Abstract
Objective
Some have suggested the superiority of biatrial versus left atrial lesions. We sought to analyze our experience.
Methods
We retrospectively reviewed 305 consecutive patients from 2007 to 2011. Rhythm success was defined as freedom from atrial fibrillation (AF) or flutter determined by 12-lead electrocardiograms at 3-month intervals. Lesions sets were pulmonary vein isolation (PVI), left-extended (PVI + mitral valve annulus [MV] lesion ± left atrial appendage lesion [LAA]) or biatrial-extended (right atrial ablation + PVI + MV ± LAA).
Results
The success rates of PVI, left-extended, and biatrial-extended lesions were as follows: at 3 months, 56.7%, 74.7%, and 79.4% (P = .003); at 6 months, 56.9%, 72.9%, and 74.6% (P = .02); at 9 months, 54.6%, 72.5%, and 83.3% (P < .001); and at 12 months, 52.6%, 76.1%, and 80.0% (P < .001). Biatrial lesions had a higher rate of pacemaker placement than did left atrial lesions (16.5% vs 7.5%; P = .02). When we grouped patients by left lesion (PVI, PVI + MV, PVI + MV + LAA) irrespective of right atrial ablation, success was as follows: 3 months, 57.9%, 71.1%, and 87.8% (P < .01); 6 months, 58.1%, 71.6%, and 77.6% (P = .03); 9 months, 55.9%, 71.3%, and 89.6% (P < .01); and 12 months, 54.1%, 74.7%, and 83.7% (P < .01).
Conclusions
PVI is associated with lower rhythm success than an extended left atrial lesion set. The addition of a right atrial lesion to an extended left atrial lesion set does not improve efficacy, but it does increase the rate of pacemaker placement for sinus dysfunction. Adding an LAA lesion may confer additional efficacy when added to a lesion set that includes PVI + MV.
Atrial fibrillation (AF), the most common arrhythmia, is prevalent in 5.9% of persons older than 65 years.1 The absolute number of persons with AF will continue to rise as our population ages. In addition to the discomforting symptoms of palpitations, shortness of breath, anxiety, and reduced exercise tolerance, AF gives rise to hemodynamic and physiologic changes resulting in tachycardia-induced cardiomyopathy or thrombus formation and subsequent stroke. AF is an independent predictor of mortality.2 Furthermore, the economic burden of AF treatment in the United States is significant with an annual cost of $6.65 billion.3
A variety of surgical approaches to AF treatment have been introduced. In an effort to decrease procedure complexity, time, and risk,4 the cut-and-sew Cox maze III procedure has been replaced with modified procedures that use ablative energy sources. Some have called this variation the Cox maze IV procedure. Although the Cox maze IV procedure replicates the full biatrial lesion pattern of the cut-and-sew maze,5 a variety of less extensive variations, including procedures that include only left atrial lesions, are frequently performed.
Despite the popularity of ablative procedures limited only to the left atrium, some have suggested that left atrial lesion sets are inadequate and inferior to more complete, biatrial approaches.6-10 For this reason, we sought to analyze our experience with a variety of approaches for biatrial versus left atrial AF ablation.
Methods
From January 2007 to November 2011, 305 consecutive patients underwent surgical ablation for AF at 1 institution. In the majority of cases, the procedure was performed in conjunction with other cardiac procedures. Concomitant procedures included mitral valve surgery (33.3%, n = 100), aortic valve surgery (15.1%, n = 46), coronary artery bypass graft (3.9%, n = 12), bypass grafting plus valve (18.0%, n = 55), 2 or more valves (17.7%, n = 54), stand-alone ablation surgery (4.6%, n = 14), and other (7.8%, n = 24). Baseline demographics are detailed in Table 1.
