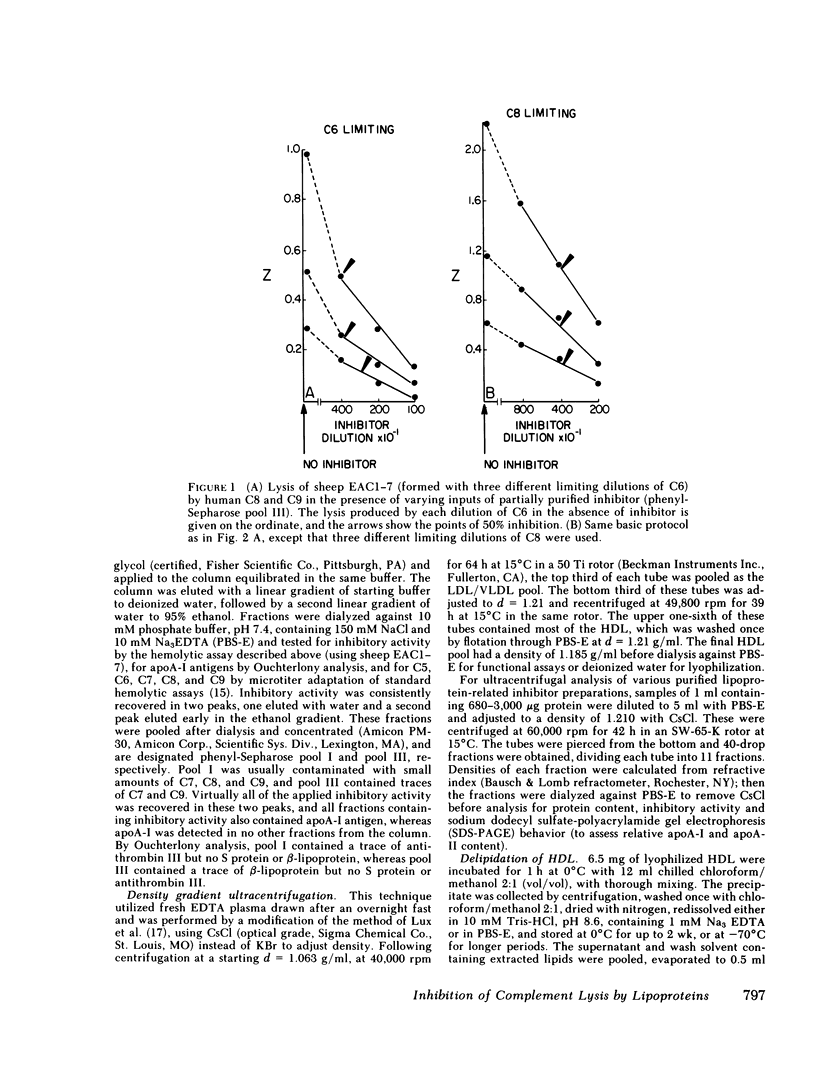
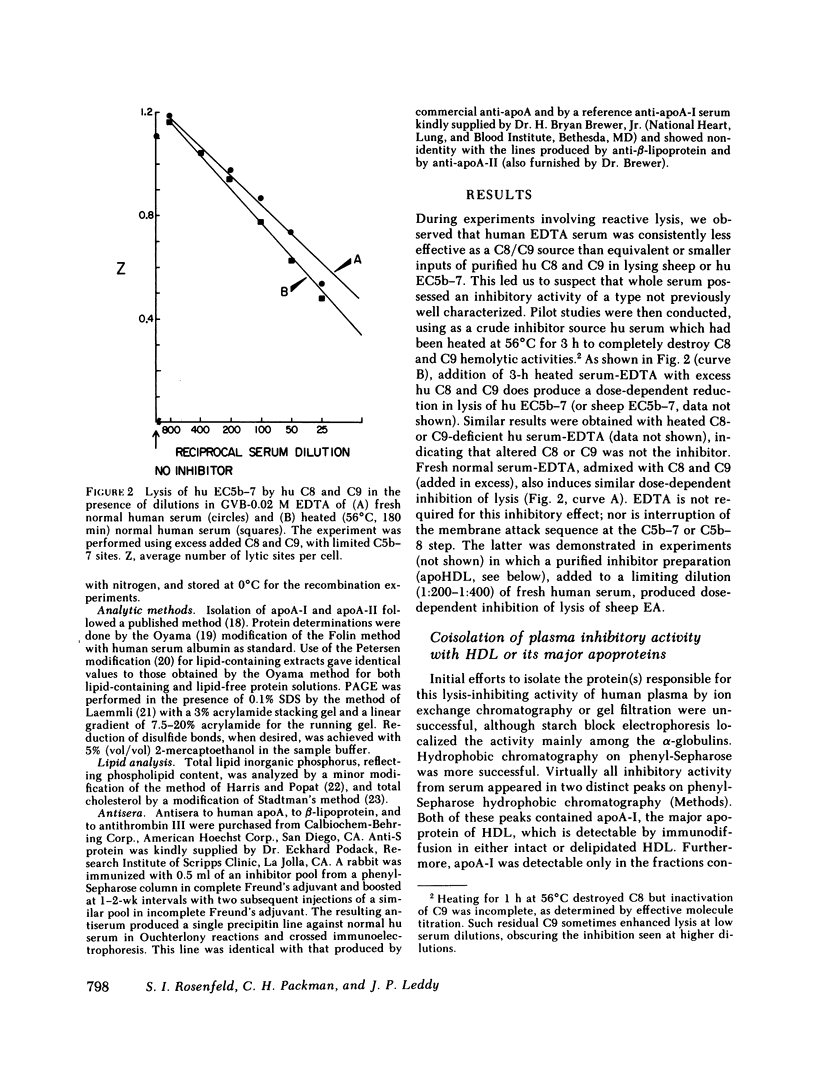
Abstract
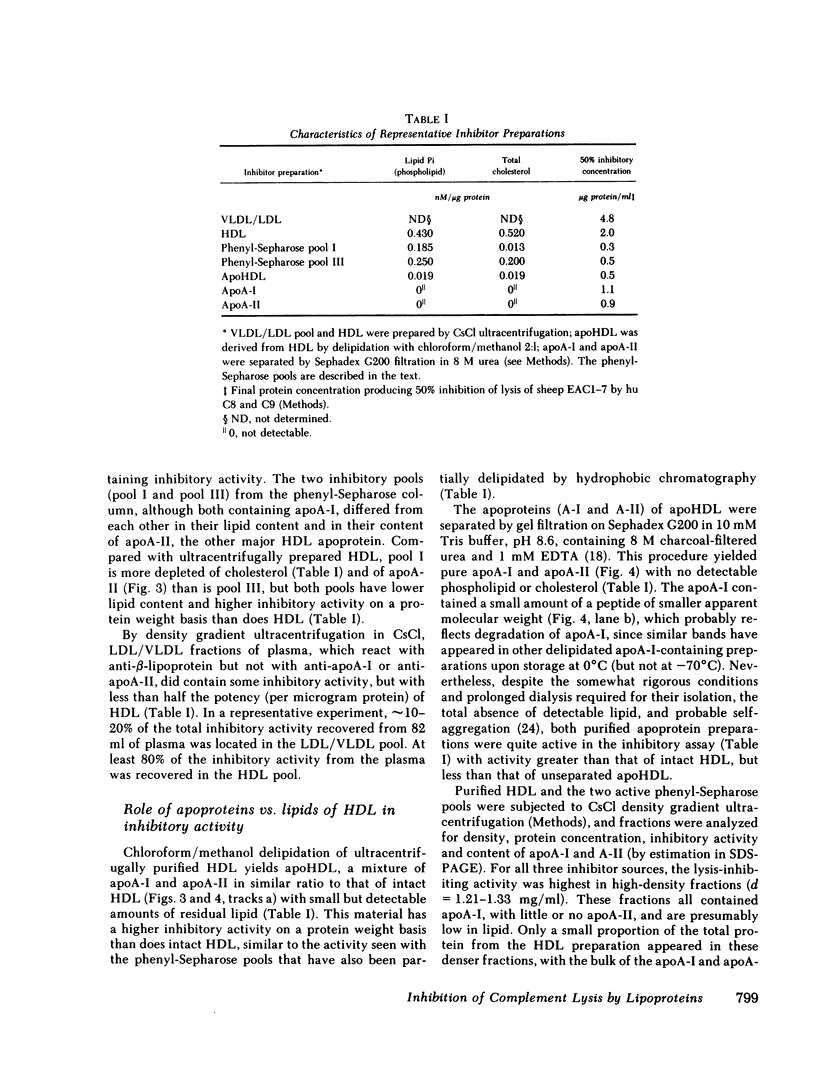
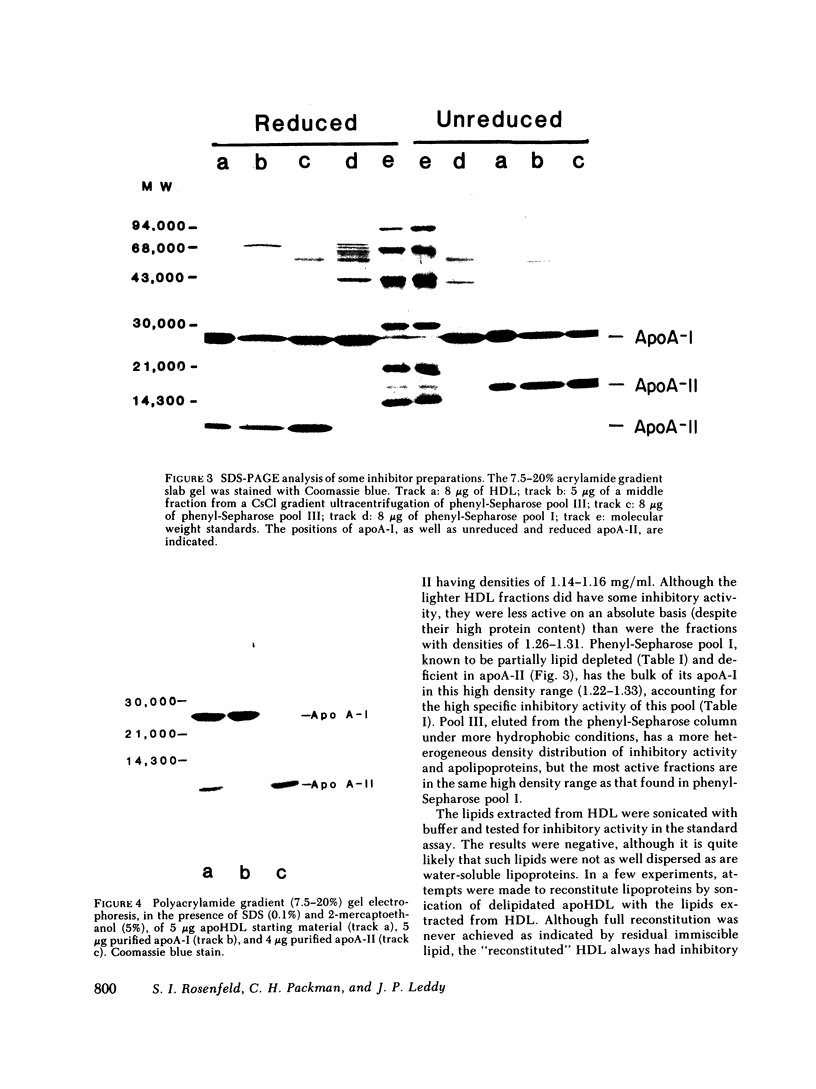
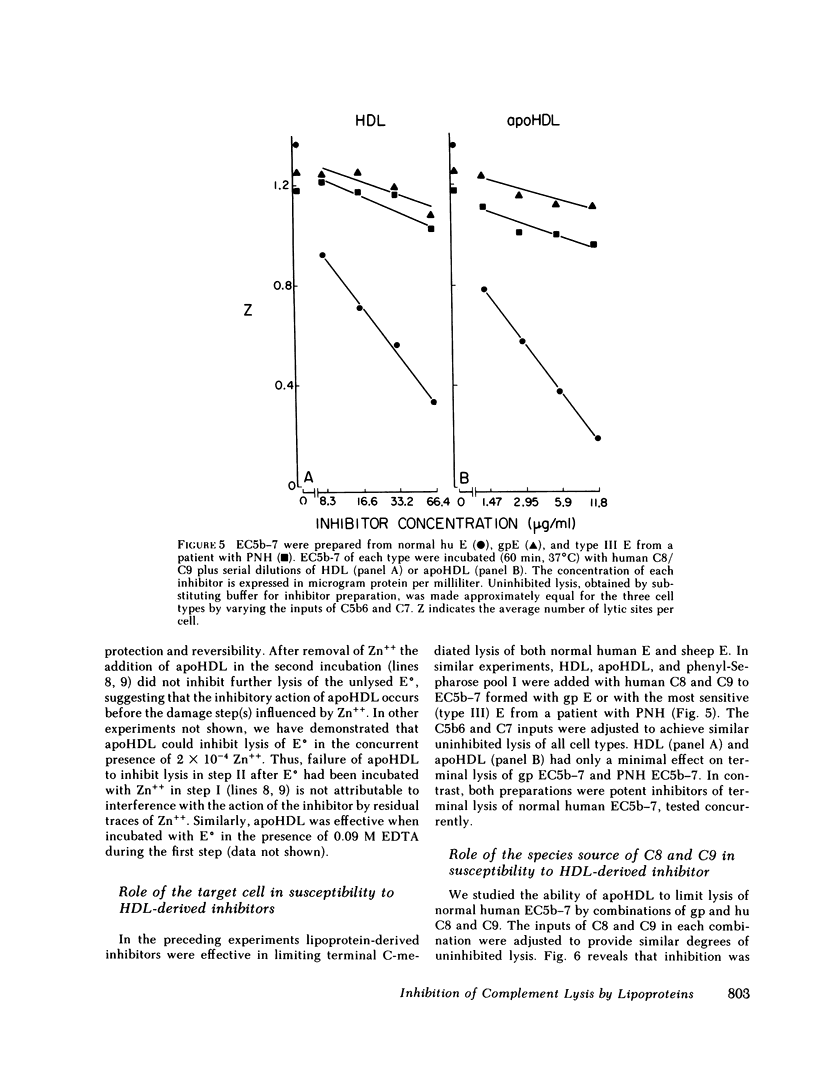
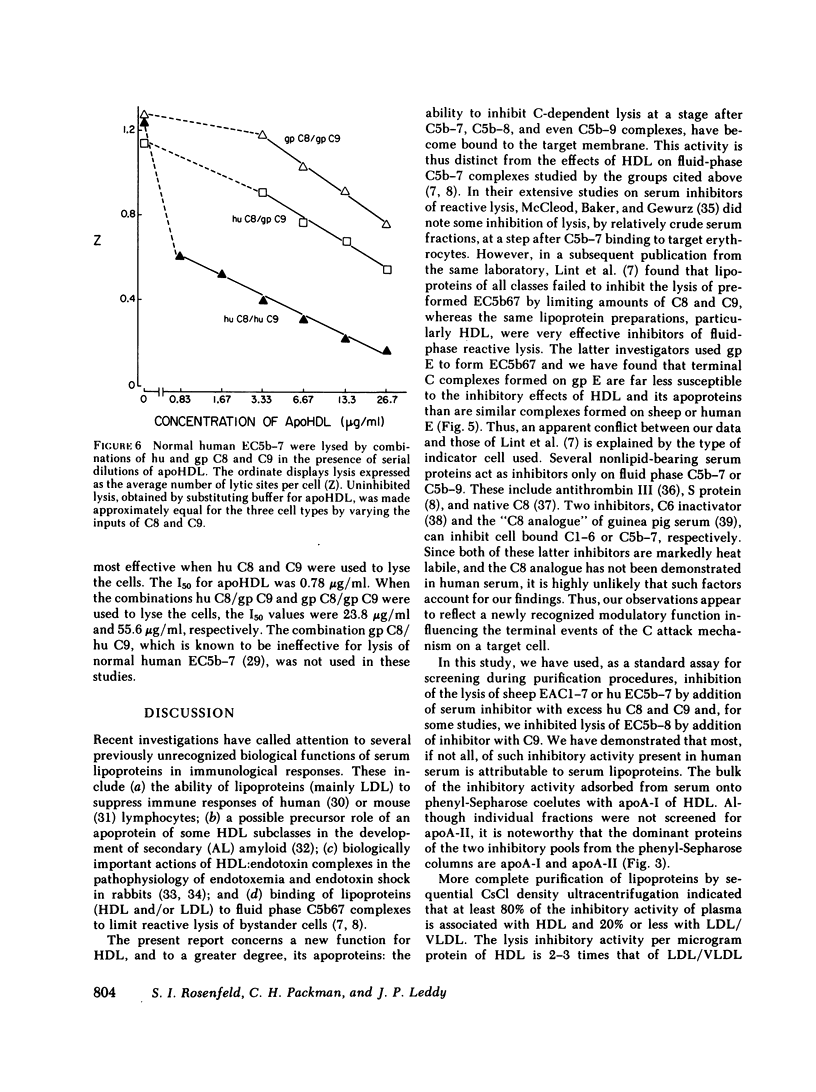
Human serum lipoproteins are known to participate in or modify several immunologically relevant responses, including the inhibition of target cell lysis initiated by fluid-phase C5b-7 (reactive lysis). We now report that human high density lipoproteins (HDL) can inhibit the complement (C) lytic mechanism after C5b-7, C5b-8, and