Abstract
Tandem duplication is a wide-spread phenomenon in plant genomes and plays significant roles in evolution and adaptation to changing environments. Tandem duplicated genes related to certain functions will lead to the expansion of gene families and bring increase of gene dosage in the form of gene cluster arrays. Many tandem duplication events have been studied in plant genomes; yet, there is a surprising shortage of efforts to systematically present the integration of large amounts of information about publicly deposited tandem duplicated gene data across the plant kingdom. To address this shortcoming, we developed the first plant tandem duplicated genes database, PTGBase. It delivers the most comprehensive resource available to date, spanning 39 plant genomes, including model species and newly sequenced species alike. Across these genomes, 54 130 tandem duplicated gene clusters (129 652 genes) are presented in the database. Each tandem array, as well as its member genes, is characterized in complete detail. Tandem duplicated genes in PTGBase can be explored through browsing or searching by identifiers or keywords of functional annotation and sequence similarity. Users can download tandem duplicated gene arrays easily to any scale, up to the complete annotation data set for an entire plant genome. PTGBase will be updated regularly with newly sequenced plant species as they become available.
Database URL: http://ocri-genomics.org/PTGBase/.
Introduction
Angiosperms are an excellent example of a group of plants that provide a sound base for understanding gene duplication (GD) in higher eukaryotes. The history of divergence of the two major classes of angiosperms, i.e. monocots and dicots, goes beyond 125–140 million years ago (MYA) to 170–235 MYA, when the natural tendency of angiosperms towards chromosomal duplication and subsequent gene loss led to much more rapid structural evolution (1–3). All angiosperms underwent polyploidization events, and the fraction of recently duplicated genes is higher in plants than in other eukaryotes (4). These genes originate as a result of at least six different modes of duplication including whole genome, tandem, proximal, DNA-based transposition, retrotransposition and dispersed duplications (5). Among these, tandem duplication refers to the generation of tandem arrays consisting of identical sequences in close genomic proximity and occurs due to unequal chromosomal crossing over (6). In plant genomes, tandem duplication events occur more frequently than other duplication modes and produce greater gene copy number and allelic variation. It is true that the tandem duplication phenomenon affects a small number of genes (∼10% of Arabidopsis or rice genes), but its contribution to the expansion of plant gene families is more significant. In Arabidopsis and rice, genes controlling stress tolerance and membrane functions were mostly involved in tandem duplication events (7, 8). Furthermore, tandem GDs have played roles in the evolution of different traits in various plant families like disease resistance in Solanaceae and Brassicaceae (9, 10), signal transduction in legumes (11), glucosinolate biosynthesis diversification in the mustard family (12), and defense response and secondary metabolism like indole alkaloid biosynthesis and tropane, piperidine and pyridine alkaloid biosynthesis in Brassica oleracea and Brassica rapa (13).
In Arabidopsis and Brassica species, tandem GD events occurred throughout the evolutionary history, and a whole-genome triplication (WGT) event in Brassica did not affect the occurrence of tandem duplication. About 43, 47 and 56% of nucleotide binding site (NBS)-encoding disease resistance (R) genes in B. oleracea, B. rapa and Arabidopsis thaliana, respectively, were generated through tandem GD events; this shows that the rate of tandem duplicated genes is higher in Arabidopsis thaliana than in Brassica species. Additionally, it was speculated that in Brassicaceae, tandem GD played a more important role in the generation of NBS-encoding R genes than a whole-genome duplication (WGD) event (13, 14). As far as the expression pattern of duplicated genes is concerned, it may follow different outcomes: neo-functionalization (acquire new expression state), subfunctionalization (partitioning of original ancestral function) or pseudo-genization (complete loss of expression) (15–17). In addition, the fate of duplicate retention depends upon certain features like its function, complexity, expression level, network connectivity and dominance of the parental genome (18–25). In angiosperms, tandem duplication-derived evolution can be well studied in Brassicaceae because each of the Brassica genomes underwent WGD events and an additional WGT event (specific to the Brassicaceae family); additionally, the close evolutionary relationship between Brassica species will facilitate the understanding of the fate of duplicate loss or retention and expression divergence (13, 26).
With the development of sequencing technology, more and more plant genomes were sequenced and released, which provides an opportune chance for researchers to study plant tandem duplicated genes further. Currently, several genomic or transcriptomic data resources for tandem repeats are available online, including STRBase (http://www.cstl.nist.gov/biotech/strbase) (27), TRbase (http://trbase.ex.ac.uk/) (28), TRDB (https://tandem.bu.edu/cgi-bin/trdb/trdb.exe.) (29), TassDB (http://helios.informatik.uni-freiburg.de/TassDB/) (30), VNTRDB (http://vntr.csie.ntu.edu.tw/) (19) and a tandem repeats database for bacterial genomes (http://minisatellites.u-psud.fr) (31). These databases focus on human, bacterial, and some other animal genomes instead of genome sequenced plant species. Tandem repeat DNA sequences, for example SSR and LTR etc., are compiled in these databases except genes performed identical or similar molecular functions. Here, we present PTGBase (freely available at http://ocri-genomics.org/PTGBase/), a database of tandem duplicated genes in assembled pseudomolecules of genome-sequenced plant species, and we demonstrate how this database allows straightforward but flexible searches for tandem duplicated genes or gene clusters in combination with identifiers or keywords of functional annotation (see online supplementary material for Supplementary Table 1). PTGBase is a resource platform through which tandem duplicated genes can be well studied via both intra- and intergenome comparisons to gain insights into their evolutionary history and further explore orthologous and paralogous genes.