Table 1. Preoperative and operative characteristics.
| Preoperative characteristics | Biatrial (% of patients) | Left atrial (% of patients) | P value |
|---|---|---|---|
| No. | 91 | 214 | — |
| Age (y) | 59 ± 28 | 68 ± 12 | <.01 |
| Female gender | 40.7 | 45.8 | .41 |
| White race | 64.8 | 78 | .02 |
| Body mass index | 28 ± 6 | 27 ±6 | .6 |
| Current smoker | 3.3 | 7.5 | .17 |
| Diabetes | 17.6 | 18.7 | .82 |
| Hypertension | 55 | 73.8 | <.01 |
| Hyperlipidemia | 27.5 | 35.6 | .23 |
| Stroke | 9.9 | 8.9 | .78 |
| Coronary artery disease | 30.8 | 42.1 | .06 |
| Congestive heart failure | 41.8 | 34.6 | .23 |
| Previous cardiac surgery | 23.1 | 13.1 | .03 |
| Depression | 5.5 | 5.1 | .89 |
| Left atrial size (cm) | 4.9 ± 0.8 | 5.1 ± 0.9 | .14 |
| AF chronicity: paroxysmal | 33.7 | 31.8 | .75 |
| Operative characteristics | |||
| Sternotomy approach | 87.6 | 76.9 | .03 |
| Radiofrequency | 16.5 | 36.9 | <.01 |
| Cryoablation | 80.2 | 33.6 | <.01 |
| Microwave | 3.3 | 29 | <.01 |
| Laser | 0 | 0.47 | .51 |
| Crossclamp time (min) | 98 ± 41 | 86 ± 41 | .02 |
| CPB time (min) | 152 ± 34 | 136 ± 54 | .03 |
AF, Atrial fibrillation; CPB, cardiopulmonary bypass.
Surgical ablation was performed biatrially or on the left atrium only. The decision to perform biatrial versus left atrial ablation was determined by each individual surgeon. All right-sided ablation lesion sets were standardized to include 3 lesions to the tricuspid valve annulus, right atrial free wall, and intercaval line (Figure 1). Left atrial ablation procedures were categorized as either pulmonary vein isolation only (PVI) or “extended left atrial lesion set,” consisting of PVI isolation plus a lesion to the mitral valve annulus (MV), with or without a lesion to the left atrial appendage (LAA).
Figure 1.
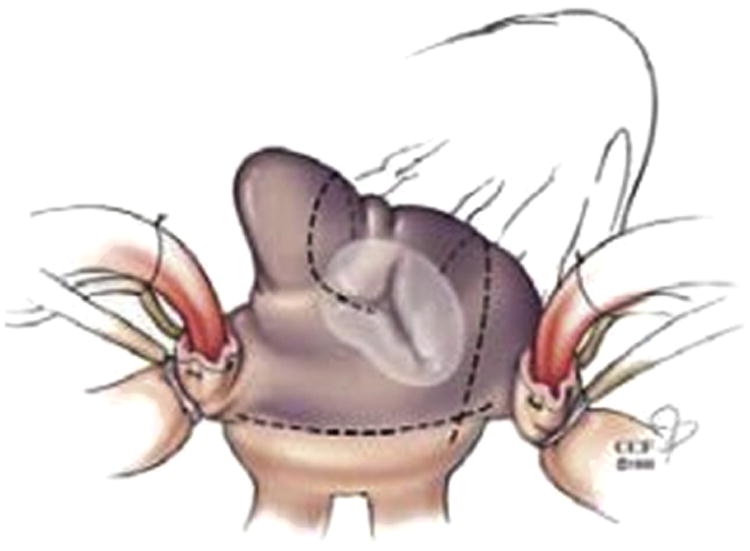
Right atrial lesion set (courtesy of Marc Gillinov, MD).
Ninety-one patients received a biatrial lesion set, and 214 patients underwent left atrial ablation only. Energy modality for ablation consisted of cryoablation (n = 145), radiofrequency (n = 94), microwave (n = 65), and laser (n = 1). Operative characteristics are detailed in Table 1. Our primary end point was rhythm success defined as freedom from AF or atrial flutter. This was determined by 12-lead electrocardiogram at 3, 6, 9, and 12 months after surgical ablation. Follow-up was 87%, 87%, 85%, and 84% at these time points, respectively. Missing data points at various time points were left blank and not counted as procedure success or failure. There was no difference among groups as divided by lesion set with regard to completeness of follow-up. Freedom from antiarrhythmics was not incorporated in the primary end point. Secondary end points included mortality and hospital length of stay, as well as postoperative myocardial infarction, stroke, and pacemaker placement. This study was approved by our institutional review board and used informed consent.
