Abstract
Hypothesis
A functional vestibular prosthesis can be implanted in human such that electrical stimulation of each semicircular canal produces canal-specific eye movements while preserving vestibular and auditory function.
Background
A number of vestibular disorders could be treated with prosthetic stimulation of the vestibular end organs. We have previously demonstrated in rhesus monkeys that a vestibular neurostimulator, based on the Nucleus Freedom cochlear implant, can produce canal-specific electrically evoked eye movements while preserving auditory and vestibular function. An investigational device exemption has been obtained from the FDA to study the feasibility of treating uncontrolled Ménière’s disease with the device.
Methods
The UW/Nucleus vestibular implant was implanted in the perilymphatic space adjacent to the three semicircular canal ampullae of a human subject with uncontrolled Ménière’s disease. Pre and postoperative vestibular and auditory function were assessed. Electrically evoked eye movements were measured at two time points postoperatively.
Results
Implantation of all semicircular canals was technically feasible. Horizontal canal and auditory function were largely, but not totally, lost. Electrode stimulation in two of three canals resulted in canal-appropriate eye movements. Over time, stimulation thresholds increased.
Conclusions
Prosthetic implantation of the semicircular canals in humans is technically feasible. Electrical stimulation resulted in canal-specific eye movements, although thresholds increased over time. Preservation of native auditory and vestibular function, previously observed in animals, was not demonstrated in a single subject with advanced Ménière’s disease.
Keywords: Vestibular implant, vestibular prosthesis, vestibular implantation, vestibular neurostimulator, Ménière’s disease, semicircular canals
INTRODUCTION
There is a pressing need for new modalities to treat vestibular dysfunction. Aside from canal plugging for superior canal dehiscence, vestibular rehabilitation, and the popularization of intratympanic injections for Ménière’s disease, the treatment for vestibular pathology has remained essentially unchanged for nearly four decades. Destructive procedures such as intratympanic gentamicin, labyrinthectomy, and vestibular nerve section are still commonly the best option for patients with episodic conditions such as Ménière’s disease who have failed to respond to less invasive management.
Over the same time period, cochlear implantation has become a remarkably successful tool for treating sensorineural hearing loss. Interest has turned to using analogous technology for treating vestibular disorders.
Several groups have developed and implanted vestibular prostheses in rodents (1,2) and non-human primates (3–5). The primary target condition has been bilateral vestibular hypofunction (BVH), a less common but debilitating disorder marked by disabling oscillopsia. Such “sensor-based” devices employ accelerometers and gyroscopes to detect 3D motion of the head and deliver an encoded signal to the ampullary nerves.
Efforts to translate this technology to human clinical trials have been hampered by the complexity of the sensor-based technology and the concern over greater risk than benefit to patients. One concern is the possibility of cochlear damage with vestibular implantation. Most patients with BVH, for example, deny a history of hearing loss (6) and at least 25% have normal audiometry for their age (7).
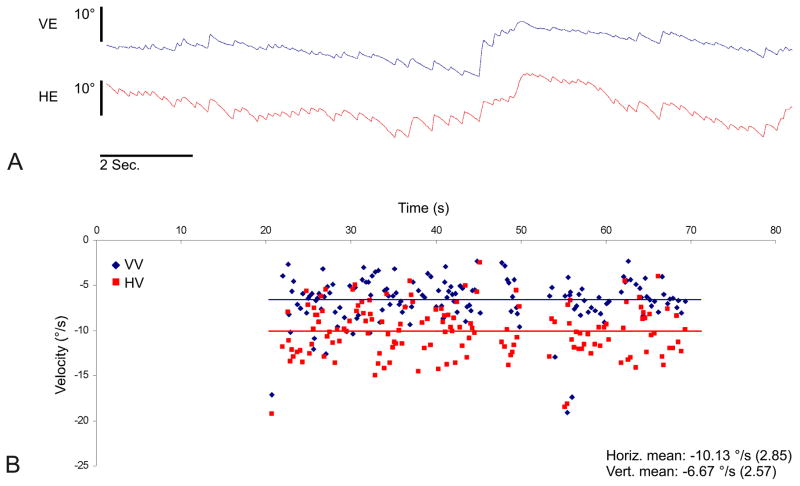
Our group has targeted unilateral uncontrolled Ménière’s disease as an ideal condition for human translation of vestibular implant technology. During Ménière’s attacks, it has been hypothesized that acute ruptures in the membranous labyrinth result in contamination of the potassium-poor perilymph with potassium-rich endolymph. The pathologically high potassium levels in the perilymph blocks neural transmission (8). Vertiginous symptoms may ensue from a sudden imbalance in vestibular output between the diseased side, where there is an acute loss of the baseline tonic signal, and the healthy side, where there is a normal baseline tonic signal. Nystagmography has suggested this latter hypothesis, showing contralaterally directed beats in 6 of 9 Meniere’s patients with spontaneous nystagmus during attacks (9). A subsequent study demonstrated contralaterally directed nystagmus during acute Meniere’s attacks in eight patients followed by a less intense after-nystagmus in the ipsilateral direction (10). Our own group has also noted contralaterally directed nystagmus in a single patient during an acute Meniere’s attack. (Fig. 1). The literature, however, is not definitive regarding the direction of nystagmus during acute attacks (11) presumably because of the heterogeneous nature of the underlying disorder, and changes over time during an attack.
FIG. 1.
Videonystagmography of a patient with left-sided Ménière’s disease during an acute attack illustrating hypofunction of the ipsilateral vestibular system. A. Vertical (blue) and horizontal (red) eye position traces. B. Vertical (blue) and horizontal (red) slow-phase velocity (SPV) over time. Each point denotes the velocity of a single slow phase, while the lines represent the average slow-phase velocity over the course of the recording.
If vertigo is due to a sudden loss of vestibular tonic output, a patient could activate the device during an attack in order to restore the absent signal. This could, in turn, halt or reduce the disabling symptoms. The prosthesis would thus act as a vestibular “pacemaker”, replacing missing afferent information with a pre-programmed and patient-controlled stimulus (12–14). Even if vertigo is due to an increase in vestibular output, then the device could potentially be programmed to override this pathologic signal. For example, through the use of high-rate, low amplitude stimuli it is theoretically possible to induce a partial depolarization block (15) and thereby reduce the rate of firing of a pathologically hyperfunctional canal. Alternatively, appropriate charge-balanced asymmetric pulse could accomplish the same goal, although this is not currently possible with our existing device.
For Ménière’s patients who are otherwise considering destructive procedures as their next therapeutic option, a vestibular implant could represent an attractive alternative. Furthermore, a device that could abort attacks while preserving native auditory and vestibular function would represent a significant advance in care.
The UW/Nucleus vestibular implant is a multistage device that can function as a pacemaker or a sensor-based prosthesis. The stimulator was designed in our laboratories and fabricated by Cochlear, Ltd. Longitudinal studies in rhesus macaques have demonstrated robust, precise, and specific stimulation of individual semicircular canal afferents as well as preservation of native canal rotational sensitivity and hearing over time periods greater than a year (4,13,16).