Implementation
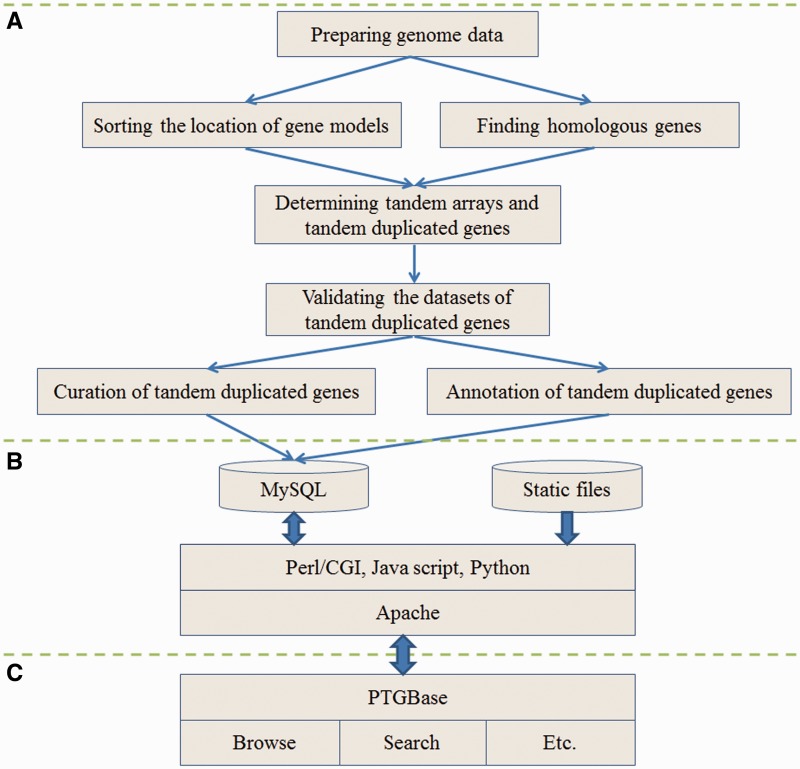
PTGBase implementation was divided into the following three steps: generate the tandem duplicated genes, set the server configuration and develop a user-friendly interface (Figure 1). Basic datasets of tandem duplicated genes were curated and analyzed by in-house Perl and Python scripts. All basic and annotation information of tandem duplicated genes were stored in the MySQL relational database and static files. PTGBase run on a CentOS operation system with the Apache HTTP server environment and MySQL relational database management system. A user-friendly web interface was developed by Perl and JavaScript programming language. The graphical views of the distribution of tandem duplicated genes on assembled pseudomolecules were developed by Perl GD module from the Comprehensive Perl Archive Network (http://www.cpan.org/) (32). A customized basic local alignment search tool (BLAST), which was downloaded from standard National Center for Biotechnology Information (NCBI) BLAST software package, is implemented to allow users to retrieve homologous genes or regions in corresponding species (33).
Figure 1.
Schematic illustration of the PTGBase sitemap. (A) Analysis flowchart to generate the tandem arrays and tandem duplicated genes. (B) Diagram of the PTGBase web server. (C) Web interface of the PTGBase sitemap.
Construction and contents
Database source
Currently, PTGBase contains 39 plant species with sequenced genomes from important plant families such as Poaceae, Fabaceae, Rosaceae and Brassicaceae. The plant species collected in PTGBase not only include key model plant species for basic scientific research but also important cash crops and food farm crops. Among these 39 plant species, genome data of 28 plant species were downloaded from species-specific databases, including the Arabidopsis Information Resource (http://www.arabidopsis.org/) (34) and the Brassica oleracea Genome Database (http://ocri-genomics.org/bolbase/) (35). Genome data of the remaining 11 plant species, which were sequenced by the Joint Genome Institute of the US Department of Energy, were downloaded from the Plant Comparative Genomics portal of the Department of Energy's Joint Genome Institute (http://genome.jgi-psf.org/) (36). We extracted four types of files to generate tandem duplicated genes, including sequence files of assembled pseudomolecules, gene model coding sequence files, protein sequence files of gene models and general feature format (GFF) files containing the location of gene models in assembled pseudomolecules (Table 1).
Table 1.