The LAA was managed in a few ways. If the base of the appendage was noted to be broad, it was left open. Otherwise, the appendage was oversewn with a 2-layer closure. Alternatively, it was amputated with a stapler or excised and oversewn.
Statistical Analysis
Outcomes were compared using χ2 or Fisher's exact tests for categorical variables and the Student t test for continuous variables expressed as mean ± standard deviation. Differences in baseline demographics or operative characteristics were accounted for by stepwise backward logistic regression. Univariate variables with a significance of P <.10 were entered in a backward stepwise logistic regression. Stata version 11 (Stat Corporation, College Station, Tex) was used for statistical analysis.
Results
Patient Characteristics
Our surgical cohort consisted of 305 patients, of whom 29.8% (n = 91) had biatrial ablation and 70.2% (n = 214) had left atrial ablation. Baseline characteristics were similar between groups, except the left-sided ablation cohort tended to be older (68 ± 12 vs 59 ± 28; P < .01) and more frequently white (78.0% vs 64.8%; P = .02) and hypertensive (73.8% vs 55.0%; P < .01), and patients in the biatrial group had a higher incidence of prior cardiac surgery (23.1% vs 13.1%; P = .03). With regard to operative characteristics, biatrial ablation was more frequently performed via sternotomy as opposed to a minimally invasive approach (87.6% vs 76.9%; P = .03) and with an equal distribution of energy modalities, including cryoablation (33.6%), radiofrequency (36.9%) and microwave (29%). The biatrial ablation group had longer cardiopulmonary bypass times (152 ± 34 minutes vs 136 ± 54 minutes; P = .03) and crossclamp times (98 ± 41 minutes vs 86 ± 41 minutes; P = .02). Patients whose concomitant procedure was mitral valve surgery were more likely to have left atrial lesions (38% vs 21%; P < .01).
Biatrial Versus Left Atrial Lesions Sets
First, we compared patients having biatrial lesions with those having left atrial ablations. Rhythm success was superior in the biatrial cohort at all time points (80.3% vs 64.0% at 3 months, P = .01; 75.7% vs 63.4% at 6 months, P = .06; 84.1% vs 61.9% at 9 months, P < .01; and 80.9% vs 62.4% at 12 months, P <.01).
Because a range of left atrial lesion sets was used in our patient cohort, from isolated PVI to more extensive left atrial lesion patterns, we sought to determine the contribution of this variability to rhythm success. In our biatrial cohort, 95% (n = 86) had extended left-sided lesions as compared with 40% (n = 86) in the left-sided ablation group. In the biatrial group, 40 patients had 3 lesions (PVI + MV + LAA), 46 patients had 2 lesions (PVI + MV), and 5 patients had only PVI. In the left-sided ablation group, 22 patients had 3 lesions, 64 patients had 2 lesions, and 128 patients had only PVI (Figure 2).
Figure 2.
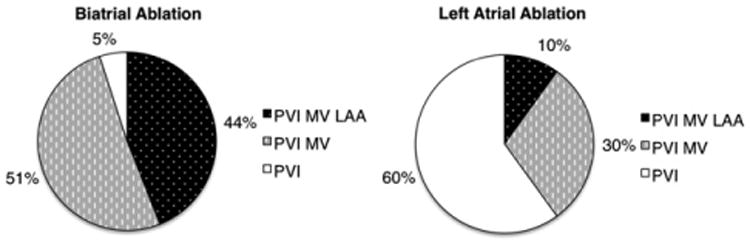
Distribution of left lesions in biatrial and left atrial lesion groups. PVI, Pulmonary vein isolation; MV, mitral valve annulus; LAA, left atrial appendage.
Next, the cohort was divided into 3 groups: PVI only (PVI), extended left atrial lesions (eLA), and biatrial with extended left atrial lesions (BeLA). The success rates of PVI, eLA, and BeLA lesions were as follows: 3 months, 56.7%, 74.7%, and 79.4% (P = .003); 6 months, 56.9%, 72.9%, and 74.6% (P = .02); 9 months, 54.6%, 72.5%, and 83.3% (P < .001); and 12 months, 52.6%, 76.1%, and 80.0% (P < .001). Through 12 months, PVI was inferior to eLA or BeLA lesions. However, there was no difference in success over this time period between patients receiving biatrial or left-sided cohorts when the left lesions were more complete (eLA) (Figure 3).