Our group has received FDA approval to enroll up to ten subjects with uncontrolled Ménière’s disease with the UW/Nucleus vestibular implant as part of a feasibility trial. We report here on the first human subject implanted with a vestibular prosthesis for a therapeutic purpose.
METHODS
Device
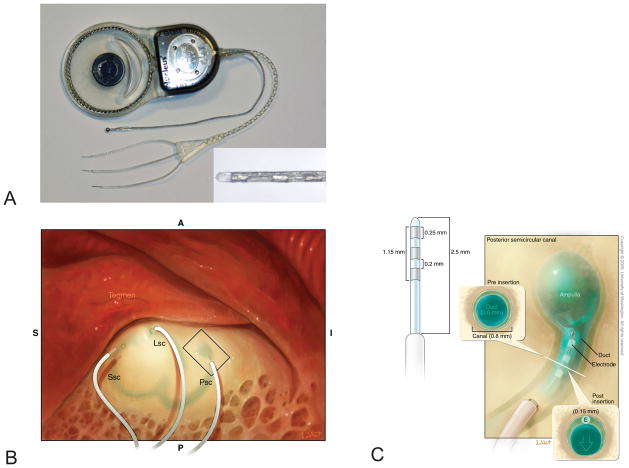
The UW/Nucleus Vestibular Implant consists of a modified Nucleus Freedom cochlear implant (Cochlear Ltd; Sydney, Australia). The receiver/stimulator is attached to a custom trifurcating array with three electrodes per array (i.e., nine electrodes total; Fig 2A). Each electrode is 150 μm in diameter, which is smaller than that of a Hybrid (electroacoustic) cochlear implant. The length of the electrode array inserted into the labyrinth is 2.5 mm. Measurements appear in Fig 2C and are further detailed in a previous report (13).
FIG. 2.
Design of the UW/Nucleus vestibular implant. A. Photograph displaying trifurcating electrode array with 3 electrodes per array (inset) as well as a ball ground electrode. The case serves as an additional ground electrode. B. Electrodes are implanted into the semicircular canal perilymphatic space adjacent to the ampulla. C. Enlargement of the box in B illustrating the theorized minimal distortion of the membranous labyrinth by the implanted electrode.
The device also includes a ball ground electrode and a ground contained within the receiver/stimulator casing (Fig 2A). The device was fabricated by Cochlear, Ltd (Lane Cove, Australia). Additional details have been described previously (4,13,17).
Human Subject
Subject Characteristics
The subject was a 56 year-old male technology executive who met inclusion criteria for the FDA-approved UW/Nucleus vestibular implant feasibility study (see below for regulatory details). His symptoms met AAO-HNS criteria for definite Ménière’s disease. His first episode of vertigo was approximately eight years prior to implantation. He had failed medical treatment with a low salt diet and hydrochlorothiazide/triamterene. He had not had intratympanic aminoglycoside or steroid injections. One year prior to vestibular implantation, he underwent an endolymphatic shunt, which was followed by symptomatic remission for five months, followed by recurrence of debilitating vertigo episodes every few days. This occurred despite continuation of the low salt diet and diuretic. A revision shunt was not considered since the result was not long lasting. At this point, the patient was offered the options of intratympanic gentamicin, labyrinthectomy, vestibular nerve section, or enrollment in the vestibular implant clinical trial. The patient chose to proceed to vestibular implantation. Preoperative audiologic and vestibular function are documented in Figs. 3–4.
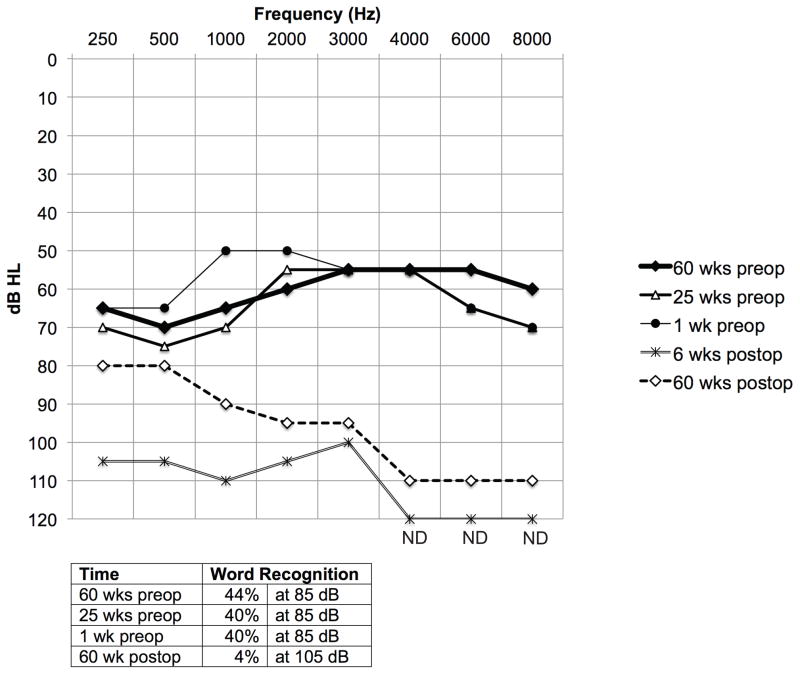
FIG. 3.
Audiometric data. Composite audiogram, including pure tone air conduction thresholds (above) and word recognition scores (below) for the implanted ear. Pre and postop refer to placement of the UW/Nucleus vestibular implant. ND = not detected.
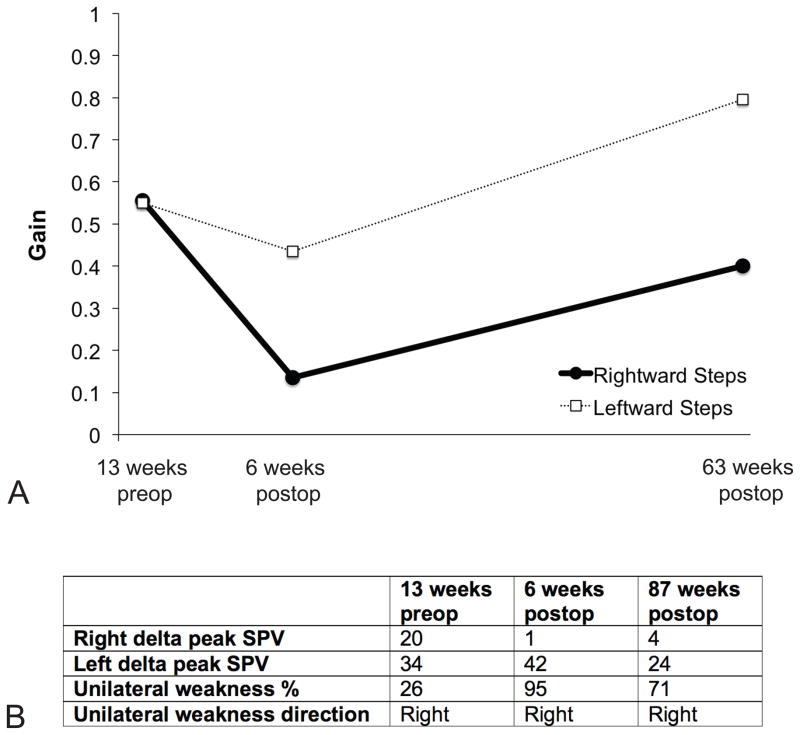
FIG. 4.