even C5b-9 have been bound to the target membrane. This inhibitory activity of serum or plasma copurifies in hydrophobic chromatography with antigenically detected apolipoprotein A-I (apoA-I), the major HDL apoprotein, and with HDL in CsCl density gradient ultracentrifugation. Although HDL is more active than its apoproteins in fluid-phase inhibition of C5b-7-initiated reactive lysis, the HDL apoproteins are more effective after C5b-7, C5b-8, or C5b-9 have become bound to human or sheep erythrocytes (E). Highly purified HDL apoproteins, apoA-I and apoA-II, both have greater inhibitory activity than whole HDL on a protein weight basis, and some evidence has been obtained that apoA-I dissociating spontaneously from HDL may be the principal inhibitory moiety in physiological situations. HDL lipids themselves are inactive. The HDL-related inhibitors are ineffective when incubated with EC5b-7 and removed before C8 and C9 are added, and only minimally effective on cell-bound C5b-8 sites before C9 is added. They exert their most prominent inhibitory activity after C9 has been bound to EC5b-8 at low temperature, but before the final temperature-dependent, Zn++-inhibitable membrane damage steps have occurred. Therefore, HDL or its apoproteins do not act to repair already established transmembrane channels, but might interfere either with insertion of C9 into the lipid bilayer or with polymerization of C9 at C5b-8 sites. This heat-stable inhibitory activity can be demonstrated to modify lysis of erythrocytes in whole serum, i.e., it does not depend upon artificial interruption of the complement membrane attack sequence at any of the above-mentioned stages. Contributions of the target membrane itself to the mechanism of inhibition are suggested by the observations that, in contrast to sheep or normal human E, lysis of guinea pig E or human E from patients with paroxysmal nocturnal hemoglobinuria is inhibited poorly.
This is the first description of a naturally occurring plasma inhibitor acting on the terminal, membrane-associated events in complement lysis. Although further study is required to assess the physiologic or immunopathologic significance of this new function of HDL, the HDL apoproteins or their relevant fragments should be useful experimentally as molecular probes of the lytic mechanism.
