Abstract
Lytic granules in natural killer (NK) cells represent a dangerous cargo that is targeted for secretion to destroy diseased cells. The appropriate management of these organelles enables the mounting of a precise and valuable host defense. The process of NK cell adhesion to a target cell through engagement of the integrin LFA-1 (lymphocyte function–associated antigen 1) promotes lytic granule organization through complex cellular mechanics and a signaling pathway characterized by Zhang et al. in this issue of Science Signaling. A limited, partially overlapping set of signaling molecules can be distinguished for their ability to promote the convergence of NK cell lytic granules on the microtubule organizing center and their polarization as they progress en masse toward the interface with a target cell.
Natural killer (NK) cells are cytolytic lymphocytes that survey the host environment for stressed or diseased cells, and they are required for the maintenance of human health. NK cells use various cell surface receptors to discern patterns of health relative to disease, with a balance toward the latter providing signals that stimulate NK cell function (1, 2). The recognition of these patterns occurs at the interface between an NK cell and its potential target, otherwise known as the NK cell immunological synapse. Integration and balancing of signals at the NK cell immunological synapse is critical, because under basal conditions, human NK cells maintain abundant effector machinery that is capable of cell destruction. Specifically, NK cells contain specialized lysosome-related organelles known as lytic granules, which are filled with cytotoxic molecules and can be targeted for secretion onto another cell. Thus, the ability of the NK cell to organize, control, and direct lytic granules is essential, and it is governed through a coordinated, tightly regulated series of cell biological steps to achieve the ultimate goal of focused degranulation (3). One of these steps is the polarization of lytic granules and the microtubule organizing center (MTOC) to the immunological synapse. In NK cells, polarization of lytic granules is preceded by their convergence on the MTOC in a separate step (4).
In this issue of Science Signaling, Zhang et al. performed one of the most comprehensive studies to date of the signals that direct control of NK cell lytic granules (5). Before this work, it was known that ligation of the NK cell integrin LFA-1 (lymphocyte function–associated antigen 1), a member of the β2 family of integrins, promotes the convergence of lytic granules on the MTOC, as well as the polarization of lytic granules and the MTOC to the immunological synapse (6, 7). The NK cell immunoglobulin G receptor CD16 promotes degranulation, but not granule polarization, toward a target cell (6). These observations uncoupled lytic granule polarization from degranulation. A number of signaling molecules were defined as being required for lytic granule polarization in NK cells, including extracellular signal–regulated kinase 2 (ERK2), the tyrosine kinase Pyk2, adenosine diphosphate ribosylation factor-like 8b (Arl8b), and the guanine nucleotide exchange factor Vav1 (3). Although lytic granule convergence was only more recently appreciated, this process depends on a more limited set of signals, including Src family kinases (7).
Zhang et al. compared phosphotyrosine profiles in NK cells in response to ligation of LFA-1 or CD16 (5). Using mass spectrometry to identify tyrosine-phosphorylated proteins or their binding partners, they defined only 23 proteins as being specific to LFA-1 signaling. With the use of bioinformatics analysis, Zhang et al. further identified connections among 11 proteins with two major network nodes, Paxillin and Pyk2, which connected seven and four proteins, respectively. Each identified protein was evaluated biologically through small interfering RNA (siRNA)–mediated gene targeting and direct observation of the positions of lytic granules relative to the immunological synapse and MTOC in fixed cell microscopic assays. Because LFA-1 is required by NK cells for optimal adhesion to target cells, Zhang et al. also performed cell adhesion assays. Silencing of integrin-linked kinase (ILK), γ-parvin, Leupaxin, RhoGEF7, Cdc42, Par6, Adenomatous Polyposis Coli (APC), CLIP-170, or Pyk2 in NK cells impaired the polarization of lytic granules and the MTOC toward target cells. Of these candidates, silencing of only Pyk2 also inhibited the formation of NK cell–target cell conjugates.