Vestibular function data. A. Rotational chair velocity step testing. B. Caloric data. Delta peak SPV indicates the difference between the peak warm slow phase velocity and the peak cold slow phase velocity values. Note that the right ear was implanted. SPV = slow phase velocity.
Vestibular Implantation
A postauricular incision was made and flaps were raised to expose the mastoid cortex. A well was drilled and a subperisoteal pocket was created for the receiver/stimulator. A simple mastoidectomy was then performed to expose the incus and vestibular labyrinth. The semicircular canals were thinned (blue lined) with a diamond burr and fenestrated with a pick. The location of the labyrinthotomy was chosen based on a visual estimate of the distance to the ampulla and the length of the electrode. The electrodes were inserted 2.5 mm into the perilymphatic space with jeweler’s forceps and positioned adjacent to each of the three ampullae (Figs. 2B, 2C). Meticulous care was taken to avoid traumatizing the canals in order to preserve residual canal rotational sensitivity. Systemic dexamethasone was administered preoperatively, perioperatively, and postoperatively.
Electrically-evoked vestibular compound action potentials (ECAPs) were measured using standard Neural Response Telemetry software to facilitate and confirm optimal electrode placement (17). After the initial placement of electrodes in each semicircular canal, adequate ECAPs could not be obtained. All three electrodes were removed and fenestrations were extended closer to the ampullae. The electrodes were inserted until they reached their hub. Adequate ECAPs were then obtained from the superior and lateral canals, but not the posterior canal. Fascia was then used to seal the labyrinthotomy and Tisseel (fibrin sealant) was used to fill the mastoid, further securing the electrodes. A tie-down stitch was additionally employed. The wound was then closed in the standard fashion for cochlear implant surgery.
Vestibular Testing
Vestibular testing was performed in a clinical vestibular laboratory. The gain and time constant were measured for rotary chair velocity step testing 13 weeks pre-implantation as well as 6 and 63 weeks post-implantation. Step testing included four 100 deg/s steps with 45 s of sustained velocity. Caloric testing was performed 13 weeks pre-implantation as well as 6 and 87 weeks post-implantation.
Vestibular Implant Stimulation
All recording was performed in darkness. Electrically evoked eye movements were measured with videonystagmography (VNG) at 2, 6, and 63 weeks postoperatively. Only the distal-most electrode on each array within a given canal was employed.
Stimulation was provided with 2 s pulse trains of constant current and frequency. Monopolar stimulation was employed and both the ball and case were used as grounds. Pulses were biphasic with a width of 100 μs per phase and a gap of 8 μs. Pulse frequencies were 300 or 600 pulses per second (PPS). A variety of currents were presented at increasing levels but kept well below the safe charge density for these small surface area electrodes.
Mapping and Assessment of Efficacy in Ménière’s Attacks
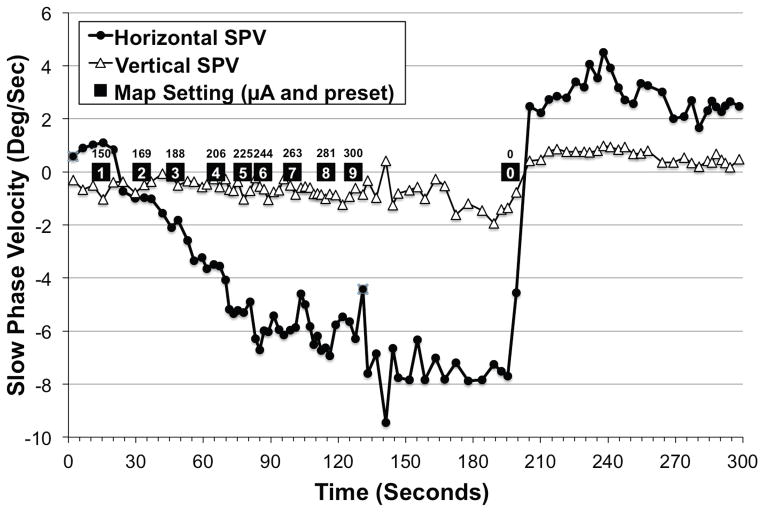
Six weeks post-implantation, the prosthesis was programmed with a take-home map created with custom software. Nine level settings (Fig. 8) were provided in increments of 25 μA, ranging from 150 μA (level 1), which was the lowest current that elicited a sense of rotation, to 350 μA (level 9), which produced the most severe vertigo the subject could comfortably tolerate for short periods. Level 5 (250 μA) approximated the intensity of his typical Ménière’s attack. All levels provided stimulation from the distal-most horizontal canal electrode at 600 PPS.
FIG. 8.
Slow phase velocity over time as “take-home” map settings are increased from “volume 1” (programmed to 150 μA) to “volume 9” (programmed to 300 μA). This simulates the sequence of stimuli the device would produce if the user were to titrate the prosthetic stimulation current to the intensity of a Ménière’s attack. The depicted simulation was performed in the laboratory in the absence of a Ménière’s attack at 6 weeks postoperatively. Positive values denote either rightward or upward slow phase velocity. Note that the subject’s actual take home map ranged from 150 μA to 350 μA.
The patient was instructed to progress through the map settings during an acute attack until symptoms were minimized or eliminated. Map settings with currents too low would be insufficient to curtail an attack. Map settings with currents too high (i.e., higher than the contralateral vestibular system’s tonic output) could theoretically produce vertigo in the opposite direction. Map settings in between could theoretically neutralize attacks.
Data Analysis
Eye movements were analyzed in Spike2 (CED; Cambridge, England) and MATLAB (MathWorks; Nattick, MA) using interactive analysis programs. For each elicited nystagmus response we marked the slow-phase segments by desaccading the record. For each segment, we estimated the mean velocity from the slope of a line fit to the eye position trace (based on a least-square error criterion). The effectiveness of the electrical stimulation was expressed in terms of the mean of these slow phase velocities (SPVs).
All error bars represent standard error of the mean (SEM).
Electrically Evoked Compound Action Potentials
Vestibular electrical evoked compound action potentials (ECAPs) were collected using the standard clinical software Custom Sound EP 1.3 for Nucleus Freedom cochlear implants. The details of setting stimulating and recording parameters have been previously described (17). Briefly, a forward masking paradigm along with an artifact reduction pulse was adopted to reduce stimulus artifact. The stimulating electrode was always set to the distal-most electrode in the array of the target canal. Current intensity in units of Clinical Level (CL) was gradually increased to find the threshold of activation. Vestibular ECAPs were monitored during intraoperative surgery and then reexamined 2 weeks post implantation. Follow-up ECAPs were collected at 63 weeks.