Full text
PDF







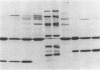
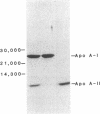
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Katz S., Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med. 1981 Dec 1;154(6):1779–1794. doi: 10.1084/jem.154.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Lavin L., Ziskind M., Koffler D. Cutaneous localization of the membrane attack complex in discoid and systemic lupus erythematosus. N Engl J Med. 1982 Feb 4;306(5):264–270. doi: 10.1056/NEJM198202043060503. [DOI] [PubMed] [Google Scholar]
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Okada M., Borsos T. Differences in cell-bound C8 sites on chicken erythrocytes measured by their reactivity with guinea pig and human C9. J Immunol. 1980 Dec;125(6):2818–2822. [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J., Borsos T. Studies on the terminal stages of immune hemolysis. III. Distinction between the insertion of C9 and the formation of a transmembrane channel. J Immunol. 1978 May;120(5):1721–1725. [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J., Borsos T. Studies on the terminal stages of immune hemolysis. IV. Effect of metal salts. J Immunol. 1979 Apr;122(4):1209–1213. [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Studies on the terminal stages of antibody-complement-mediated killing of a tumor cell. I. Evidence for the existence of an intermediate, T. J Immunol. 1976 May;116(5):1272–1275. [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Immunoregulatory plasma low density lipoprotein: the biologic activity and receptor-binding specificity is independent of neutral lipids. J Immunol. 1981 Mar;126(3):1008–1012. [PubMed] [Google Scholar]
- FRANK M. M., RAPP H. J., BORSOS T. STUDIES ON THE TERMINAL STEPS OF IMMUNE HEMOLYSIS. II. RESOLUTION OF THE E TRANSFORMATION REACTION INTO MULTIPLE STEPS. J Immunol. 1965 Feb;94:295–300. [PubMed] [Google Scholar]
- Giavedoni E. B., Dalmasso A. P. The indiction by complement of a change in KSCN-dissociable red cell membrane lipids. J Immunol. 1976 Apr;116(4):1163–1169. [PubMed] [Google Scholar]
- Hammer C. H., Shin M. L., Abramovitz A. S., Mayer M. M. On the mechanism of cell membrane damage by complement: evidence on insertion of polypeptide chains from C8 and C9 into the lipid bilayer of erythrocytes. J Immunol. 1977 Jul;119(1):1–8. [PubMed] [Google Scholar]
- Hsu K. H., Ghanta V. K., Hiramoto R. N. Immunosuppressive effect of mouse serum lipoproteins. I. In vitro studies. J Immunol. 1981 May;126(5):1909–1913. [PubMed] [Google Scholar]
- Hu V. W., Esser A. F., Podack E. R., Wisnieski B. J. The membrane attack mechanism of complement: photolabeling reveals insertion of terminal proteins into target membrane. J Immunol. 1981 Jul;127(1):380–386. [PubMed] [Google Scholar]
- Hänsch G. M., Hammer C. H., Vanguri P., Shin M. L. Homologous species restriction in lysis of erythrocytes by terminal complement proteins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5118–5121. doi: 10.1073/pnas.78.8.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Kinoshita T., Okada M., Akiyama Y. Release of phospholipids from complement-mediated lesions on the surface structure of Escherichia coli. J Immunol. 1977 Jul;119(1):65–72. [PubMed] [Google Scholar]
- Kopp W. C., Burrell R. Evidence for antibody-dependent binding of the terminal complement component to alveolar basement membrane. Clin Immunol Immunopathol. 1982 Apr;23(1):10–21. doi: 10.1016/0090-1229(82)90066-6. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Weisz-Carrington P., Nussenzweig R. S. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979 Sep;37(3):416–423. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lint T. F., Behrends C. L., Gewurz H. Serum lipoproteins and C567-INH activity. J Immunol. 1977 Sep;119(3):883–888. [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Gockerman J. P. Measurement of the third component of complement bound to red blood cells in patients with the cold agglutinin syndrome. J Clin Invest. 1973 Feb;52(2):493–501. doi: 10.1172/JCI107206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Levy R. I., Gotto A. M., Fredrickson D. S. Studies on the protein defect in Tangier disease. Isolation and characterization of an abnormal high density lipoprotein. J Clin Invest. 1972 Oct;51(10):2505–2519. doi: 10.1172/JCI107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey J. B., Gotto A. M., Jr, Pownall H. J. Human plasma high density apolipoprotein A-I: effect of protein-protein interactions on the spontaneous formation of a lipid-protein recombinant. Biochem Biophys Res Commun. 1981 Mar 31;99(2):466–474. doi: 10.1016/0006-291x(81)91768-x. [DOI] [PubMed] [Google Scholar]