Statistics of tandem duplicated genes of genome-sequenced plant species in PTGBase
| Latin name | Common name | Genome size | Gene number | Number of TD genes | Number of TD clusters | Release version |
|---|---|---|---|---|---|---|
| Arabidopsis lyrata | Lyraterockcress | 206.7M | 32 670 | 3609 | 1485 | Version 1.0 (Apr 2011) |
| Arabidopsis thaliana | Arabidopsis | 125M | 35 386 | 3503 | 1383 | TAIR 9.0 (Jun 2009) |
| Aureococcus anophagefferens | Heterokont algae | 57M | 11 501 | 176 | 84 | JGI 1.0 (Sep 2007) |
| Brachypodium distachyon | Purple false brome | 260M | 31 029 | 3233 | 1326 | Phytozome v6.0 |
| Brassica oleracea | Cabbage | 630M | 45 758 | 3443 | 1514 | Version 1.0 |
| Brassica rapa | Chinese cabbage | 485M | 41 173 | 4501 | 1918 | Version 1.1 |
| Cajanus cajan | Pigeonpea | 833M | 48 680 | 3837 | 1736 | IIPG v1.0 |
| Carica papaya | Papaya | 370M | 27 950 | 1937 | 822 | ASGPB v1.0 |
| Chlamydomonas reinhardtii | Green algae | 130M | 17 113 | 1220 | 521 | Version 4.2 |
| Chlorella variabilis NC64A | Microalgae | 46M | 9 791 | 451 | 207 | JGI 1.0 (Sep 2010) |
| Cicer arietinum | Chickpea | 738M | 28 269 | 1396 | 610 | Version 1.0 |
| Citrullus lanatus | Watermelon | 425M | 23 440 | 2248 | 863 | Version 1.0 |
| Citrus sinensis | Orange | 367M | 36 450 | 3253 | 1261 | CITRUS v1.0 (2012) |
| Cucumissativus | Cucumber | 350M | 26 682 | 2077 | 830 | Phytozome v6.0 |
| Fragariavesca | Strawberry | 240M | 34 809 | 3823 | 1582 | GDR v1.0 |
| Glycine max | Soybean | 1,100M | 75 778 | 5764 | 2384 | v1.1 (Jun 2013) |
| Gossypium raimondii | Cotton | 761.4M | 40 976 | 4674 | 1866 | Version 2.1 |
| Linum usitatissimum | Flax | 373M | 43 484 | 4169 | 1823 | Phytozome v9.1 v1.0 |
| Lotus japonicus | Lotus | 472M | 42 399 | 1211 | 543 | Release 2.5 |
| Malus × domestica Borkh. | Apple | 742M | 63 541 | 16 602 | 6638 | GDR v1.0 |
| Medicago truncatula | Barrel medic | 500M | 53 423 | 3761 | 1653 | Mt3.5 v3 (Jun 2011) |
| Musa acuminata | Banana | 523M | 36 549 | 1352 | 571 | CIRAD v1.0 |
| Oryza sativa L. ssp.japonica | Rice | 466M | 67 393 | 4544 | 1931 | IRGSP v1.0 |
| Phaeodactylum tricornutum | Diatom algae | 27.4M | 10 402 | 440 | 206 | JGI 2.0 (May 2007) |
| Physcomitrella patens | Moss | 480M | 38 354 | 797 | 381 | Version 1.6 (Jan 2008) |
| Populus trichocarpa | Western poplar | 480M | 45 033 | 5224 | 2084 | JGI 2.0 (Feb 2010) |
| Prunus mume | Plum flower | 280M | 31 390 | 5059 | 1985 | prunusmumegenome v1.0 |
| Ricinus communis | Castor bean | 350M | 31 221 | 2613 | 1075 | Release 0.1 (May 2008) |
| Selaginella moellendorffii | Selaginella | 212M | 22 285 | 1676 | 748 | Version 1.0 (Dec 2007) |
| Sesamum indicum | Sesame | 357M | 27 148 | 2848 | 1126 | Version 1.0 |
| Setaria italica | Millet | 490M | 38 801 | 4879 | 2027 | Phytozome v9.1 |
| Solanum lycopersicum | Tomato | 900M | 34 727 | 4173 | 1640 | Version 2.3 |
| Solanum tuberosum | Potato | 844M | 39 031 | 4504 | 1839 | Version 3.4 |
| Sorghum bicolor | Sorghum | 730M | 29 448 | 4256 | 1664 | Sbi 1.4 (Dec 2007) |
| Thellugiella parvula | Rockface star-violet | 140M | 27 132 | 2316 | 980 | Thellungiella v2.0 |
| Theobroma cacao | Cacao | 430M | 46 143 | 3867 | 1604 | Release 0.9 (Sep 2010) |
| Vitis vinifera | Grape vine | 490M | 26 346 | 3668 | 1405 | Genoscope (Aug 2007) |
| Volvox carteri | Green alga | 138M | 15 285 | 1402 | 595 | JGI 1.0 (Jun 2007) |
| Zea mays ssp. mays L. | Maize | 2300M | 63 293 | 2837 | 1220 | Release 5a (Nov 2010) |
TD, tandem duplicated.