Figure 3.
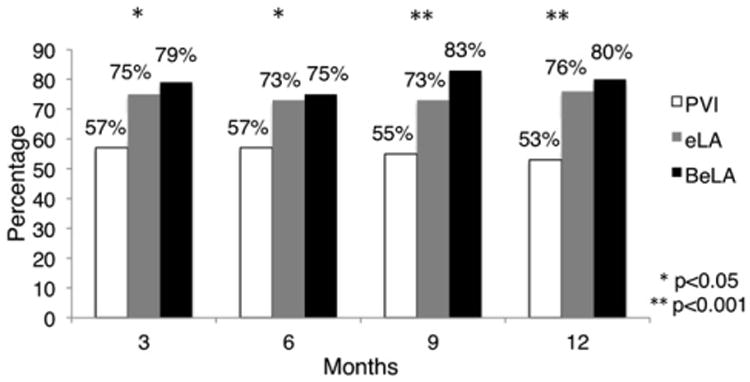
Rhythm success with pulmonary vein isolation (PVI), extended left atrial lesions (eLA), and biatrial with extended left atrial lesions (BeLA).
To account for differences among group characteristics, we performed a stepwise backward multivariable logistic regression. This model took into account differences in demographic characteristics including age, race, history of hypertension, and previous cardiac surgery as well as differences in operative characteristics including differences in energy modality, cardiopulmonary bypass time, and crossclamp time. As compared with a biatrial extended lesion set, PVI had inferior rhythm success at 3 months (odds ratio [OR], 0.34; confidence interval [CI], 0.17-0.69; P = .003), 6 months (OR, 0.45; CI, 0.23-0.88; P = .02), 9 months (OR, 0.24; CI, 0.11-0.51; P = .001), and 12 months (OR, 0.28; CI, 0.13-0.58; P = .001). In our multivariable analysis, there was no difference between eLA and BeLA groups with respect to rhythm success.
Concomitant Mitral Surgery Subset
To minimize the confounding influence of concomitant procedure heterogeneity, we repeated the analysis in the subset of our patients who had concomitant mitral valve surgery (n = 100). In our mitral valve cohort, 19 patients had BeLA, 44 had eLA, and 37 had PVI. There were no differences in baseline characteristics for this subset of patients. With respect to operative characteristics, the only significant difference was the more frequent use of cryoablation as in the BeLA group versus the eLA group (84.2% vs 45.0%; P <.01). Rhythm success in the PVI, eLA, and BeLA groups was as follows: at 3 months, 62.1%, 79.5%, and 93.8% (P = .05); at 6 months, 65.5%, 82.1%, and 87.5% (P = .15); at 9 months, 65.5%, 84.2%, and 93.3% (P = .06); and at 12 months, 62.1%, 86.5%, and 86.7% (P = .04). The univariate analysis demonstrated the inferiority of PVI and no difference between eLA and BeLA lesion sets and was confirmed on multivariable logistic regression that took into account differences in energy modality.
Extent of Left Atrial Lesion Set
Next, we sought to investigate further the impact of the extent of the left atrial ablation on rhythm success. That is, did performing 3 left lesions confer greater efficacy than 2 left lesions, irrespective of whether right-sided ablation was performed? To that end, we grouped patients by left lesion set irrespective of right atrial ablation. Rhythm success for PVI, 2 left lesions, and 3 left lesions was as follows: 3 months, 57.9%, 71.1%, and 87.8% (P = .001); 6 months, 58.1%, 71.6%, and 77.6% (P = .03); 9 months, 55.9%, 71.3%, and 89.6% (P <.001); and 12 months, 54.1%, 74.7%, and 83.7% (P <.001) (Figure 4). Multivariable analysis to account for differences in baseline characteristics and operative characteristics confirmed the inferiority of PVI as compared with 3 left lesions—rhythm success for PVI as compared with the 3 left lesion group was as follows: OR, 0.19; CI, 0.08-0.49 at 3 months (P = .001); OR, 0.40; CI 0.18-0.87 at 6 months (P = .02); OR, 0.15; CI, 0.05-0.40 at 9 months (P < .001); and OR, 0.23; CI, 0.10-0.54 at 12 months (P = .002). Multivariable analysis also suggested that 2 left lesions were inferior to 3 left lesions, at least at the 3-month (OR, 0.34; CI, 0.13-0.90; P = .03) and 9-month (OR, 0.29; CI, 0.10-0.81; P = .02) time points.
