve to increase the attachment area for the long extensor muscles, allowing for improved extension and lateral rotation of the head.
It is worth noting that all variables strongly affecting PC1, PC2, and PC4 are associated with the neurocranium, and variables affecting PC3 are all associated with the splanchnocranium. It has been thoroughly demonstrated within our own lineage, and mammals more broadly, that these basicranial and facial regions demonstrate strong levels of phenotypic independence (see, for example, Porto et al., 2009; Drake & Klingenberg, 2010; Sanger et al., 2012; Klingenberg, 2013) While this pattern is debated within humans and other great apes (Singh et al., 2012; Mitteroecker et al., 2012; Martínez-Abadías et al., 2012), some similarities in skull transformation between humans and rabbits have been noted in the literature (DuBrul, 1950; Moore & Spence, 1969). Moore & Spence (1969) highlighted that both humans and rabbits transform the facial regions relative to the basicranium, but also pointed out that the transformation seems to be driven in the facial regions within rabbits, whereas it seems to be driven from the basicranium in humans. While our data do not directly address modularity or developmental pathways within leporid skulls, it would be useful to understand how relative transformation between the facial and basicranial regions within leporids, which seems to influence facial tilt, could be explained mechanistically by these developmental trajectories.
Most importantly, it is striking that facial tilt does distinguish generalist locomotors clearly from more active taxa within leporids. This suggests that FT represents a meaningful biological metric among leporids, but may also summarize a specific aspect of cranial shape not recognized, but alluded to, within our linear variables. While our linear measurements failed to strongly discern differences among locomotor groups, this may be a function of the limited ability of these variables to capture important shape differences among crania within leporids due to the highly transformed nature of their skulls (e.g., pronounced dorsal arching). Nonetheless, our linear variables do separate taxonomic groups, as has been done in other studies (see, for example, Palacios et al., 2008; Pintur et al., 2014).
Our study demonstrates that the dorsal arching found within leporid skulls, mainly represented here as facial tilt, has a strong relationship with how these animals moved. Facial tilt is related to a complex transformation of nearly all aspects of the leporid skull, including basicranial rearrangement and facial changes in the diastema region. Our linear variables, in addition to distinguishing taxonomic groups, also capture some aspects of these changes related to locomotion. Based on the changes in orbit orientation that are associated with increased facial tilt, it is likely that skull transformations in crown leporids are driven by a need for increased visual perception of substrate.
Supplemental Information
Acknowledgments
We are grateful to Neil Duncan and Eileen Westwig of the American Museum of Natural History and Jim Dines of the Natural History Museum of Los Angeles County for access to specimens in their care. We thank Christopher Heesy and Kevin Middleton for helpful discussions, and Margaret Metz for assistance with R.
Funding Statement
This project was initiated under a fellowship from the AMNH awarded to Brian P. Kraatz. Nicholas Brumacod completed work on this project while enrolled in Western University of Health Sciences’ MSMS program, and received a summer fellowship from the Graduate School at that university to continue that work. Travel to the AMNH by Matthew J. Wedel was made possibly by funds from the Department of Anatomy, Western University of Health Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Brian P. Kraatz and Mathew J. Wedel are Academic Editors for PeerJ.
Author Contributions
Brian P. Kraatz conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Emma Sherratt performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Nicholas Bumacod performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Mathew J. Wedel conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
References
- Adams (2014a).Adams DC. A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Systematic Biology. 2014a;63(5):685–697. doi: 10.1093/sysbio/syu030. [DOI] [PubMed] [Google Scholar]
- Adams (2014b).Adams DC. A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution. 2014b;68:2675–2688. doi: 10.1111/evo.12463. [DOI] [PubMed] [Google Scholar]
- Adams et al. (2014).Adams DC, Collyer ML, Otarola-Castillo E, Sherratt E. Geomorph: software for geometric morphometric analyses. (R package version 2.1) 2014 Available at http://cran.r-project.org/web/packages/geomorph/index.html .