Finding tandem duplicated genes
In this study, we focused on the tandem duplicated functional genes on the same assembled pseudomolecules excluding one or more unrelated genes within a tandem array, which were generated by tandem duplication or other tandem repeat events. These tandem duplicated functional genes performed identical or similar molecular functions in the process of plant growth, development and adaptation to the environment. In order to get the most accurate datasets of tandem duplicated genes in plants, we designed the following major steps to obtain the tandem duplicated genes from assembled pseudomolecules by procedures consisting of 26 in-house Perl and Python scripts. (i) Finding homologous genes: according to the phylogenetic relationship of all species in different subgroups, at least three species which were belonged to two different layers of phylogenetic tree were recognized as target genomes and one species was regarded as the last common ancestor of other species. The orthoMCL software was used to classify orthologous groups with E-value ≤ 1e-20 and inflation parameter of 1.5, which intended to detect the homologous genes descended for a single gene in the last common ancestor of all species (i.e. the genes descended for a single gene in the last common ancestor of all species under consideration) (33, 37). (ii) Sorting the location of gene models: the GFF file was a key genome sequencing file contained the location of predicted gene models for genome sequenced plant species. Based on GFF files of target genomes, target gene models were sorted by descending order according to the gene location on assembled pseudomolecules. (iii) Determining the tandem duplicated arrays: checking if two or more genes from the same orthologous group are next to each other in target genome. This would allow users to know that the genes are related to each other through duplication without needing to identify the precise taxonomic level at which the GD occurred. (iv) validating the datasets: for the known function duplicated genes, InterPro was employed to validate function of tandem duplicated genes by classifying them into different families and predicting conserved domains and important sites; for the unknown function duplicate genes, a duplicate gene pair was just that the two genes (next to each other in the genome) need to have a sequence coverage percentage ≥80%. (v) Out-putting the standard format datasets: in-house Perl and Python scripts were used to format the output file with cluster names and gene lists. At last, 54 130 tandem arrays (129 652 genes) were generated and curated manually for further analysis (Figure 1A).
Functional annotation
We generated comprehensive functional annotation of tandem duplicated genes. In PTGBase, all tandem duplicated genes were annotated by performing Blast2GO, a tool for the functional annotation of sequences and the analysis of annotation data (http://www.blast2go.com/), with stringent parameters (38). For each tandem duplicated gene, PTGBase offered complete Gene Ontology (GO) annotation, including GO identifier, term, and corresponding name space. In order to obtain the protein functional classification of tandem duplicated genes, InterPro was employed to provide functional analysis of tandem duplicated genes by classifying them into different families and predicting conserved domains and important sites (39). Every tandem duplicated gene was annotated by the COG database (40). For every tandem duplicated gene, this database supplied InterPro identifiers, functional description and names of member databases in which protein sequences of tandem duplicated genes were classified into families and conserved domain or motif types, as well as identifiers of corresponding member databases in InterPro (Table 2).
Table 2.
Functional classification of tandem duplicated genes in PTGBase
| Latin name | Common name | Numbers of tandem duplicated genes | InterPro |
Gene Ontology |
||
|---|---|---|---|---|---|---|
| Gene number | Percentage (%) | Gene number | Percentage (%) | |||
| Arabidopsis lyrata | Lyraterockcress | 3609 | 3490 | 96.70 | 2428 | 67.28 |
| Arabidopsis thaliana | Arabidopsis | 3503 | 3453 | 98.57 | 2579 | 73.62 |
| Aureococcus anophagefferens | Heterokont algae | 176 | 160 | 90.91 | 121 | 68.75 |
| Brachypodium distachyon | Purple false brome | 3233 | 3149 | 97.40 | 2401 | 74.27 |
| Brassica oleracea | Cabbage | 3443 | 3287 | 95.47 | 2345 | 68.11 |
| Brassica rapa | Chinese cabbage | 4501 | 4238 | 94.16 | 3200 | 71.10 |
| Cajanus cajan | Pigeonpea | 3837 | 3532 | 92.05 | 2346 | 61.14 |
| Carica papaya | Papaya | 1937 | 1824 | 94.17 | 1353 | 69.85 |
| Chlamydomonas reinhardtii | Green algae | 1220 | 1068 | 87.54 | 552 | 45.25 |
| Chlorella variabilis NC64A | Microalgae | 451 | 438 | 97.12 | 263 | 58.31 |
| Cicer arietinum | Chickpea | 1396 | 1284 | 91.98 | 971 | 69.56 |
| Citrullus lanatus | Watermelon | 2248 | 2168 | 96.44 | 1679 | 74.69 |
| Citrus sinensis | Orange | 3253 | 3105 | 95.45 | 2385 | 73.32 |
| Cucumis sativus | Cucumber | 2077 | 2024 | 97.45 | 1520 | 73.18 |
| Fragaria vesca | Strawberry | 3823 | 3594 | 94.01 | 2649 | 69.29 |
| Glycine max | Soybean | 5764 | 5646 | 97.95 | 4335 | 75.21 |
| Gossypium raimondii | Cotton | 4674 | 4357 | 93.22 | 3235 | 69.21 |
| Linum usitatissimum | Flax | 4169 | 4061 | 97.41 | 3012 | 72.25 |