Regulatory Approvals
The study protocol was reviewed and approved by the Western Institutional Review Board. An investigational device exemption (IDE) was granted by the US Food and Drug Administration for implantation of the UW/Nucleus Vestibular Implant as part of a clinical feasibility trial in ten subjects.
RESULTS
Perioperative Findings
Implantation was well tolerated. The subject had nystagmus several hours after surgery; however, vertigo was minimal. On postoperative day one, no significant nystagmus was observed clinically and the subject was ambulatory and eating well. The subject was discharged home less than 24 hours after surgery.
For five weeks after implantation, the subject continued to have typical, discrete Meniere’s attacks every second to third day. The attack frequency, duration, and quality were similar to before implantation. After five weeks, at about the time his first take-home processor could be provided, his attacks ceased. He had only one subsequent attack, which occurred six months postoperatively.
Auditory Testing
Six weeks post-implantation, the subject had a pure tone threshold shift of 40 dB at lower frequencies and undetectable thresholds at higher frequencies. At 60 weeks post-implantation the thresholds partially recovered. At lower frequencies they were within 20 dB of the preoperative baseline and at higher frequencies they were within 50 dB. The word recognition score dropped from 40% at 85 dB preoperatively to 4% at 105 dB 60 weeks postoperatively. (Fig. 3) The subject’s contralateral ear had essentially normal hearing for his age both pre and postoperatively (data not shown).
Vestibular Testing
Rotational chair velocity step testing revealed a normal gain 13 weeks preop. Between this time point and 6 weeks post-implantation, the gain declined, particularly for steps towards the implanted ear (i.e., rightward steps). At 63 weeks post-implantation, the gain recovered for rightward steps, however there was a persistent asymmetry. (Fig. 4A) The time constants became indeterminate at 6 weeks post-implantation for steps toward the implanted ear, and were shortened for steps away from the implanted ear, but recovered by 63 weeks.
Caloric testing indicated a 26% unilateral weakness preoperatively. This weakness increased to 95% at 6 weeks postoperatively and recovered slightly to 71% at 87 weeks. At all time points, the weakness was rightward. (Fig. 4B)
Electrically Evoked Eye Movements
Canal-appropriate, electrically evoked eye movements were elicited by individual canal electrodes.
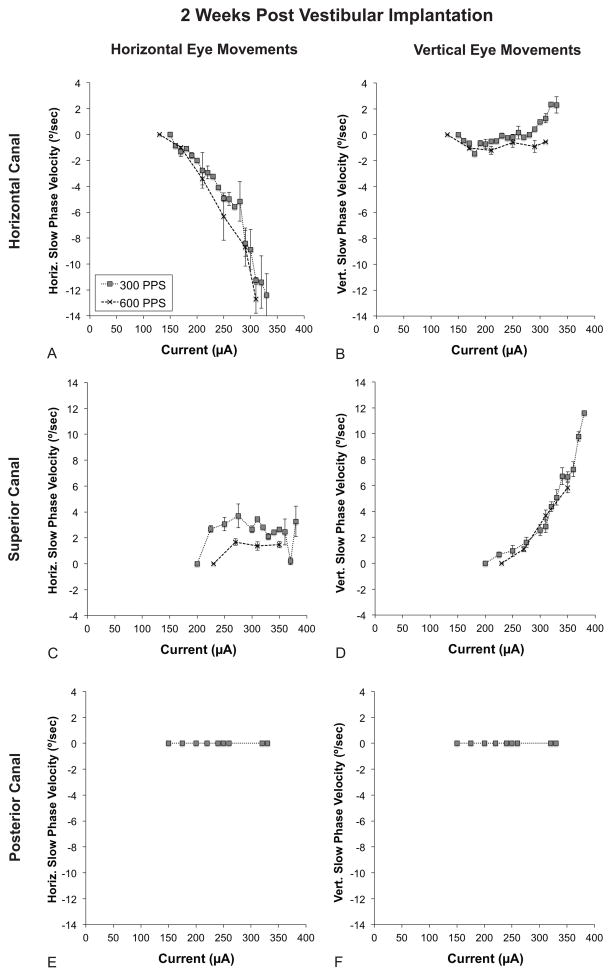
Stimulation of the horizontal canal electrode resulted in a horizontally oriented nystagmus with the slow phase directed away from the implanted ear. Two weeks post-implantation, the threshold for measurable nystagmus was approximately 150 μA. Increasing current resulted in higher slow phase velocities (i.e., the velocity of the slow phase component of the nystagmus; SPV). Doubling the pulse frequency from 300 pulses per second (PPS) to 600 PPS resulted in slightly higher SPVs at a given current. (Fig. 5A)
FIG. 5.
Electrically evoked eye movements at an early time point (2 weeks post-implantation). All graphs depict slow phase velocity (SPV) versus current. Positive values denote either rightward or upward slow phase velocity. Refer to the inset in A for trace labels. A. Horizontal SPV during stimulation of the horizontal canal electrode. B. Vertical SPV during stimulation of the horizontal canal electrode. C. Horizontal SPV during stimulation of the superior canal electrode. D. Vertical SPV during stimulation of the superior canal electrode. E. Horizontal SPV during stimulation of the posterior canal electrode. F. Vertical SPV during stimulation of the posterior canal electrode. Note that the ranges of the X and Y-axes are the same for all graphs. Error bars represent SEM. PPS = pulses per second.
Vertical nystagmus was seen with horizontal canal stimulation but only at the higher stimulation currents. The vertical SPV was only a small fraction of the horizontal SPV. (Fig. 5B)
Stimulation of the superior canal electrode resulted in a vertically oriented nystagmus with the slow phase directed upwards. The threshold for detectable nystagmus for superior canal stimulation was 200 μA, higher than for horizontal canal stimulation. Higher pulse frequency did not result in faster SPVs. There was no current-dependent increase in horizontal SPV above the threshold for electrically evoked vertical nystagmus. (Fig. 5C)
No nystagmus was observed with stimulation of the posterior canal electrode despite a prominent and canal appropriate subjective sense of vertigo. (Fig. 5E–F)
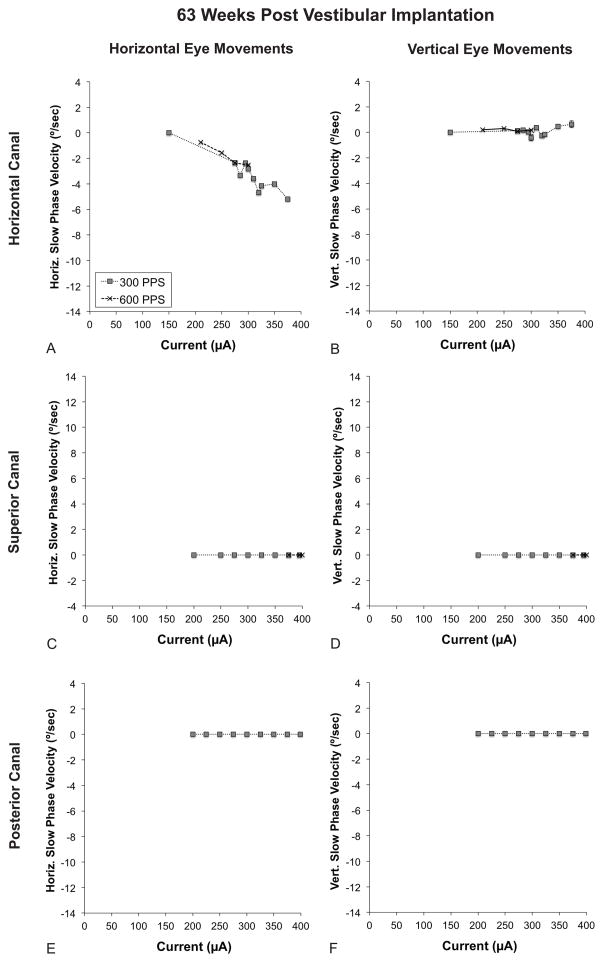
In order to assess the ability to persistently generate electrically evoked eye movements after long-term implantation, we repeated the experiment at 63 weeks post-implantation. The threshold for eliciting horizontal nystagmus with stimulation of the horizontal canal electrode increased from 150 μA to 200 μA. (Fig. 6A) As with the earlier time point, there was minimal vertical nystagmus. (Fig. 6B).