- Aerts et al. (2000).Aerts P, Van Damme R, Vanhooydonck B, Zaaf A, Herrel A. Lizard locomotion: how morphology meets ecology. Netherlands Journal of Zoology. 2000;50(2):261–277. doi: 10.1163/156854200505865. [DOI] [Google Scholar]
- Alves & Hackländer (2008).Alves PC, Hackländer K. Lagomorph species: geographical distribution and conservation status. In: Alves PC, Ferrand N, Hackländer K, editors. Lagomorph biology: evolution, ecology, and conservation. Heidelberg: Springer; 2008. pp. 395–405. [Google Scholar]
- Asher et al. (2005).Asher RJ, Meng J, Wible JR, McKenna MC, Rougier GW, Dashzeveg D, Novacek MJ. Stem Lagomorpha and the antiquity of Glires. Science. 2005;307(5712):1091–1094. doi: 10.1126/science.1107808. [DOI] [PubMed] [Google Scholar]
- Barone, Pavaux & Blin (1973).Barone R, Pavaux PC, Blin B. Atlas d’anatomie du lapin [Atlas of rabbit anatomy] Paris: Masson; 1973. [Google Scholar]
- Barros, Herrel & Kohlsdorf (2011).Barros F, Herrel A, Kohlsdorf T. Head shape evolution in Gymnophthalmidae: does habitat use constrain the evolution of cranial design in fossorial lizards? Journal of Evolutionary Biology. 2011;24(11):2423–2433. doi: 10.1111/j.1420-9101.2011.02372.x. [DOI] [PubMed] [Google Scholar]
- Blomberg, Garland & Ives (2003).Blomberg S, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57(4):717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Bramble (1989).Bramble DM. Axial-appendicular dynamics and the integration of breathing and gait in mammals. American Zoologist. 1989;29(1):171–186. [Google Scholar]
- Cartmill (1970).Cartmill M. PhD dissertation. 1970. The orbits of arboreal mammals. [Google Scholar]
- Cervantes, Lorenzo & Hoffmann (1990).Cervantes FA, Lorenzo C, Hoffmann RS. Romerolagus diazi. Mammalian Species. 1990:1–7. doi: 10.2307/3504131. [DOI] [Google Scholar]
- Chapman & Flux (1990).Chapman JA, Flux JE, editors. Rabbits, hares and pikas: status survey and conservation action plan. Gland, Switzerland: International Union for Conservation of Nature and Natural Resources; 1990. [Google Scholar]
- Chapman & Flux (2008).Chapman JA, Flux JE. Introduction to the Lagomorpha. In: Alves PC, Ferrand N, Hackländer K, editors. Lagomorph biology: evolution, ecology, and conservation. Heidelberg: Springer; 2008. pp. 1–9. [Google Scholar]
- Cox (2008).Cox PG. A quantitative analysis of the Eutherian orbit: correlations with masticatory apparatus. Biological Reviews. 2008;83(1):35–69. doi: 10.1111/j.1469-185X.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- Cox & Jeffery (2008).Cox PG, Jeffery N. Geometry of the semicircular canals and extraocular muscles in rodents, lagomorphs, felids and modern humans. Journal of Anatomy. 2008;213:583–596. doi: 10.1111/j.1469-7580.2008.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer (1947).De Beer GR. How animals hold their heads. Proceedings of the Linnean Society of London. 1947;159(2):125–139. doi: 10.1111/j.1095-8312.1947.tb00491.x. [DOI] [Google Scholar]
- Dice (1933).Dice LR. Some characters of the skull and skeleton of the fossil hare, Palaeolagus haydeni. Papers of the Michigan Academy of Sciences and Arts and Letters. 1933;18:301–306. [Google Scholar]
- Drake & Klingenberg (2010).Drake AG, Klingenberg CP. Large-scale diversification of skull shape in domestic dogs: disparity and modularity. The American Naturalist. 2010;175(3):289–301. doi: 10.1086/650372. [DOI] [PubMed] [Google Scholar]
- DuBrul (1950).DuBrul EL. Posture, locomotion and the skull in Lagomorpha. American Journal of Anatomy. 1950;87(2):277–313. doi: 10.1002/aja.1000870205. [DOI] [PubMed] [Google Scholar]
- Fostowicz-Frelik (2007).Fostowicz-Frelik Revision of Hypolagus (Mammalia: Lagomorpha) from the Plio-Pleistocene of Poland: qualitative and quantitative study. Annals of Zoology. 2007;57(3):541–590. [Google Scholar]
- Gambaryan & Hardin (1974).Gambaryan PP, Hardin H. How mammals run: anatomical adaptations. New York: Wiley; 1974. p. 357. [Google Scholar]