| Lotus japonicus | Lotus | 1211 | 1177 | 97.19 | 888 | 73.33 |
| Malus × domestica Borkh. | Apple | 16 602 | 14 085 | 84.84 | 10 361 | 62.41 |
| Medicago truncatula | Barrel medic | 3,761 | 3498 | 93.01 | 2544 | 67.64 |
| Musa acuminata | Banana | 1352 | 1328 | 98.22 | 1082 | 80.03 |
| Oryza sativa L. ssp.japonica | Rice | 4544 | 4374 | 96.26 | 3110 | 68.44 |
| Phaeodactylum tricornutum | Diatom algae | 440 | 406 | 92.27 | 206 | 46.82 |
| Physcomitrella patens | Moss | 797 | 720 | 90.34 | 530 | 66.50 |
| Populus trichocarpa | Western poplar | 5224 | 5100 | 97.63 | 3839 | 73.49 |
| Prunus mume | Plum flower | 5059 | 4805 | 94.98 | 3551 | 70.19 |
| Ricinus communis | Castor bean | 2613 | 2556 | 97.82 | 1908 | 73.02 |
| Selaginella moellendorffii | Selaginella | 1676 | 1483 | 88.48 | 954 | 56.92 |
| Sesamum indicum | Sesame | 2848 | 2697 | 94.70 | 2073 | 72.79 |
| Setaria italica | Millet | 4879 | 4582 | 93.91 | 3255 | 66.71 |
| Solanum lycopersicum | Tomato | 4173 | 3960 | 94.90 | 2962 | 70.98 |
| Solanum tuberosum | Potato | 4504 | 4008 | 88.99 | 2932 | 65.10 |
| Sorghum bicolor | Sorghum | 4256 | 4169 | 97.96 | 3133 | 73.61 |
| Thellugiella parvula | Rockface star-violet | 2316 | 2230 | 96.29 | 1686 | 72.80 |
| Theobroma cacao | Cacao | 3867 | 3787 | 97.93 | 2858 | 73.91 |
| Vitis vinifera | Grape vine | 3668 | 3596 | 98.04 | 2909 | 79.31 |
| Volvox carteri | Green alga | 1402 | 1087 | 77.53 | 615 | 43.87 |
| Zea mays ssp. mays L. | Maize | 2837 | 2379 | 83.86 | 1647 | 58.05 |
Web interface and usage
Major modules provided by PTGBase
PTGBase is an integrated plant tandem duplicated genes database that provides not only a comprehensive platform to study plant tandem duplicated genes but also the materials for researchers to further study plant genome evolution. A powerful web-based user interface was designed based on different classifications of major function modules. Each of the major functional modules provided a specific capability for retrieving information about tandem duplicated genes from the database or viewing the tandem duplicated genes in the context of either the phylogenetic or genome sequence comparisons. The two sorting menus show the sum of tandem duplicated gene clusters available from PTGBase by names of plant species. The species names are linked to a list of the associated tandem duplicated gene clusters containing additional information. Depending on the respective focus of data mining, a precise query for identifiers and a fuzzy query for keywords of functional annotations were designed for automated data retrieval of tandem duplicated gene clusters and flexible functional annotations. Moreover, additional functional modules were designed to enrich the content of PTGBase and supplied a comprehensive resource platform of tandem duplicated genes for the community.
Browse module to show overall view of tandem duplicated genes and clusters
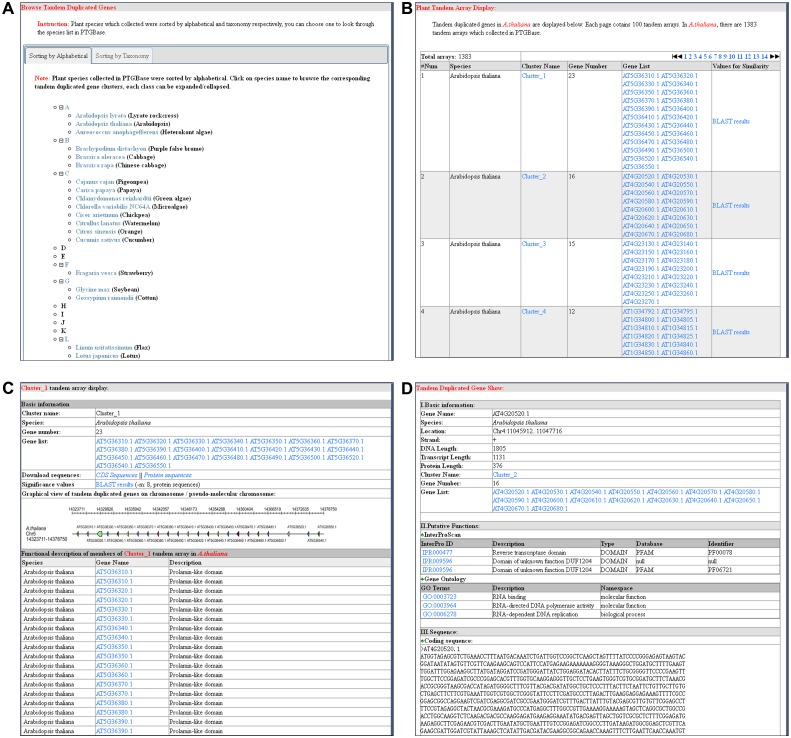
Multilayer browse modules were developed to display a comprehensive resource of tandem duplicated genes compiled in PTGBase (Figure 2). There are 39 plant species deposited in PTGBase; standardizing the order of these plant species will bring more convenience to select the data for species of interest. The browse module offers two major navigation tabs to show plant tandem duplicated gene clusters: (i) alphabetical sorting and (ii) sorting by taxonomy (Figure 2A). In the alphabetical sorting, all species are sorted alphabetically, and every class can be expanded or collapsed by clicking the corresponding icons. Following the evolutionary relationship deposited in corresponding genome papers and the NCBI taxonomy database, we constructed the phylogenetic tree among plant species in PTGBase. In the sorting by taxonomy tab, a phylogenetic tree is provided to show plant species that supply a clear evolutionary pedigree for users to further study the evolutionary history of tandem duplicated genes. In the two tabs, users can select a species of interest and click the species name to retrieve the tandem duplicated gene clusters in the selected species. Tandem duplicated gene clusters are shown with the following five pieces of information: species to which the clusters belong, cluster name, number of genes in the clusters, gene list in clusters, and significance values for sequence similarities (Figure 2B). Clicking the hyperlink of the cluster name allows users to obtain the information of this whole tandem duplicated gene cluster which includes the number of genes in the cluster, coding and protein sequences of duplicated genes, significance values for sequence comparison, distribution of the duplicated genes on assembled pseudomolecules, and functional features of duplicated genes (Figure 2C). The distribution of duplicated genes on pseudomolecules provides a valuable hint about the formation of the duplicated genes on assembled pseudomolecules and improves the understanding of the tandem duplicated genes. The summary of functional features of duplicated genes indicates the functional types of duplicated genes that are clustered together on assembled pseudomolecules. Clicking the hyperlink of the duplicated gene name displays basic information, putative function, and sequence information in detail (Figure 2D). Multi-level browse functional modules will allow more opportunities for users to identify useful information and understand tandem duplicated genes clearly.