- McLeod B., Baker P., Gewurz H. Studies on the inhibition of C56-initiated lysis (reactive lysis). III. Characterization of the inhibitory activity C567-INH and its mode of action. Immunology. 1975 Jan;28(1):133–149. [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., Yamamoto K. I., Lint T. F. Restriction of complement-mediated membrane damage by the eighth component of complement: a dual role for C8 in the complement attack sequence. J Immunol. 1979 Sep;123(3):1245–1252. [PubMed] [Google Scholar]
- Nilsson U. R., Tomar R. H., Taylor F. B., Jr Additional studies on human C5: development of a modified purification method and characterization of the purified product by polyacrylamide gel electrophoresis. Immunochemistry. 1972 Jul;9(7):709–723. doi: 10.1016/0019-2791(72)90015-8. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Packman C. H., Rosenfeld S. I., Jenkins D. E., Jr, Thiem P. A., Leddy J. P. Complement lysis of human erythrocytes. Differeing susceptibility of two types of paroxysmal nocturnal hemoglobinuria cells to C5b-9. J Clin Invest. 1979 Aug;64(2):428–433. doi: 10.1172/JCI109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977 Dec;119(6):2024–2029. [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. I., Kelly M. E., Leddy J. P. Hereditary deficiency of the fifth component of complement in man. I. Clinical, immunochemical, and family studies. J Clin Invest. 1976 Jun;57(6):1626–1634. doi: 10.1172/JCI108433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. I., Packman C. H., Jenkins D. E., Jr, Countryman J. K., Leddy J. P. Complement lysis of human erythrocytes. III. Differing effectiveness of human and guinea pig C9 on normal and paroxysmal nocturnal hemoglobinuria cells. J Immunol. 1980 Nov;125(5):2063–2068. [PubMed] [Google Scholar]
- Rouault T. A., Rosse W. F., Bell S., Shelburne J. Differences in the terminal steps of complement lysis of normal and paroxysmal nocturnal hemoglobinuria red cells. Blood. 1978 Feb;51(2):325–330. [PubMed] [Google Scholar]
- Sahashi K., Engel A. G., Lambert E. H., Howard F. M., Jr Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol. 1980 Mar;39(2):160–172. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]
- Scanu A., Toth J., Edelstein C., Koga S., Stiller E. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 1969 Aug;8(8):3309–3316. doi: 10.1021/bi00836a027. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Patsch J. R., Packard C. J., Gotto A. M., Jr, Taunton O. D. Dynamic properties of human high density lipoprotein apoproteins. J Lipid Res. 1978 Mar;19(3):383–389. [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Abramovitz A. S., Mayer M. M. On the mechanism of membrane damage by C: exposure of hydrophobic sites on activated C proteins. J Immunol. 1977 Oct;119(4):1358–1364. [PubMed] [Google Scholar]
- Stolfi R. L. An analogue of guinea pig C8: in vitro generation and inhibitory activity. J Immunol. 1970 May;104(5):1212–1219. [PubMed] [Google Scholar]
- Sundsmo J. S., Müller-Eberhard H. J. Neoantigen of the complement membrane attack complex of cytotoxic human peripheral blood lymphocytes. J Immunol. 1979 Jun;122(6):2371–2378. [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. A., Spitznagel J. K. Characteristics of complement-dependent release of phospholipid from Escherichia coli. Infect Immun. 1971 Jul;4(1):23–28. doi: 10.1128/iai.4.1.23-28.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. I., Gewurz G. The complex of C5b and C6: isolation, characterization, and identification of a modified form of C5b consisting of three polypeptide chains. J Immunol. 1978 Jun;120(6):2008–2015. [PubMed] [Google Scholar]
- Yamamoto K. I. Lytic activity of C5-9 complexes for erythrocytes from the species other than sheep: C9 rather than C8-dependent variation in lytic activity. J Immunol. 1977 Oct;119(4):1482–1485. [PubMed] [Google Scholar]