Figure 4.
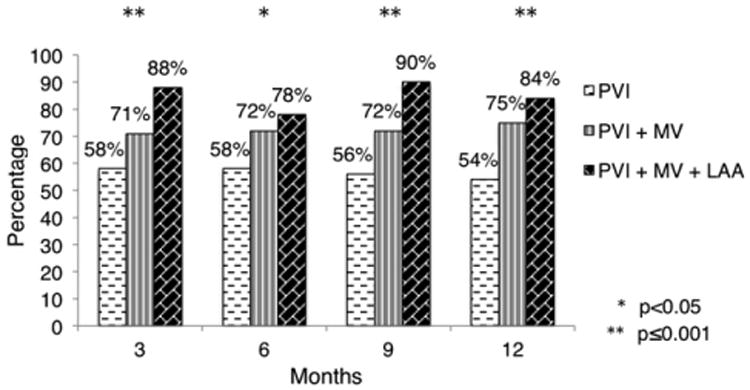
Rhythm success stratified by completeness of left atrial lesion. PVI, Pulmonary vein isolation; MV, mitral valve annulus; LAA, left atrial appendage.
Again, we repeated this analysis in our mitral valve subset. The success of PVI, 2 left lesions, and 3 left lesions, regardless of right atrial ablation, was 62.1%, 79.0%, and 94.1% at 3 months (P = .04), 65.5%, 79.0%, and 94.1% at 6 months (P = .08), 65.5%, 81.1%, and 100% at 9 months (P = .02), and 62.1%, 83.3%, and 93.8% at 12 months (P = .03). On multivariable logistic regression, PVI was inferior to 3 left lesions at 3 months (OR, 0.10; CI, 0.01-0.88; P = .04), 6 months (OR, 0.12; CI, 0.01-1.0; P = .05), and 12 months (OR, 0.08, CI, 0.01-0.9; P = .04), whereas there was no difference in efficacy between 2 left lesions versus 3 left lesions at 3 months (OR, 0.23; CI, 0.03-2.0; P = .19), 6 months (OR, 0.23; CI, 0.03-2.0; P = .19), and 12 months (OR, 0.35; CI, 0.04-3.4; P = .36).
Morbidity
With respect to our secondary outcomes, patients having biatrial ablation lesions had a higher rate of pacemaker placement than did those having only left atrial lesions (16.5% vs 7.5%; P = .02). We further investigated the reason for pacemaker placement to clarify whether the pacemaker placement was related to the concomitant procedure or the ablation (Table 2). Performing a 2-valve procedure at the same time as surgical ablation was a risk factor for pacemaker placement. However, there was no difference in the frequency or the type of double valve procedures between the groups to account for the different rates of pacemaker placement. In addition, other operative parameters including bypass time, energy modality, and surgeon were not predictors of postoperative pacemaker placement. In the overall patient cohort, although there was no difference in the incidence of atrioventricular block as the indication for pacemaker insertion between the biatrial versus left atrial ablation groups (4.4% vs 3.7%; P = .79), the incidence of sinus bradycardia or arrest was significantly higher in the biatrial group (12.1 % vs 3.7%; P < .01). In the mitral valve subset, there was also a higher rate of pacemaker placement in the biatrial cohort as compared with the left atrial ablation cohort (26.3% vs 8.6%; P = .03). Again, there was no difference in the frequency of atrioventricular block as the indication for pacemaker placement in the biatrial versus the left atrial ablation groups (5.3% vs 3.7%; P = .76), but there was a much higher rate of sinus dysfunction in the biatrial group (21.1% vs 4.9%; P = .02).
Table 2. Indications for Pacemaker Placement.
| Indications | eLA | BeLA | MV cohort eLA | MV cohort BeLA |
|---|---|---|---|---|
| AV bock | 3.7% | 4.4% | 3.7% | 5.3% |
| Sinus Dysfunction | 3.7% | 12.1% | 4.9% | 21.1% |
eLA, Extended left atrial lesions; BeLA, biatrial with extended left atrial lesions; MV, mitral valve annulus; AV, atrioventricular.