To screen for the signals that were needed for lytic granules to converge on the MTOC, Zhang et al. used the KHYG-1 NK cell line, which has persistently converged granules. Through siRNA-mediated gene targeting and fixed-cell microscopy, they determined that knockdown of Pyk2, Leupaxin, Cdc42, or CLIP-170 led to dispersion of the preconverged granules in KHYG-1 cells. In contrast, knockdown of ILK, γ-parvin, RhoGEF7, or APC had no effect, despite these proteins having defined roles in promoting lytic granule polarization. Therefore, within the limited signals specific to LFA-1 ligation in NK cells, the work of Zhang et al. not only identifies the requirements for polarization and convergence of lytic granules, but also defines a potential branch point where each function is accessed (Fig. 1).
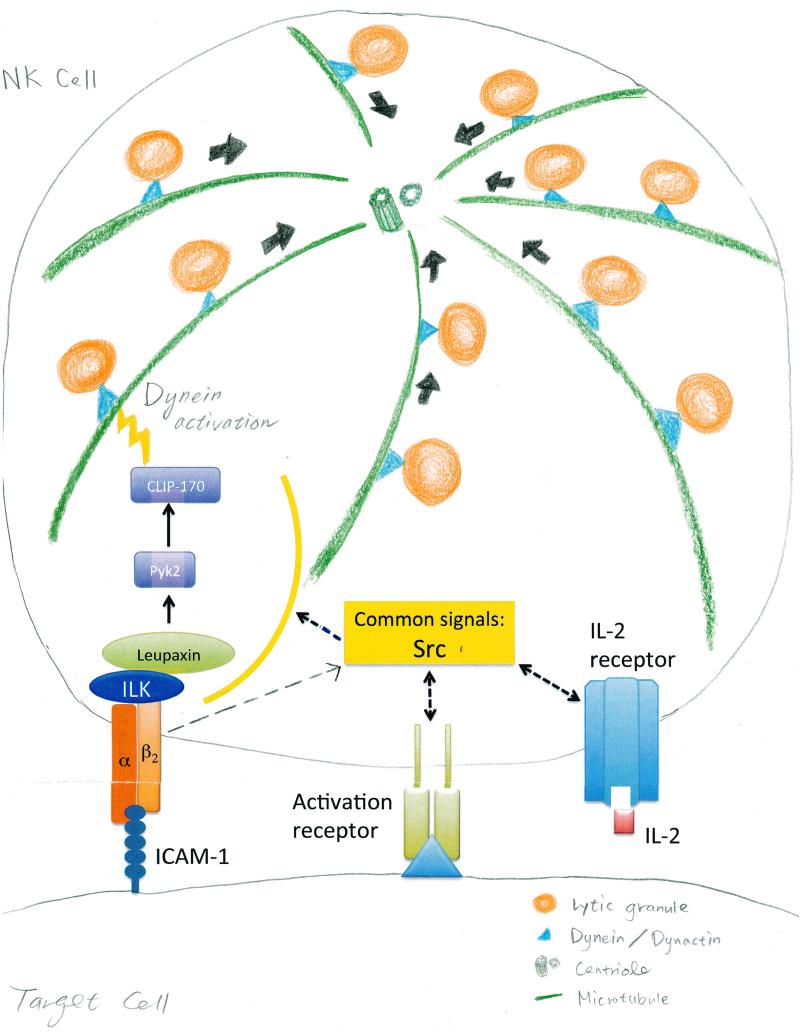
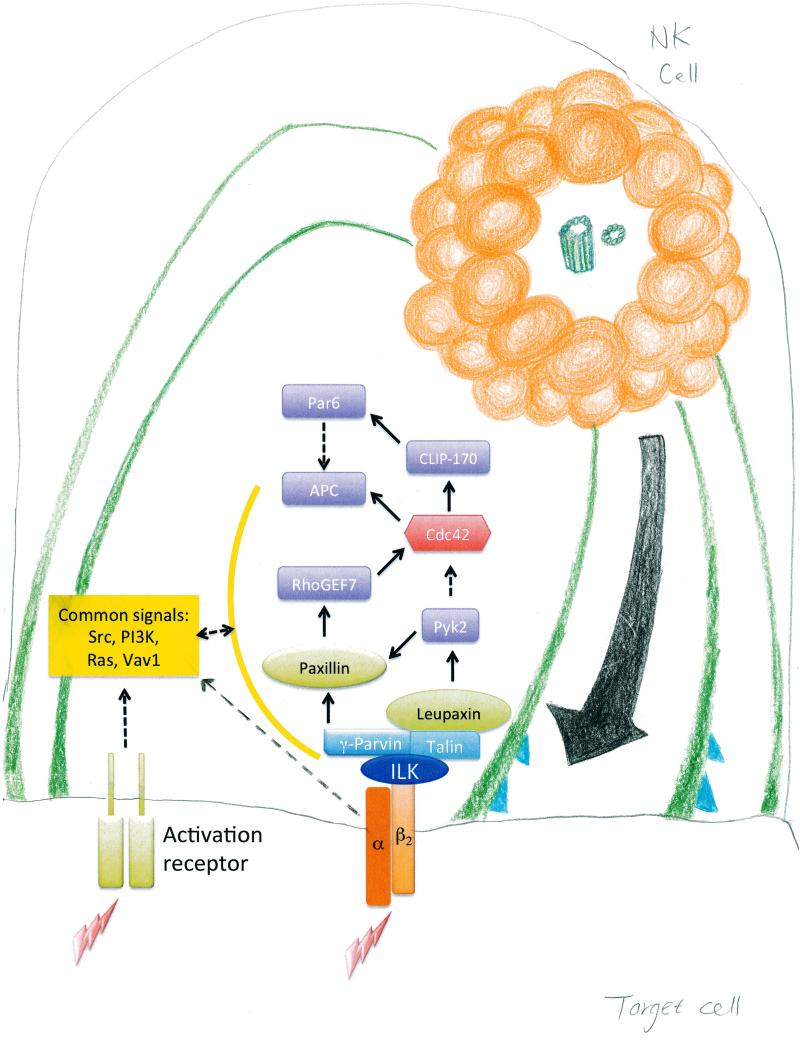
Fig. 1. Signaling requirements for LFA-1–dependent granule convergence and polarization in NK cell cytotoxicity.
(A) After engagement of LFA-1, lytic granules in NK cells converge on the MTOC through the minus-end–directed function of the dynein motor complex and signaling by Src family kinases (4, 7). This process can also be activated by stimulating NK cells with interleukin-2 (IL-2) (7). Zhang et al. demonstrated that the activation of ILK, leupaxin, Pyk2, and CLIP-170 in response to LFA-1 engagement was specifically required for the convergence of NK cell lytic granules on the MTOC. Although other NK cell–activating receptors promote convergence, the unbiased subtractive proteomics performed by Zhang et al. has defined these signals as at least minimally required by LFA-1 in inducing convergence function, which thus distinguishes them from those involved in the general activation of NK cells by CD16. ICAM-1, intercellular adhesion molecule–1. (B) Engagement of LFA-1 also stimulates the polarization of lytic granules and the