- Gans (1974).Gans C. Biomechanics: an approach to vertebrate biology. Ann Arbor: University of Michigan Press; 1974. p. 272. [Google Scholar]
- Garland (1983).Garland T., Jr The relation between maximal running speed and body mass in terrestrial mammals. Journal of Zoology. 1983;199:157–170. doi: 10.1111/j.1469-7998.1983.tb02087.x. [DOI] [Google Scholar]
- Heesy (2004).Heesy CP. On the relationship between orbit orientation and binocular visual field overlap in mammals. Anatomical Record. 2004;281A:1104–1110. doi: 10.1002/ar.a.20116. [DOI] [PubMed] [Google Scholar]
- Heesy (2005).Heesy CP. Function of the mammalian postorbital bar. Journal of Morphology. 2005;264(3):363–380. doi: 10.1002/jmor.10334. [DOI] [PubMed] [Google Scholar]
- Heesy (2008).Heesy CP. Ecomorphology of orbit orientation and the adaptive significance of binocular vision in primates and other mammals. Brain, Behavior and Evolution. 2008;71(1):54. doi: 10.1159/000108621. [DOI] [PubMed] [Google Scholar]
- Heesy, Ross & Demes (2007).Heesy CP, Ross CF, Demes B. Oculomotor stability and the functions of the postorbital bar and septum. In: Ravosa MJ, Dagosto M, editors. Primate origins: adaptations and evolution. New York: Springer; 2007. pp. 257–283. [Google Scholar]
- Hildebrand (1988).Hildebrand M. Analysis of vertebrate structure. 3rd ed. New York: John Wiley and Sons; 1988. [Google Scholar]
- Hoffmeister & Zimmerman (1967).Hoffmeister DF, Zimmerman EG. Growth of the skull in the cottontail (Sylvilagus floridanus) and its application to age determination. American Midland Naturalist. 1967;78:198–206. doi: 10.2307/2423380. [DOI] [Google Scholar]
- Hopkins & Davis (2009).Hopkins SB, Davis EB. Quantitative morphological proxies for fossoriality in small mammals. Journal of Mammalogy. 2009;90:1449–1460. doi: 10.1644/08-MAMM-A-262R1.1. [DOI] [Google Scholar]
- Hunt (1974).Hunt RM. The auditory bulla in Carnivora: an anatomical basis for reappraisal of carnivore evolution. Journal of Morphology. 1974;143(1):21–75. doi: 10.1002/jmor.1051430103. [DOI] [PubMed] [Google Scholar]
- Igarashi et al. (2000).Igarashi N, Yamamura K, Yamada Y, Kohno S. Head movements and neck muscle activities associated with the jaw movement during mastication in the rabbit authors. Brain Research. 2000;871(1):151–155. doi: 10.1016/S0006-8993(00)02433-1. [DOI] [PubMed] [Google Scholar]
- Ivanoff (2001).Ivanoff DV. Partitions in the carnivoran auditory bulla: their formation and significance for systematics. Mammal Review. 2001;31(1):1–16. doi: 10.1046/j.1365-2907.2001.00069.x. [DOI] [Google Scholar]
- Iwaniuk et al. (2008).Iwaniuk AN, Heesy CP, Hall MI, Wylie DR. Relative Wulst volume is correlated with orbit orientation and binocular visual field in birds. Journal of Comparative Physiology A. 2008;194(3):267–282. doi: 10.1007/s00359-007-0304-0. [DOI] [PubMed] [Google Scholar]
- Jeffery & Cox (2010).Jeffery N, Cox PG. Do agility and skull architecture influence the geometry of the mammalian vestibulo-ocular reflex? Journal of Anatomy. 2010;216(4):496–509. doi: 10.1111/j.1469-7580.2010.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg (2013).Klingenberg CP. Cranial integration and modularity: insights into evolution and development from morphometric data. Hystrix. 2013;24(1):43–58. doi: 10.4404/hystrix-24.1-6367. [DOI] [Google Scholar]
- Liao, Zhang & Liu (2007).Liao J, Zhang Z, Liu N. Effects of altitudinal change on the auditory bulla in Ochotona daurica (Mammalia, Lagomorpha) Journal of Zoological Systematics and Evolutionary Research. 2007;45(2):151–154. doi: 10.1111/j.1439-0469.2006.00401.x. [DOI] [Google Scholar]
- Lieberman, Ross & Ravosa (2000).Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function, and integration. American Journal of Physical Anthropology. 2000;113(s 31):117–169. doi: 10.1002/1096-8644(2000)43:31+<117::AID-AJPA5>3.3.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Maddison & Maddison (2015).Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. (Version 3.02) 2015 Available at http://mesquiteproject.org .