There was no difference between biatrial or left-sided ablation groups with respect to hospital length of stay (11.2 ± 10.9 vs 10.5 ± 9.1; P = .58) or 30-day mortality (3.8% vs 3.2%; P = .80). There was no difference with regard to major postoperative complications including myocardial infarction (0% vs 0.5%; P = 1.0) and stroke (3.8% vs 3.7%; P = .97).
Discussion
In our initial analysis of our 305-patient cohort, we found a higher success rate through 12 months in those receiving biatrial versus left atrial lesion sets. This finding was consistent with those of other studies that have concluded that biatrial ablation is superior to left-sided lesions. However, these studies grouped all left lesions together8,11 and incorporated energy modalities no longer in use. When the left lesion set has been deconstructed, there have been a myriad of left lesion sets that have been compared with biatrial lesion sets. These lesion sets have included ganglionic plexi ablation,6 coronary sinus lesions,7 as well as cavotricuspid isthmus lesions.10 The results have been conflicting with reports of superior efficacy in the biatrial group6,7 versus no difference in efficacy.9,12,13 It is difficult to compare studies given the heterogeneity of lesions performed.
Further analysis of our detailed lesion set data revealed that the overwhelming majority of the patients in our biatrial cohort had extended left lesions sets (PVI + MV ± LAA), whereas the majority of those in the left-sided cohort had only PVI. Thus, we expanded our investigation to determine whether the superior efficacy observed in the biatrial cohort was attributable to the addition of a right-sided lesion, or rather to the more frequent presence of an extended left lesion set. When we broke patients down by extensiveness of the left atrial lesion, we found that the efficacy of eLA lesion set and the biatrial lesion set (BeLA) were similar and that both were superior to PVI alone. This finding conflicts with reports of satisfactory outcomes with PVI14-16 and corroborates another study that linked PVI alone to inferior outcomes.17 This may be related to the high proportion of persistent AF in our PVI cohort.
Although there were no differences in the groups with respect to important known predictors of ablation efficacy, such as left atrial size and chronicity of AF, we performed a multivariable analysis to account for other demographic and technical differences. Even after accounting for these potential confounding factors in our multivariable analysis, we found that there was no difference in success rate between an extended left atrial lesion set (eLA) and a biatrial lesion set (BeLA). These findings were also confirmed in the more homogeneous group of patients having mitral surgery as their concomitant procedure. Thus, in our patient cohort, it appears that the superior efficacy of biatrial ablation over left atrial ablation was a result of the fact that an extended left atrial lesion set (which is superior to PVI alone) was more commonly used in the biatrial procedures.
In addition, we found that 3 left lesions may confer an additional efficacy benefit as compared with 2 left lesions. Other studies have noted the MV lesion as an important addition to PVI.17,18 In some cases, the LAA is an important trigger of AF.19 Our data suggests that the LAA lesion may be an important component of the left atrial lesion set in addition to the MV lesion.
We wanted to investigate the risk factors associated with postoperative pacemaker placement. We found that performing 2 valve procedures at the same time as surgical ablation was a predictor of postoperative pacemaker placement. However, there was no difference in either the frequency or type of double valve procedures in the biatrial versus the left atrial ablation cohorts. To further adjust for possible confounders, we examined pacemaker placement in our mitral valve cohort and it confirmed our finding of biatrial ablation as a predictor of postprocedure sinoatrial node dysfunction. Other operative characteristics including bypass time, energy modality, and surgeon were not predictors of postoperative pacemaker placement.
The reported rates of pacemaker placement in the literature range from 5% to 10%,14,20,21 but few studies have reported the rate of pacemaker placement after right atrial lesions.7,22 We observed a higher rate of pacemaker placement in our biatrial cohort. There were no differences between the left-sided cohort and the biatrial cohort with respect to postprocedure atrioventricular block, and the increased pacemaker requirement in the biatrial group was related an increased incidence of sinus dysfunction. These findings were confirmed in the concomitant mitral surgery subset as well. We23 have previously published our findings noting that postoperative pacemaker placement after surgical ablation was an independent predictor of short-term mortality. Thus, this complication is not necessarily without consequence.