FIG. 6.
Electrically evoked eye movements at a late time point (63 weeks post-implantation). All graphs depict slow phase velocity (SPV) versus current. Positive values denote either rightward or upward slow phase velocity. Refer to the legend in part A for tracing labels. A. Horizontal SPV during stimulation of the horizontal canal electrode. B. Vertical SPV during stimulation of the horizontal canal electrode. C. Horizontal SPV during stimulation of the superior canal electrode. D. Vertical SPV during stimulation of the superior canal electrode. E. Horizontal SPV during stimulation of the posterior canal electrode. F. Vertical SPV during stimulation of the posterior canal electrode. The scale of the X and Y-axes is the same as in Fig. 5. Error bars represent SEM. PPS = pulses per second.
Unfortunately, electrically evoked eye movements could not be generated at 63 weeks with currents up to 400 μA with the superior canal electrode. (Fig. 6C–D) The posterior canal electrode continued to not produce nystagmus at 63 weeks. At this time it also no longer produced any percept of vertigo. (Fig. 6E–F)
Subjective Sensations
During stimulation of the horizontal canal electrode, the subject felt a sense of rolling to the right at lower currents and rightward yaw rotation at higher currents. The velocity and amplitude of the perceived yaw rotation increased with increased current level. This percept persisted 63 weeks post-implantation despite less pronounced eye movements. With stimulation of the superior canal electrode, the subject felt movement down and to the left. Despite the absence of nystagmus with posterior canal stimulation, a sense of vertigo was present at certain currents at the early postoperative time point. Interestingly, the subject never became unsteady or nauseated during the seated stimulation. In addition, the subject denied any auditory side effects during stimulation 2 and 6 weeks post-implantation. However, on 63 weeks post-implantation, he experienced a loud high-pitched tone at 295 μA and 300 PPS for stimulation with the horizontal canal electrode.
Facial Movement and Electrically Evoked Compound Action Potentials
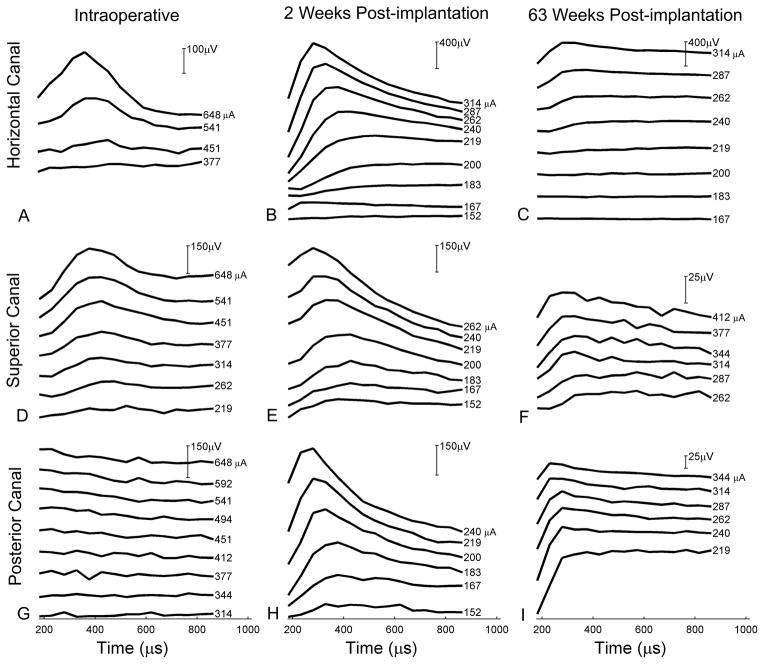
Facial muscle or lid twitches were not observed during three separate testing sessions at even the highest currents. A strong ECAP was observed for the superior and horizontal canal electrodes at 2 weeks postop. At 63 weeks postop, however, the ECAP for the horizontal canal electrode was less pronounced and for the superior canal was substantially smaller. (Fig. 7)
FIG. 7.
Electrically evoked compound action potentials (ECAPs). A. Horizontal canal intraop. B. Horizontal canal at 2 weeks postop. C. Horizontal canal at 63 weeks postop. D. Superior canal intraop. E. Superior canal at 2 weeks postop. F. Superior canal at 63 weeks postop. G. Posterior canal intraop. H. Posterior canal at 2 weeks postop. I. Posterior canal at 63 weeks postop. Note that the Y-axis scale varies (indicated by vertical bar at upper-right of graphs).
Vertigo Control During Ménière’s Episodes
After the subject was provided with the take-home stimulation map, he had one attack of vertigo as of the writing of this manuscript. The attack occurred at six months postoperatively, was mild in intensity, lasted one hour, and occurred at home. A map setting of 1 (150 μA, 600 PPS) subjectively suppressed symptoms. Using a map setting of 2 (175 μA, 600 PPS) worsened the vertigo. Turning off the device during the attack resulted in increased symptoms. These sensations were reproducible, such that cycling through the intensity settings resulted in the same response pattern. The subject repeatedly cycled through these settings during the hour-long attack.
An illustration of horizontal slow phase velocities as map settings were progressively increased in the laboratory (and in the absence of a Ménière’s attack) appears in Fig. 8.
Comparison Rhesus Macaque
Our group recently implanted all three semicircular canals of a single healthy adolescent rhesus macaque with the same device in an analogous fashion to the human subject. Comparison between the human Meniere’s patient and this non-diseased monkey may provide additional insight into the function of the vestibular implant. These data are available on the journal’s website. (Supplemental Figs. 1–5).
DISCUSSION
Previous reports from our research group have demonstrated the feasibility of implanting the rhesus macaque vestibular system and preserving both vestibular and auditory function (4,13,16), Phillips et al, submitted). In this study, we report on the first human implanted with a vestibular prosthesis for therapeutic purposes.