MTOC to the immunological synapse in NK cells. Polarization of the MTOC is likely to depend on synaptic dynein through microtubule end-capture shrinkage (10). Zhang et al. determined that a limited set of signals partially overlapping with those involved in convergence are necessary downstream of LFA-1 to promote polarization. Again, the subtractive proteomics eliminated signals common to general NK cell activation by CD16. For granule polarization to the immunological synapse, LFA-1 engagement caused the recruitment of ILK into close proximity with the cytoplasmic tail of the β2 subunit of LFA-1, which further activated the downstream signaling molecules γ-parvin, Leupaxin, RhoGEF7, and Pyk2. RhoGEF7, a direct activator of Cdc42, and Pyk2, an indirect activator of Cdc42 (through inhibition of RhoGAP proteins), then stimulate activation of the Cdc42 signaling pathway, which establishes cell polarity during cell migration and division (12). Within the Cdc42 signaling pathway, Par6, APC, and CLIP-170 are required for the polarization of granules and the MTOC in human NK cells. PI3K, phosphatidylinositol 3-kinase.
To achieve these definitive results, Zhang et al. used particular model systems and made certain assumptions that will be important to expand upon in future studies. All signals common to LFA-1 and CD16 were eliminated from the analysis, and there are likely to be important candidates, contributors, and regulators still within that group of molecules. Because the discovery process relied on a single time and procedure for NK cell activation, other candidates might be further distinguished in additional kinetic or signal-strength analyses. Similarly, understanding the interrelatedness and activation sequence of the identified proteins may help generate a more comprehensive model of NK cell activation that can enable other activating, and even inhibitory, receptor functions to be integrated.