As this experience encompasses several years and as energy modalities in favor have changed over the years, there is a certain heterogeneity to our experience. We tried to account for these differences in energy modality in a number of ways. First, we limited our experience to the past 5 years to encompass more contemporary energy modalities and, indeed, radiofrequency and cryoablation constituted the majority of our experience. Second, we accounted for differences in energy modality in our multivariable model. Third, we performed a subanalysis on our more homogenous MV cohort.
There has been the suggestion that procedure success is dependent on the operator. We investigated this question in our experience. Surgical ablation has been performed at Columbia since the late 1990s, and our AF database has maintained records since 1999. The operators who constituted the majority of procedures performed (91.5%) had the same level of experience. Operator was not a predictor of procedure success.
Our study has several limitations. First, although demographic and operative data were collected prospectively in our clinical research database, all analyses were performed retrospectively. Consequently, our findings support an association between events rather than a definitive cause and effect relationship. Also, the specific procedures performed in our patients were not based on randomized assignment or even specific predetermined criteria, but rather on clinical practice norms and surgeon preference. Although we attempted to take known differences in patient characteristics and operative details into account by applying multivariable statistical techniques, our study remains vulnerable to the pitfalls of a nonrandomized, retrospective analysis, including unknown confounders and selection, procedure, and detection bias. Second, we determined rhythm success by quarterly 12-lead electrocardiographic analysis. Although this method allowed for excellent follow-up rates and represented the only practical way of getting this degree of data completeness given the demographic and geographic profile of our patients, it is subject to the risk of underdetection of arrhythmias and therefore overestimation of success rates. Third, our follow-up was only short term, although we are actively collecting long-term data. Fourth, although this study represents a large single-center cohort, the division of patients into subgroups limited our study power. Finally, our cohort includes a heterogeneous population with respect to concomitant procedures. This lends itself to several confounders including, potentially, differences in arrhythmia pathophysiology. A subanalysis of the mitral valve cohort attempted to correct for this, but the reduced study numbers further decreased the statistical power of this analysis.
In summary, we found that PVI is associated with lower efficacy than more extensive left atrial lesion sets and that the addition of right atrial lesions to an extended left atrial lesion set does not improve efficacy, but increases the rate of pacemaker implantation for sinus dysfunction. Given the limitations outlined above, our results are certainly not definitive. However, inasmuch as our study does describe a substantial real-world experience with a variety of lesion sets in a representative cardiac surgical population with AF, we believe that our findings are worth pursuing in a more rigorous way. We eagerly await the results of an ongoing National Institutes of Health trial studying the efficacy of left atrial and biatrial lesion sets in patients with AF who are undergoing mitral valve surgery.
Abbreviations and Acronyms
- AF
atrial fibrillation
- BeLA
biatrial with extended left atrial lesions
- eLA
extended left atrial lesions
- LAA
left atrial appendage
- MV
mitral valve annulus
- PVI
pulmonary vein isolation
Footnotes
Disclosures: Authors have nothing to disclose with regard to commercial support.
Read at the 92nd Annual Meeting of The American Association for Thoracic Surgery, San Francisco, California, April 28-May 2, 2012.