The surgery and stimulation sessions were very well tolerated by the human subject. Recovery was no more complicated than cochlear implantation and vertigo was minimal. Specifically, there were far milder vestibular signs and symptoms than after typical labyrinthectomy.
Vestibular and auditory function declined postoperatively and only partially recovered. This is in contrast to monkeys that underwent analogous two or three canal implantation with the same device by our group (13,16). In a recent monkey that had all three canals implanted, vestibular and auditory function declined far less postoperatively and then recovered to near normal levels (Supplemental Figs. 1–2) This latter monkey’s auditory results were similar to those of a previous study from a different group, which revealed a small 5–10 dB drop in hearing in 4 rhesus monkeys with 3 implanted canals (18). However, in a larger study by our laboratory using implantation techniques identical to the current study, only 3 of 8 monkeys with 2–3 canals implanted lost some hearing in the implanted ear. One of these loses was only mild (16).
The physical specifications of the implant used in the previous monkey studies and the present study were identical. Since the human labyrinth and temporal bone are markedly larger than that of the rhesus macaque, one might expect, if anything, less trauma and resultantly less loss of native function in the human. Hybrid (electroacoustic) cochlear implant electrodes, for example, are designed to be narrower than standard cochlear implant electrodes to allow greater preservation of native auditory function (19). The greater loss of function and poorer recovery could also be a species difference. For example, humans may possess a lesser ability to recover from perturbations of the vestibulocochlear system than rhesus macaques.
Another critical difference is that the implanted monkeys had normal vestibular systems, whereas the human in the present study had a diseased system. It is possible that beyond a certain level of damage, the labyrinth is less robust to further insult. The inability to recover from, or adapt to, foreign body implantation within the bony labyrinth may also be inherently related to the pathophysiology of Ménière’s disease. For example, while auditory function often recovers after a spontaneous drop early on in the disease course, drops in advanced disease (which includes the subject), may not reverse. Thus the vestibulocochlear end organs in Ménière’s disease may simply be less resistant to insult. Canal plugging for benign paroxysmal vertigo or superior canal dehiscence, for example, completely occludes the membranous labyrinth and rarely has an effect on auditory function (20,21). Alternatively, hydrops of the membranous labyrinth may decrease the perilymphatic space into which the electrode is inserted, increasing risk of compression or injury to the membranous labyrinth. Luetje has previously reported that hydropic ears poorly tolerate fenestration of the posterior semicircular canal (22). Likewise, the danger of stapedectomy in the setting of hydrops is well known (23).
Trauma from extending the fenestration in order to obtain optimal electrode placement could also have contributed to inner ear injury. However, monkeys that were reimplanted once or twice using the same technique still largely had preservation of native function (16). Lastly, a recent study of the converse scenario of vestibular insult after cochlear implantation revealed a significant loss in only 1 of 28 subjects (3.6%) (24). This demonstrates that it is possible to implant one organ without affecting neighboring organs. Further studies are needed to determine the degree of vestibular and auditory preservation in human vestibular implantation.
Since a take-home processor was provided, the subject had only one Ménière’s attack, which was mild. He was able to suppress symptoms at the lowest programmed current. Dialing up the current increased symptoms, presumably because the ipsilateral tonic vestibular output became too high (i.e., higher than the contralateral vestibular system’s tonic output). Turning off the implant also resulted in increased symptoms. While this sole incident of subjective data are very limited, it offers some early encouragement that the device may have functional utility in halting Ménière’s attacks. We can not rule out the possibility that the effect was placebo in nature. More data from stimulation during attacks are needed.
It is also possible that the postoperative subtotal reduction of vestibular function caused a remission in attacks. Indeed this is the precise goal of intratympanic gentamicin (ITG). While both vestibular implantation and ITG carry the risk of vestibular impairment, the former has been designed to attempt to avoid it, while the latter is designed to cause it. In this single subject, it is likely that the cessation of Ménière’s attacks more than five weeks postoperatively was due to a decrement in vestibular function at that time. However even if implanting a Ménière’s ear proves to consistently diminish vestibular function, implantation still carries a major theoretical advantage. For example, with ITG, recurrence of vertigo episodes at later time points requires repeat administration with its associated inconvenience, side effects, and risks. With vestibular implantation, however, any recurrence of vertigo episodes could potentially be treated with the device at home.
The definitive intervention for uncontrolled Ménière’s disease is total ablation of vestibular function through a labyrinthectomy or vestibular nerve section. Several lines of evidence suggest that this patient did not have an immediate postoperative labyrinthectomy. First, the implantation resulted in minimal postoperative vertigo. The subject had intact vestibular function in the implanted ear preoperatively, thus had the surgery resulted in a typical labyrinthectomy, he would have had far more pronounced and disabling postoperative symptoms. Second, the subject continued to have frequent, discrete Meniere’s attacks for five weeks following surgery. If the subject had a typical labyrinthectomy, attacks should have immediately ceased. Third, the patient had some recovery of postoperative vestibular and auditory function many months after surgery. Long term, however, despite a small but remarkable amount of low frequency hearing recovery, the majority of vestibular and auditory function remained absent.
During one of the subject’s stimulation sessions, a high frequency tone or vibration was perceived at high stimulation currents of the horizontal canal. This compares to a similar sound perceived during stimulation of a posterior canal electrode in a modified cochlear implant (25). Current spread to the cochlea may be responsible for these percepts. In the latter study, however, it was hypothesized that the sound was saccular in origin due to saccular projections to the cochlear nucleus (26).
The eye movement response to stimulation in the subject was qualitatively similar to that of a monkey that underwent three-canal implantation in an analogous fashion (Supplemental Fig. 3). For example, slow phase eye movement velocity (SPV) scaled with stimulation current. In general, out-of-plane eye movements were minimal, suggesting minimal current spread to neighboring canals. Previous reports in both monkeys (5,13) as well as in human vestibular implantation (25,27) have demonstrated variable degrees of current spread. Further improvement of canal-specific stimulation could be obtained by optimizing stimulation parameters. It may also be possible to create purer rotational signals by “steering” a resultant vector through stimulation of multiple canals (28).
Important differences were also present between the subject’s data and monkeys implanted by our group with the same device in an analogous fashion. First, SPVs are considerably below the peak velocities reported in monkeys by other groups and in a comparably implanted monkey in our laboratory. Likewise, thresholds for detectable nystagmus were higher in the human subject. (13) (Supplemental Fig. 3) There are several potential explanations for these differences. The recording conditions were not identical in the two species. Stimulation in monkeys took place in an environment where either rotation or electrical stimulation could occur, increasing the similarities between fictive and real rotation and possibly potentiating the VOR response. Stimulation in the human took place on a fixed bench. In addition, the monkeys had no known vestibular lesion, whereas the human subject had advanced Ménière’s disease. Degeneration of Scarpa’s ganglion cells in Ménière’s disease (29), for example, may result in decreased sensitivity to stimulation. Finally, the anatomy of the monkey and human semicircular canals differ in that there is potentially more opportunity for current to shunt away from the ampullary afferent terminals in humans due to lower resistance in the larger canal. However, this should be at least partly offset by the larger distances between ampullae and larger axonal diameters in humans.