Figure 2.
Major browsing function modules of PTGBase. (A) Overview of browsing functions for tandem arrays in PTGBase. (B) Browsing the tandem duplicated genes by tandem array in special plants. (C) Detailed annotation of a tandem duplicated gene cluster. (D) Detailed annotation of tandem duplicated genes in PTGBase.
Search module for identifiers or keywords in the database
Searching the function module of identifiers related to tandem duplicated genes or keywords of functional annotation was developed by Perl and JavaScript scripts which supplied a visual and powerful searching platform. A search navigation is available at the top of the searching function module, providing a quick and clear means for searching specific objects by identifiers or keywords in this database. According to different entry points to search, three parts were deposited in PTGBase which contained searching by identifiers or names of tandem duplicated genes, searching by identifiers of functional annotations and searching by keywords of functional annotations. In the section to search by identifiers or names of tandem duplicated genes, users can retrieve valuable information about tandem duplicated genes by inputting a gene ID, a whole tandem array of tandem duplicated genes by supplying the name of a gene and cluster and a tandem array list by supplying gene numbers and species name. Users can also retrieve basic information about tandem duplicated genes compiled in PTGBase. In the section to search by identifiers of a functional annotation, users can supply a GO ID or InterPro accession number to obtain tandem duplicated genes with those annotations among species that can be used to understand certain functional types of clustered genes on assembled pseudomolecules. Moreover, the search module also allows users to use keywords of functional annotations to search tandem duplicated genes among species. A powerful fuzzy search function was developed that permits users using simple keyword of a functional annotation to traverse the whole annotation database to obtain all duplicated genes containing the simple keyword in their functional description.
Sequence similarity search by nucleic acid or amino acid sequence(s)
Customized WWWBLAST modules were designed for users to implement online sequence comparison conveniently in PTGBase (33). The query is a nucleic acid or an amino acid sequence. By uploading a sequence file or pasting a sequence directly, users can find homologous duplicated genes or syntenic regions from compiled genome datasets by selecting an appropriate BLAST program and designated plant species. Thus, by implementing a sequence similarity search, users can obtain not only the putative annotation of the query sequence but also the location of the query sequence on assembled pseudomolecules by homology sequence comparison. For BLAST hits, hyperlinks to the annotation pages in PTGBase and cross-links to annotation pages in the species-specific database have been added in this database for users to get more annotations of query sequence.
Download tandem duplicated genes data and contribution to PTGBase
PTGBase supplies a convenient download module for users to retrieve useful information about tandem duplicated genes. First, users can download a compressed file containing tandem duplicated gene clusters and coding or protein sequences of tandem duplicated genes by selecting a target plant species in the box and clicking the ‘download’ button. Second, genome data of genome-sequenced plant species collected in PTGBase can be downloaded freely, and the data policy of the released genome should be obeyed. The downloadable genome data contain coding and protein sequences and a GFF file containing the location of gene models on assembled pseudomolecules. If users want other files of genome data for species of interest, they can access the hyperlinks of the species-specific expert database or JGI, which is supplied by our database, to obtain complete genome sequencing data for the plant species of interest.
In order to supply an excellent data resource of tandem duplicated genes in plants for the community, PTGBase asked users to submit the tandem duplicated genes to this database and enriched the contents of tandem duplicated genes in PTGBase. The procedures to generate the tandem duplicated genes should follow the pipeline of PTGBase. Moreover, we can help users obtain tandem duplicated genes for their species of interest. After curation, the newly available data of tandem duplicated genes will be included in PTGBase.