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart R. Prevalence, age distribution, and gender of patients with atrial fibrillation. Arch Intern Med. 1995;155:469–73. [PubMed] [Google Scholar]
- 2.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation. N Engl J Med. 1982;306:1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–56. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 4.Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL, et al. The Cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Onorati F, Mariscalco G, Rubino AS, Serraino F, Santini F, Musazzi A, et al. Impact of lesion sets on mid-term results of surgical ablation procedure for atrial fibrillation. J Am Coll Cardiol. 2011;57:931–40. doi: 10.1016/j.jacc.2010.09.055. [DOI] [PubMed] [Google Scholar]
- 7.Kim JB, Bang JH, Jung SH, Choo SJ, Chung CH, Lee JW. Left atrial ablation versus biatrial ablation in the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2011;92:1397–405. doi: 10.1016/j.athoracsur.2011.05.066. [DOI] [PubMed] [Google Scholar]
- 8.Barnett SD, Niv A. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg. 2006;131:1029–35. doi: 10.1016/j.jtcvs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Deneke T, Khargi K, Grewe PH, von Dryander S, Kuschkowitz F, Lawo T, et al. Left atrial versus bi-atrial maze operation using intraoperatively cooled-tip radio-frequency ablation in patients undergoing open-heart surgery. J Am Coll Cardiol. 2002;39:1644–50. doi: 10.1016/s0735-1097(02)01836-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Meng X, Li H, Cui Y, Han J, Xu C. Prospective randomized comparison of left atrial and biatrial radiofrequency ablation in the treatment of atrial fibrillation. Eur J Cardiothorac Surg. 2009;35:116–22. doi: 10.1016/j.ejcts.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Melo J, Santiago T, Aguiar C, Berglin E, Knaut M, Alfieri O, et al. Surgery for atrial fibrillation in patients with mitral valve disease: results at five years from the International Registry of Atrial Fibrillation Surgery. J Thorac Cardiovasc Surg. 2008;135:863–9. doi: 10.1016/j.jtcvs.2007.08.069. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy PM, Kruse J, Shalli S, Ilkhanoff L, Goldberger JJ, Kadish AH, et al. Where does atrial fibrillation fail? Implications for increasing effectiveness of ablation. J Thorac Cardiovasc Surg. 2010;139:860–7. doi: 10.1016/j.jtcvs.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht A, Kalil RA, Schuch L, Abrahao R, Sant'Anna JRM, de Lima G, et al. Randomized study of surgical isolation of the pulmonary veins for correction of permanent atrial fibrillation associated with mitral valve disease. J Thorac Cardiovasc Surg. 2009;138:454–9. doi: 10.1016/j.jtcvs.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Beyer E, Lee R, Lam BK. Point: Minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: early multicenter results. J Thorac Cardiovasc Surg. 2009;137:521–6. doi: 10.1016/j.jtcvs.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Edgerton JR, McClelland JH, Duke D, Gerdisch MW, Steinberg BM, Bronleewe SH, et al. Minimally invasive surgical ablation of atrial fibrillation: six-month results. J Thorac Cardiovasc Surg. 2009;138:109–14. doi: 10.1016/j.jtcvs.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 16.Nasso G, Bonifazi R, Del Prete A, Del Prete G, Lopriore V, Bartolomucci F, et al. Long-term results of ablation for isolated atrial fibrillation through a right minithoracotomy: toward a rational revision of treatment protocols. J Thorac Cardiovasc Surg. 2011;142:e41–6. doi: 10.1016/j.jtcvs.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Gillinov AM, Bhavani S, Blackstone EH, Rajeswaran J, Svensson L, Navia JL, et al. Surgery for permanent atrial fibrillation: impact of patient factors and lesion set. Ann Thorac Surg. 2006;82:502–14. doi: 10.1016/j.athoracsur.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Minakata K, Yunoki T, Yoshikawaa E, Katsu M, Oda T, Ujino K. Predictors of success of the modified maze procedure using radiofrequency device. Asian Cardiovasc Thorac Ann. 2011;19:33–8. doi: 10.1177/0218492310394876. [DOI] [PubMed] [Google Scholar]
- 19.Biase LD, Burkhardt D, Mohanty P, Sanchez J, Mohanty S, Horton R, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 20.Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson B, O'Brien SM, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2008;85:909–15. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 21.Mohr FW, Fabricius AM, Falk V, Autschbach R, Doll N, von Oppell U, et al. Curative treatment of atrial fibrillation with intraoperative radiofrequency ablation: short-term and midterm results. J Thorac Cardiovasc Surg. 2002;123:919–27. doi: 10.1067/mtc.2002.120730. [DOI] [PubMed] [Google Scholar]
- 22.Ablage A, Peterffy M, Kallner G. The biatrial cryo-maze procedure for treatment of atrial fibrillation: a single-center experience. Scand Cardiovascu Jl. 2011;45:112–9. doi: 10.3109/14017431.2010.547595. [DOI] [PubMed] [Google Scholar]
- 23.Worku B, Pak SW, Cheema F, Russo M, Housman B, Van Patten D, et al. Incidence and predictors of pacemaker placement after surgical ablation for atrial fibrillation. Ann Thorac Surg. 2011;92:2085–90. doi: 10.1016/j.athoracsur.2011.07.058. [DOI] [PubMed] [Google Scholar]