- Martínez-Abadías et al. (2012).Martínez-Abadías N, Esparza M, Sjøvold T, González-José R, Santos M, Hernández M, Klingenberg CP. Pervasive genetic integration directs the evolution of human skull shape. Evolution. 2012;66(4):1010–1023. doi: 10.1111/j.1558-5646.2011.01496.x. [DOI] [PubMed] [Google Scholar]
- Matthee et al. (2004).Matthee CA, Van Vuuren BJ, Bell D, Robinson TJ. A molecular supermatrix of the rabbits and hares (Leporidae) allows for the identification of five intercontinental exchanges during the Miocene. Systematic Biology. 2004;53(3):433–447. doi: 10.1080/10635150490445715. [DOI] [PubMed] [Google Scholar]
- Mitteroecker et al. (2012).Mitteroecker P, Gunz P, Neubauer S, Müller G. How to explore morphological integration in human evolution and development? Evolutionary Biology. 2012;39(4):536–553. doi: 10.1007/s11692-012-9178-3. [DOI] [Google Scholar]
- Moore & Spence (1969).Moore WJ, Spence TF. Age changes in the cranial base of the rabbit (Oryctolagus cuniculus) The Anatomical Record. 1969;165(3):355–361. doi: 10.1002/ar.1091650305. [DOI] [PubMed] [Google Scholar]
- Mosimann (1970).Mosimann JE. Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. Journal of the American Statistical Association. 1970;65(330):930–945. doi: 10.1080/01621459.1970.10481136. [DOI] [Google Scholar]
- Mosimann & James (1979).Mosimann JE, James FC. New statistical methods for allometry with application to Florida redwinged blackbirds. Evolution. 1979;33:444–459. doi: 10.2307/2407633. [DOI] [PubMed] [Google Scholar]
- Noble, Kowalski & Ravosa (2000).Noble VE, Kowalski EM, Ravosa MJ. Orbit orientation and the function of the mammalian postorbital bar. Journal of Zoology. 2000;250(3):405–418. doi: 10.1111/j.1469-7998.2000.tb00784.x. [DOI] [Google Scholar]
- Novacek (1977).Novacek MJ. Aspects of the problem of variation, origin and evolution of the eutherian auditory bulla. Mammal Review. 1977;7:131–150. doi: 10.1111/j.1365-2907.1977.tb00366.x. [DOI] [Google Scholar]
- Palacios et al. (2008).Palacios F, Angelone C, Alonso G, Reig S. Morphological evidence of species differentiation within Lepus capensis Linnaeus, 1758 (Leporidae, Lagomorpha) in Cape Province, South Africa. Mammalian Biology-Zeitschrift für Säugetierkunde. 2008;73(5):358–370. doi: 10.1016/j.mambio.2007.10.013. [DOI] [Google Scholar]
- Pavlinov & Rogovin (2000).Pavlinov II, Rogovin KA. Relation between size of pinna and auditory bulla in specialized desert rodents. Zhurnal Obshchei Biologii. 2000;61:87–101. (in Russian) [PubMed] [Google Scholar]
- Pintur et al. (2014).Pintur K, Dančević N, Štedul I, Popović N, Slijepčević V. Craniometric features of European hare (Lepus europaeus Pall.) from North-west Croatia and the island of Vir. Veterinarski arhiv. 2014;84(4):387–400. [Google Scholar]
- Porto et al. (2009).Porto A, de Oliveira FB, Shirai LT, De Conto V, Marroig G. The evolution of modularity in the mammalian skull I: morphological integration patterns and magnitudes. Evolutionary Biology. 2009;36(1):118–135. doi: 10.1007/s11692-008-9038-3. [DOI] [Google Scholar]
- Rayner (1988).Rayner JM. Form and function in avian flight. In: Johnston RF, editor. Current Ornithology. New York: Springer; 1988. pp. 1–66. [Google Scholar]
- R Core Team (2014).R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. Available at http://www.R-project.org/ [Google Scholar]