Discussion
PTGBase represents an exhaustive collection of plant tandem duplicated genes that were collected and compiled from several public databases and additional private resources. It will present an unprecedented opportunity to study gene family expansion of specific traits or phenotypes and plant intra- and intergenome evolution. When a class of genes that performs a specific function experienced tandem duplication or other tandem repeat events after the formation of a species, it will increase the gene dosage, which enhances the gene function and results in either beneficial or detrimental effects on plant growth, development or adaptation to the environment (41, 42). For example, NBS-encoding genes play an important role in resistance to diseases and are greatly influenced by tandem duplication. In a recent study, Yu et al. (2014) systematically reported that NBS-encoding genes in Brassica species experienced species-specific gene family amplification by tandem duplication after the divergence of B. rapa and B. oleracea. LRR-RLK genes is another type of disease resistance genes (R genes) and have a critical role in defense response. Argout et al. (43) reported that the Theobroma cacao genome contains at least 253 LRR-RLK genes orthologous to Arabidopsis LRR-RLK genes. According to the analysis of tandem duplicated genes for T. cacao genome in PTGBase, 46 LRR-RLK genes were generated by tandem duplication event, representing approximately 18.2% of total LRR-RLK genes in T. cacao genome. For R genes, the tandem duplication event will increase the gene dosage and the increased gene dosage might have some advantages to plant pathogen defense (14).
The emergence of tandem duplicated genes has given rise to great challenges for studying orthologous genes among species in the context of plant evolution. After the divergence of plant species from ancestral species, plant species have experienced tandem duplication events and formed species-specific tandem repeat or duplicated genes. The most straightforward way to detect orthologous genes of tandem arrays between different species is to use a sequence similarity search to classify orthologous genes among tandem arrays of different species. The expression patterns of repeat or duplicated genes reveal different outcomes: neo-functionalization, subfunctionalization and pseudogenization (15–17). Yu et al. (14) examined the expression profile of NBS-encoding genes of a tandem array and explored the hypothesis that the expression profiles of different NBS-coding members are separated into different groups that are indicative of functional divergence, but the members of an NBS-encoding tandem array performed the same function with the same gene expression pattern and shared nearly identical sequence similarity. Therefore, using a sequence similarity search was the best way to explore the orthologous genes of tandem arrays among different species until now.
Conclusions and perspectives
PTGBase is the first plant tandem duplicated genes database that embraces a wide spectrum of genome resources for genome-sequenced plant species. It not only focuses on functional genomics for each plant species but also is dedicated to comparative genomics in the context of plant phylogenetic analysis spanning a wide range of plant genomes. PTGBase provides effective data mining tools and efficient use of tandem duplicated gene information for users to retrieve useful data easily. The database will be continuously improved by updating tandem duplicated gene collections and newly detected tandem duplicated genes from available plant genomes within the framework of PTGBase. Future efforts will also develop better approaches to classify plant genes correlated with tandem duplicated events, as well as refine the structure of this database. We aim to develop and maintain a comprehensive plant tandem duplicated genes database to improve our knowledge of functional genomics, comparative genomics, and evolutionary biology by providing systematic data resources and integrative analytical frameworks and views.
Supplementary Data
Supplementary data are available at Database Online.
Acknowledgements
We thank Dr. Xin Zhou in Washington University in St. Louis for the critical reading of the manuscript and three anonymous reviewers for their useful comments on the manuscript.
Funding
This work was supported by the Oil Crops Research Institute, CAAS, China on behalf of National High Technology Research and Development Program of China (2013AA102602) and National Key Basic Research Program of China (2011CB109300). Funding for open access charge: Oil Crops Research Institute, CAAS, China.
Conflict of interest None declared.
References
- 1.Davies T.J., Barraclough T.G., Chase M.W., et al. (2004) Darwin's abominable mystery: Insights from a supertree of the angiosperms. Proc. Natl Acad. Sci. USA, 101, 1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterston R.H., Lindblad-Toh K., Birney E., et al. (2002) Initial sequencing and comparative analysis of the mouse genome. Nature, 420, 520–562. [DOI] [PubMed] [Google Scholar]
- 3.Smith S.F., Snell P., Gruetzner F., et al. (2002) Analyses of the extent of shared synteny and conserved gene orders between the genome of Fugu rubripes and human 20q. Genome Res., 12, 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockton S., Gaut B.S. (2005) Plant conserved non-coding sequences and paralogue evolution. Trends Genet., 21, 60–65. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Wang X., Paterson A.H. (2012) Genome and gene duplications and gene expression divergence: a view from plants. Ann. N Y Acad. Sci., 1256, 1–14. [DOI] [PubMed] [Google Scholar]
- 6.Kane J., Freeling M., Lyons E. (2010) The evolution of a high copy gene array in Arabidopsis. J. Mol. Evol., 70, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark R.M., Schweikert G., Toomajian C., et al. (2007) Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science, 317, 338–342. [DOI] [PubMed] [Google Scholar]
- 8.Rizzon C., Ponger L., Gaut B.S. (2006) Striking similarities in the genomic distribution of tandemly arrayed genes in Arabidopsis and rice. PLoS Comput. Biol., 2, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parniske M., Wulff B.B., Bonnema G., et al. (1999) Homologues of the Cf-9 disease resistance gene (Hcr9s) are present at multiple loci on the short arm of tomato chromosome 1. Mol. Plant Microb. Interact., 12, 93–102. [DOI] [PubMed] [Google Scholar]