In the present study, the SPVs in the horizontal canal electrode were greatly diminished at 63 weeks postoperatively compared to 2 weeks and the response was lost in the superior canal electrode at 63 weeks in the human subject. This differs from a monkey that recently underwent three-canal implantation in an analogous fashion, where the SPVs were relatively similar at the early and late recording dates. In addition, electrically evoked compound action potentials (ECAPs) in this monkey were similarly strong at both 1 and 37 weeks post-implantation. (Supplemental Fig. 4) In the human, however, the ECAPs for the horizontal canal were about half the amplitude for a given clinical level at 63 weeks compared to 2 weeks, paralleling the reduction in evoked eye movements. Similarly, for the superior canal, very weak ECAPs were observed at 63 weeks. These findings indicate decreased activation of the canal afferents in the human subject over time, which could be due to anatomical/physiologic differences in the two species or to the relative health of the implanted canals at the outset. Another possibility may relate to the monkey’s undergoing frequent stimulation trials whereas the human subject was only tested at three time points. Frequent stimulation may help to maintain the vestibular afferent input to the brain following implantation by maintaining the afferent projections to the ampullae. These pathways could be compromised either by implantation or by Ménière’s disease. An additional possibility is a change in the electrical properties separating the electrodes from the afferents. Finally, the electrodes may have migrated in the larger human canals, although this seems doubtful to have occurred more than 5 weeks postoperatively.
In summary, implantation of a human subject with a vestibular prosthesis was well tolerated and resulted in robust electrically evoked eye movements and vestibular percepts. Horizontal canal and auditory function were poorly but partly preserved. Results from monkeys suggest that greater preservation of native function, as well as long-term stimulation, are theoretically possible. Further refinements in surgical techniques, such as electrode placement closer to the ampullae and better electrode stabilization, as well as more frequent stimulation sessions, may result in improved human outcomes. These preliminary data pave the way for further investigation of vestibular implantation for both the treatment of acute Ménière’s attacks and for many other vestibular disorders.
Supplementary Material
Audiometric data comparing the human subject and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. A. Human subject composite audiogram, including pure tone air conduction thresholds (above) and word recognition scores (below) for the implanted ear. Monkey composite auditory brainstem response (ABR) for both B. implanted ear and C. non-implanted ear. Note that while otoscopy did not reveal an obvious middle ear effusion in the monkey one week post-implantation, it is possible that residual hemotympanum from surgery contributed to the transient 30–40 dB threshold shift seen in C. Pre and postop refer to placement of the UW/Nucleus vestibular implant. ND = not detected.
Vestibular function data comparing the human subject and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. A. Human gain with rotational chair velocity step testing. B. Monkey gain with rotational chair velocity step testing. C. Human caloric data. Delta peak SPV indicates the difference between the peak warm slow phase velocity and the peak cold slow phase velocity values. Note that in both subjects, the right ear was implanted. SPV = slow phase velocity.
Comparison of early electrically evoked eye movements for the human subject and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. Human data are 2 weeks post-implantation. Monkey data are 9 weeks post-implantation. All graphs depict slow phase velocity (SPV) versus current. Positive values denote either rightward or upward slow phase velocity. Refer to the inset in A for trace labels (note that solid lines are monkey and dotted/dashed lines are human). A. Horizontal SPV during stimulation of the horizontal canal electrode. B. Vertical SPV during stimulation of the horizontal canal electrode. C. Horizontal SPV during stimulation of the superior canal electrode. D. Vertical SPV during stimulation of the superior canal electrode. E. Horizontal SPV during stimulation of the posterior canal electrode. F. Vertical SPV during stimulation of the posterior canal electrode. Note that within a subject, the scale of the X and Y-axes are the same for all graphs. Error bars represent SEM. PPS = pulses per second.
Comparison of late electrically evoked eye movements for the human and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. Human data are 63 weeks post-implantation. Monkey data are 33 weeks post-implantation. All graphs depict slow phase velocity (SPV) versus current. Positive values denote either rightward or upward slow phase velocity. Refer to the legend in part A for tracing labels (note that solid lines are monkey and dotted/dashed lines are human). A. Horizontal SPV during stimulation of the horizontal canal electrode. B. Vertical SPV during stimulation of the horizontal canal electrode. C. Horizontal SPV during stimulation of the superior canal electrode. D. Vertical SPV during stimulation of the superior canal electrode. E. Horizontal SPV during stimulation of the posterior canal electrode. F. Vertical SPV during stimulation of the posterior canal electrode. The scale of the X and Y-axes is the same as in Supp. Fig. 3. Error bars represent SEM. PPS = pulses per second.
Electrically evoked compound action potentials (ECAPs) for a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. Compare to Fig. 7.
Acknowledgments
The authors thank Douglas D. Backous, MD and Barry Lia, PhD for their assistance. This work was supported by NIH/NIDCD contract N01-DC-6-005, NCRR ITHS ignition award RR00166, the Coulter Foundation, Cochlear, Ltd, and a gift from Sara Kranwinkle.
Footnotes
Financial Disclosures: JTR is a paid consultant for Cochlear Ltd, which manufactured and provided the UW/Nucleus Vestibular Implant. The company was not involved in data acquisition, analysis, or composition of this paper. JTR, JOP, CRSK, LL, KN, SMB, and the University of Washington hold intellectual property rights to the device being studied.