- Reese, Lanier & Sargis (2013).Reese AT, Lanier HC, Sargis EJ. Skeletal indicators of ecological specialization in pika (Mammalia, Ochotonidae) Journal of Morphology. 2013;274(5):585–602. doi: 10.1002/jmor.20127. [DOI] [PubMed] [Google Scholar]
- Ross (1995).Ross CF. Allometric and functional influences on primate orbit orientation and the origins of the Anthropoidea. Journal of Human Evolution. 1995;29(3):201–227. doi: 10.1006/jhev.1995.1057. [DOI] [Google Scholar]
- Ross & Martin (2007).Ross CF, Martin RD. The role of vision in the origin and evolution of primates. Evolution of Nervous Systems. 2007;4:59–78. [Google Scholar]
- Sanger et al. (2012).Sanger TJ, Mahler DL, Abzhanov A, Losos JB. Roles for modularity and constraint in the evolution of cranial diversity among Anolis lizards. Evolution. 2012;66(5):1525–1542. doi: 10.1111/j.1558-5646.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- Seckel & Janis (2008).Seckel L, Janis C. Convergences in scapula morphology among small cursorial mammals: an osteological correlate for locomotory specialization. Journal of Mammalian Evolution. 2008;15:261–279. doi: 10.1007/s10914-008-9085-7. [DOI] [Google Scholar]
- Sherratt et al. (2014).Sherratt E, Gower DJ, Klingenberg CP, Wilkinson M. Evolution of cranial shape in caecilians (Amphibia: Gymnophiona) Evolutionary Biology. 2014;41(4):528–545. doi: 10.1007/s11692-014-9287-2. [DOI] [Google Scholar]
- Singh et al. (2012).Singh N, Harvati K, Hublin JJ, Klingenberg CP. Morphological evolution through integration: a quantitative study of cranial integration in Homo, Pan, Gorilla and Pongo. Journal of Human Evolution. 2012;62(1):155–164. doi: 10.1016/j.jhevol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Stoner, Bininda-Emonds & Caro (2003).Stoner CJ, Bininda-Emonds ORP, Caro T. The adaptive significance of coloration in lagomorphs. Biological Journal of the Linnean Society. 2003;79(2):309–328. doi: 10.1046/j.1095-8312.2003.00190.x. [DOI] [Google Scholar]
- Strait & Ross (1999).Strait DS, Ross CF. Kinematic data on primate head and neck posture: implications for the evolution of basicranial flexion and an evaluation of registration planes used in paleoanthropology. American Journal of Physical Anthropology. 1999;108(2):205–222. doi: 10.1002/(SICI)1096-8644(199902)108:2<205::AID-AJPA6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Szalay (1985).Szalay FS. Rodent and lagomorph morphotype adaptations, origins, and relationships: some postcranial attributes analyzed. In: Luckett WP, Hartenberger J-L, editors. Evolutionary Relationships Among Rodents. New York: Springer; 1985. pp. 83–132. [Google Scholar]
- Thompson (1942).Thompson D W. On growth and form. 2nd ed. New York: Macmillan; 1942. [Google Scholar]
- Vidal, Graf & Berthoz (1986).Vidal PP, Graf W, Berthoz A. The orientation of the cervical vertebral column in unrestrained awake animals. Experimental Brain Research. 1986;61:549–559. doi: 10.1007/BF00237580. [DOI] [PubMed] [Google Scholar]
- Wake (1993).Wake MH. The skull as a locomotor organ. In: Hanken J, Hall BK, editors. The Skull, volume 3: Functional and Evolutionary Mechanisms. Chicago: University of Chicago Press; 1993. pp. 197–240. [Google Scholar]
- Webb (1984).Webb PW. Body form, locomotion and foraging in aquatic vertebrates. American Zoologist. 1984;24(1):107–120. [Google Scholar]
- Young et al. (2014).Young JW, Danczak R, Russo GA, Fellmann CD. Limb bone morphology, bone strength, and cursoriality in lagomorphs. Journal of Anatomy. 2014;225:403–418. doi: 10.1111/joa.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.