Additionally, Zhang et al. used a cell line with preconverged granules to evaluate lytic granule convergence, and thus, they defined signals that are required for the maintenance of convergence as opposed to those needed for activation of this process. The maintenance of convergence, however, has potential to be a separate and equally interesting step in the control of lytic granules in NK cells. Hence, further evaluation of the signal requirements for the initiation of convergence may prove informative. Zhang et al. also imply the existence of specific signaling thresholds that might need to be achieved to polarize, as opposed to converge, lytic granules. This indicates the need for more detailed studies of NK cell signal strength, combined with more accurate quantitative measurements of lytic granule positioning. It will be important to appreciate how this previously uncharacterized signaling pathway might be used by other immune cells, and even other non–immune cell types, that manage intracellular cargoes.
Regardless, Zhang et al. have identified a signaling pathway for defined steps in the maturation of the NK cell immunological synapse. This study has some important additional implications for the understanding of the immunological synapse and for cell biology in general. Both the processes of MTOC polarization and lytic granule convergence on the MTOC depend on the dynein motor protein complex (4, 8–10). Although some insights have been derivative from studies in other types of lymphocytes, MTOC polarization requires dynein function at the immunological synapse, in which end-captured microtubules are shortened to promote approximation to the synaptic membrane (10). In contrast, lytic granule convergence depends on microtubule minus-end–directed cargo transport with aggressive and abrupt individual granule traffic to the MTOC (4). Thus, the distinct signaling pathways for lytic granule polarization and lytic granule convergence identified by Zhang et al. may define specific inroads for stimulating particular dynein functions or modes of dynein activation. As the topic of dynein function and its activation through cell signals is actively pursued and poorly understood (11), this finding may represent an important clue with applications across biology.
Zhang et al. used an unbiased analysis to identify critical signals required for the maturation of the NK cell immunological synapse, and in so doing, they delineated specific requirements for individual cell biological steps in this process. These insights will likely empower further mechanistic divisibility of cytolytic cell function and more specific insight into the regulation of, and access to, important NK cell activities. Additionally, this work will likely have valuable implications for the regulation of organelle positioning and secretion in other non-immune cell types needed for the maintenance of human health.
Acknowledgments
Funding: This work was supported by NIH-R01AI067946 to J.S.O.
Footnotes
Elucidation of integrin signaling networks in natural killer cells defines the mechanisms required for lytic granule clustering and polarization.
Citation: H.-T. Hsu, J. S. Orange, Distinct integrin-dependent signals define requirements for lytic granule convergence and polarization in natural killer cells. Sci. Signal. 7, pe24 (2014).
References and Notes
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. Medline doi:10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. Medline doi:10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, Forbes LR, Watkin LB, Orange JS. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol. Cell Biol. 2014;92:245–255. doi: 10.1038/icb.2013.96. Medline doi:10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol. Biol. Cell. 2010;21:2241–2256. doi: 10.1091/mbc.E09-11-0930. Medline doi:10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, March ME, Lane WS, Long EO. A signaling network stimulated by an integrin with a β2 subunit promotes the polarization of lytic granules in cytotoxic cells. Sci. Signal. 2014;7:ra96. doi: 10.1126/scisignal.2005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. Medline doi:10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, Orange JS. Rapid activation receptor– or IL-2–induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood. 2013;121:2627–2637. doi: 10.1182/blood-2012-06-437012. Medline doi:10.1182/blood-2012-06-437012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. Medline doi:10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Kapoor TM, Chen JK, Huse M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc. Natl. Acad. Sci. U.S.A. 2013;110:11976–11981. doi: 10.1073/pnas.1306180110. Medline doi:10.1073/pnas.1306180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi J, Wu X, Chung AH, Chen JK, Kapoor TM, Hammer JA. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J. Cell Biol. 2013;202:779–792. doi: 10.1083/jcb.201301004. Medline doi:10.1083/jcb.201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, López MP, Vale RD, Jülicher F, Reck-Peterson SL, Dogterom M. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell. 2012;148:502–514. doi: 10.1016/j.cell.2012.01.007. Medline doi:10.1016/j.cell.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etienne-Manneville S. Cdc42 – the centre of polarity. J. Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. Medline doi:10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]