References
- 1.Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28:572–81. doi: 10.1114/1.293. [DOI] [PubMed] [Google Scholar]
- 2.Della Santina C, Migliaccio A, Patel A. Electrical stimulation to restore vestibular function development of a 3-d vestibular prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7380–5. doi: 10.1109/IEMBS.2005.1616217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merfeld DM, Haburcakova C, Gong W, et al. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng. 2007;54:1005–15. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- 4.Phillips JO, Bierer SM, Ling L, et al. Real-time communication of head velocity and acceleration for an externally mounted vestibular prosthesis. Proceedings of the IEEE Engineering in Medicine and Biology Society. 2011;2011:3537–41. doi: 10.1109/IEMBS.2011.6090588. [DOI] [PubMed] [Google Scholar]
- 5.Dai C, Fridman GY, Davidovics NS, et al. Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear Res. 2011;281:74–83. doi: 10.1016/j.heares.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie MB, Minor LB. Prognosis in bilateral vestibular hypofunction. Laryngoscope. 1999;109:35–41. doi: 10.1097/00005537-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Vibert D, Liard P, Hausler R. Bilateral idiopathic loss of peripheral vestibular function with normal hearing. Acta Otolaryngol (Stockh) 1995;115:611–5. doi: 10.3109/00016489509139375. [DOI] [PubMed] [Google Scholar]
- 8.Schuknecht HF. Meniere’s disease, pathogenesis and pathology. Am J Otolaryngol. 1982;3:349–52. doi: 10.1016/s0196-0709(82)80009-4. [DOI] [PubMed] [Google Scholar]
- 9.Aschan G, Stahle J. Nystagmus in Meniere’s disease during attacks; a nystagmographical study. Acta Otolaryngol (Stockh) 1957;47:189–201. doi: 10.3109/00016485709130333. [DOI] [PubMed] [Google Scholar]
- 10.McClure JA, Lycett P. Recovery nystagmus. The Journal of otolaryngology. 1978;7:141–8. [PubMed] [Google Scholar]
- 11.Bance M, Mai M, Tomlinson D, et al. The changing direction of nystagmus in acute Meniere’s disease: pathophysiological implications. Laryngoscope. 1991;101:197–201. doi: 10.1288/00005537-199102000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Merfeld DM, Gong W, Morrissey J, et al. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006;53:2362–72. doi: 10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein JT, Bierer S, Kaneko C, et al. Implantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol Neurotol. 2012;33:789–96. doi: 10.1097/MAO.0b013e318254ec24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall C, 3rd, Merfeld DM, Rauch SD, et al. Vestibular prostheses: the engineering and biomedical issues. J Vestib Res. 2002;12:95–113. [PubMed] [Google Scholar]
- 15.Rubinstein JT, Della Santina CC. Development of a biophysical model for vestibular prosthesis research. J Vestib Res. 2002;12:69–76. [PubMed] [Google Scholar]
- 16.Bierer SM, Ling L, Nie K, et al. Auditory outcomes following implantation and electrical stimulation of the semicircular canals. Hear Res. 2012;287:51–6. doi: 10.1016/j.heares.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie K, Bierer SM, Ling L, et al. Characterization of the electrically evoked compound action potential of the vestibular nerve. Otol Neurotol. 2011;32:88–97. doi: 10.1097/mao.0b013e3181f6ca45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai C, Fridman GY, Della Santina CC. Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys. Hear Res. 2011;277:204–10. doi: 10.1016/j.heares.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gantz BJ, Turner C, Gfeller KE, et al. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 20.Limb CJ, Carey JP, Srireddy S, et al. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969–80. doi: 10.1097/01.mao.0000235376.70492.8e. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal SK, Parnes LS. Human experience with canal plugging. Ann N Y Acad Sci. 2001;942:300–5. doi: 10.1111/j.1749-6632.2001.tb03754.x. [DOI] [PubMed] [Google Scholar]
- 22.Luetje CM. Hearing results following posterior semicircular canal injury during antesigmoid retrolabyrinthine selective vestibular nerve section. Am J Otol. 1992;13:600–2. [PubMed] [Google Scholar]
- 23.Issa TK, Bahgat MA, Linthicum FH, Jr, et al. The effect of stapedectomy on hearing of patients with otosclerosis and Meniere’s disease. Am J Otol. 1983;4:323–6. [PubMed] [Google Scholar]
- 24.Melvin TA, Della Santina CC, Carey JP, et al. The effects of cochlear implantation on vestibular function. Otol Neurotol. 2009;30:87–94. doi: 10.1097/mao.0b013e31818d1cba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyot JP, Sigrist A, Pelizzone M, et al. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann Otol Rhinol Laryngol. 2011;120:143–9. doi: 10.1177/000348941112000301. [DOI] [PubMed] [Google Scholar]
- 26.McCue MP, Guinan JJ., Jr Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. The American journal of otology. 1997;18:355–60. [PubMed] [Google Scholar]
- 27.Wall C, 3rd, Kos MI, Guyot JP. Eye movements in response to electric stimulation of the human posterior ampullary nerve. Ann Otol Rhinol Laryngol. 2007;116:369–74. doi: 10.1177/000348940711600509. [DOI] [PubMed] [Google Scholar]
- 28.Fridman GY, Davidovics NS, Dai C, et al. Vestibulo-Ocular Reflex Responses to a Multichannel Vestibular Prosthesis Incorporating a 3D Coordinate Transformation for Correction of Misalignment. J Assoc Res Otolaryngol. 2010;11:367–81. doi: 10.1007/s10162-010-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji K, Velazquez-Villasenor L, Rauch SD, et al. Temporal bone studies of the human peripheral vestibular system. Meniere’s disease. Ann Otol Rhinol Laryngol Suppl. 2000;181:26–31. doi: 10.1177/00034894001090s505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Audiometric data comparing the human subject and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. A. Human subject composite audiogram, including pure tone air conduction thresholds (above) and word recognition scores (below) for the implanted ear. Monkey composite auditory brainstem response (ABR) for both B. implanted ear and C. non-implanted ear. Note that while otoscopy did not reveal an obvious middle ear effusion in the monkey one week post-implantation, it is possible that residual hemotympanum from surgery contributed to the transient 30–40 dB threshold shift seen in C. Pre and postop refer to placement of the UW/Nucleus vestibular implant. ND = not detected.
Vestibular function data comparing the human subject and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. A. Human gain with rotational chair velocity step testing. B. Monkey gain with rotational chair velocity step testing. C. Human caloric data. Delta peak SPV indicates the difference between the peak warm slow phase velocity and the peak cold slow phase velocity values. Note that in both subjects, the right ear was implanted. SPV = slow phase velocity.
Comparison of early electrically evoked eye movements for the human subject and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. Human data are 2 weeks post-implantation. Monkey data are 9 weeks post-implantation. All graphs depict slow phase velocity (SPV) versus current. Positive values denote either rightward or upward slow phase velocity. Refer to the inset in A for trace labels (note that solid lines are monkey and dotted/dashed lines are human). A. Horizontal SPV during stimulation of the horizontal canal electrode. B. Vertical SPV during stimulation of the horizontal canal electrode. C. Horizontal SPV during stimulation of the superior canal electrode. D. Vertical SPV during stimulation of the superior canal electrode. E. Horizontal SPV during stimulation of the posterior canal electrode. F. Vertical SPV during stimulation of the posterior canal electrode. Note that within a subject, the scale of the X and Y-axes are the same for all graphs. Error bars represent SEM. PPS = pulses per second.
Comparison of late electrically evoked eye movements for the human and a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. Human data are 63 weeks post-implantation. Monkey data are 33 weeks post-implantation. All graphs depict slow phase velocity (SPV) versus current. Positive values denote either rightward or upward slow phase velocity. Refer to the legend in part A for tracing labels (note that solid lines are monkey and dotted/dashed lines are human). A. Horizontal SPV during stimulation of the horizontal canal electrode. B. Vertical SPV during stimulation of the horizontal canal electrode. C. Horizontal SPV during stimulation of the superior canal electrode. D. Vertical SPV during stimulation of the superior canal electrode. E. Horizontal SPV during stimulation of the posterior canal electrode. F. Vertical SPV during stimulation of the posterior canal electrode. The scale of the X and Y-axes is the same as in Supp. Fig. 3. Error bars represent SEM. PPS = pulses per second.
Electrically evoked compound action potentials (ECAPs) for a recent monkey that had all three semicircular canals implanted by our group with the same device in an analogous fashion. Compare to Fig. 7.