- 10.Leister D. (2004) Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance gene. Trends Genet, 20, 116–122. [DOI] [PubMed] [Google Scholar]
- 11.Bellieny-Rabelo D., Oliveira A.E., Venancio T.M. (2013) Impact of whole-genome and tandem duplications in the expansion and functional diversification of the F-box family in legumes (Fabaceae). PLoS One, 8, e55127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofberger J.A., Lyons E., Edger P.P., et al. (2013) Whole genome and tandem duplicate retention facilitated glucosinolate pathway diversification in the mustard family. Genome Biol. Evol., 5, 2155–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S., Liu Y., Yang X., et al. (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun, 5, 3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J., Tehrim S., Zhang F., et al. (2014) Genome-wide comparative analysis of NBS-encoding genes between Brassica species and Arabidopsis thaliana. BMC Genomics, 15, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Force A., Lynch M., Pickett F.B., et al. (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics, 151, 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W.-H., Gojobori T., Nei M. (1981) Pseudogenes as a paradigm of neutral evolution. Nature, 292, 237–239. [DOI] [PubMed] [Google Scholar]
- 17.Soukup S. W. (1974), Evolution by gene duplication. S. Ohno. Springer-Verlag, New York. 1970. 160 pp. Teratology, 9 250–251. 10.1002/tera.1420090224. [Google Scholar]
- 18.Blanc G., Wolfe K.H. (2004) Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell, 16, 1667–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang C.H., Chang Y.C., Underwood A., et al. (2007) VNTRDB: a bacterial variable number tandem repeat locus database. Nucleic Acids Res., 35, D416–D421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanada K., Zou C., Lehti-Shiu M.D., et al. (2008) Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol., 148, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W.K., Liu Y.L., Xia E.H., et al. (2013) Prevalent role of gene features in determining evolutionary fates of whole-genome duplication duplicated genes in flowering plants. Plant Physiol., 161, 1844–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal C., Papp B., Hurst L.D. (2001) Highly expressed genes in yeast evolve slowly. Genetics, 158, 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman B.A., Bowers J.E., Feltus F.A., et al. (2006) Buffering of crucial functions by paleologous duplicated genes may contribute cyclicality to angiosperm genome duplication. Proc. Natl Acad. Sci. USA, 103, 2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnable J.C., Springer N.M., Freeling M. (2011) Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proc. Natl Acad. Sci. USA, 108, 4069–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas B.C., Pedersen B., Freeling M. (2006) Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Res., 16, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghe G.D., Hufnagel D.E., Tang H., et al. (2014) Consequences of whole-genome triplication as revealed by comparative genomic analyses of the Wild Radish Raphanus raphanistrum and three other Brassicaceae species. The Plant Cell Online , 26, 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruitberg C.M., Reeder D.J., Butler J.M. (2001) STRBase: a short tandem repeat DNA database for the human identity testing community. Nucleic Acids Res., 29, 320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boby T., Patch A.M., Aves S.J. (2005) TRbase: a database relating tandem repeats to disease genes for the human genome. Bioinformatics, 21, 811–816. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand Y., Rodriguez A., Benson G. (2007) TRDB—the Tandem Repeats Database. Nucleic Acids Res., 35, D80–D87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiller M., Nikolajewa S., Huse K., et al. (2007) TassDB: a database of alternative tandem splice sites. Nucleic Acids Res., 35, D188–D192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Fleche P., Hauck Y., Onteniente L., et al. (2001) A tandem repeats database for bacterial genomes: application to the genotyping of Yersinia pestis and Bacillus anthracis. BMC Microbiol, 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hietaniemi J. (2008) Comprehensive perl archive network. Helsinki, Finland: CPAN, pp. 6. [Google Scholar]
- 33.Altschul S.F., Madden T.L., Schaffer A.A., et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huala E., Dickerman A.W., Garcia-Hernandez M., et al. (2001) The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Res., 29, 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J., Zhao M., Wang X., et al. (2013) Bolbase: a comprehensive genomics database for Brassica oleracea. BMC Genomics, 14, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodstein D.M., Shu S., Howson R., et al. (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res., 40, D1178–D1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L., Stoeckert C.J., Jr., Roos D.S. (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res., 13, 2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conesa A., Gotz S., Garcia-Gomez J.M., et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21, 3674–3676. [DOI] [PubMed] [Google Scholar]
- 39.Quevillon E., Silventoinen V., Pillai S., et al. (2005) InterProScan: protein domains identifier. Nucleic Acids Res., 33, W116–W120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatusov R.L., Galperin M.Y., Natale D.A., et al. (2000) The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res., 28, 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch M., Conery J.S. (2000) The evolutionary fate and consequences of duplicate genes. Science, 290, 1151–1155. [DOI] [PubMed] [Google Scholar]
- 42.Papp B., Pal C., Hurst L.D. (2003) Dosage sensitivity and the evolution of gene families in yeast. Nature, 424, 194–197. [DOI] [PubMed] [Google Scholar]
- 43.Argout X., Salse J., Aury J.M., et al. (2011) The genome of Theobroma cacao. Nat. Genet., 43, 101–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.