Platelets are central in the process of hemostasis as they maintain the integrity of the vasculature and prevent excessive interstitial bleeding. Upon endothelial injury, activated platelets form a thrombus, and in combination with the coagulation cascade they lead to the formation of a tight plug and prevention of blood loss. By an alternative mechanisms, immune cells are also capable of promoting thrombosis and consequently preventing pathogen dissemination [1]. The role of thrombosis during viral infections however remains elusive and is poorly understood.
In addition to their classical role in hemostasis and thrombosis, platelets have a less appreciated immune function. Platelets engage the innate immune system by interacting with neutrophils and monocytes and forming heterotypic aggregates with these cell populations [2]. Through their toll-like receptors, platelets can present bacteria to the neutrophils for destruction [2]. Platelets are also important in neutrophil extracellular trap formation during bacterial infections [1]. In addition to their interaction with the cells of the innate immune system, platelets are also capable of interacting with the adaptive branch of the immune system [3]. Studies have shown that platelets are capable in regulating T-cell differentiation [4] and interacting with different subsets of lymphocytes [3]. These more recent studies have shown that, in addition to their critical role in hemostasis and thrombosis, platelets are major mediators in crosscommunication with the immune system.
It is not surprising that in the setting of infections, platelets are instrumental for host survival. In an animal study, platelet depletion reduced survival of mice after LPS injection [5]. A similar outcome was observed when platelets were depleted before infection with encephalomyocarditis virus (EMCV) [6]. During infection with murine lymphocytic choriomeningitis virus, platelet presence prevented the severity of concomitant lethal hemorrhagic anemia [7]. Studies of human platelets have shown the presence of viral particles or RNA originating from Influenza [8], HIV [9], HCV [10] or Dengue [10]. Although various RNA viruses are found in circulating platelets and platelets may be first responders to certain viral infections, it is unclear if there is a common response in immunity.
Supporting the important role of platelets in immunity, in this issue of the Journal of Thrombosis and Haemostasis, Negrotto et al. report that platelets directly improve the outcome of Coxsackievirus B (CVB)-induced myocarditis [11]. CVB is a single stranded RNA human virus, infection with which may range from mild gastrointestinal distress to aseptic meningitis or myocarditis. In this study, the authors show that platelet presence is critical for the survival of mice post CVB infection and platelet depletion exaggerates the development of myocarditis. During the course of infection, platelets formed aggregates with the neutrophil/granulocyte population. The authors note that, contrary to other studies, P-selectin and vWF surface expression in mice did not change on platelets as the infection progressed. Instead, CVB infection increased platelet phosphatidylserine (PS) surface translocation without causing apoptosis. It is possible that platelet-granulocyte interactions were mediated by the increased negative charge on the platelet surface as a result of the increased phosphatidyilserine surface expression. It is not clear, however, if P-selectin is involved in the initial interaction with neutrophils or if PS is the only mediator of the platelet-neutrophil interaction during CVB infection in mice. Interestingly, CVB ex vivo infection of human platelets did increase P-selectin surface expression suggesting that there may be inherit species variations or there are differences between in vivo and ex vivo platelet activation during infection.
Importantly, CVB did not reproduce in human platelets and did not require the presence of the CAR-receptors [11]. Neutralizing the activation of platelet-integrins (αiibβiii), central for thrombus formation, moderately reduced viral titers suggesting that thrombosis may play a role in the initial sequestration of the virus [11]. Although in vitro, these experiments suggest that maybe the lack of a nucleus and reduced nucleotide pool in platelet is beneficial in the initial response to viral infections, forcing interactions with different leukocyte populations and activating the immune system. The authors also observed mild thrombocytopenia that peaked at 48 hours. Finally, the authors elegantly showed that platelet depletion led to increased viral titers in heart tissue, elevated tissue lesions and more severe myocarditis (Figure 1). In summary, this study is the first to show that lower viral titers are associated with platelet presence, CVB does not replicate in platelets, and that depletion of platelets directly affects the outcome of the severity of myocarditis.
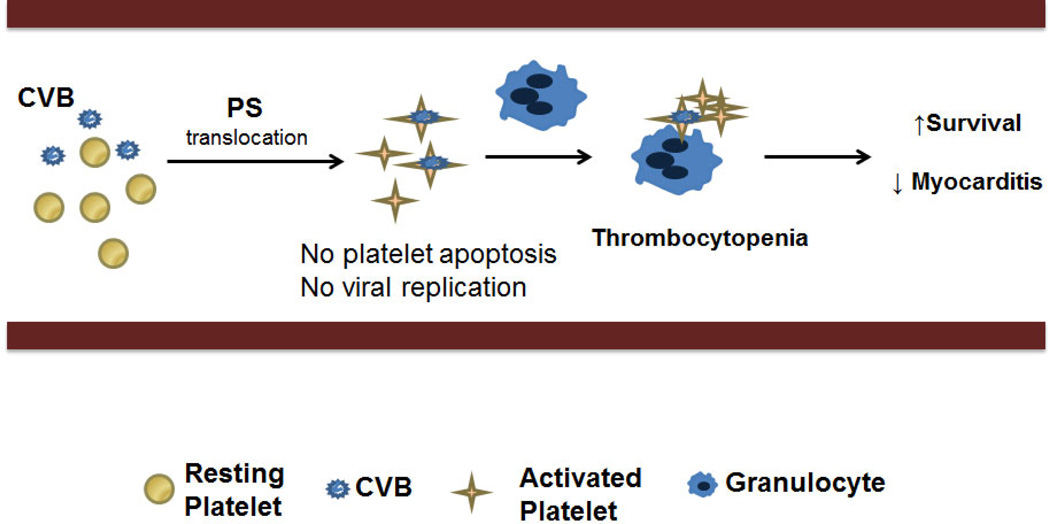
Figure 1. Platelets activate the innate immune response during Coxsackieviruses B (CVB)-infection.
During the course of infection, platelets interact with the CVB particles. This interaction results in platelet phosphatidylserine (PS) membrane-translocation, platelet-granulocyte aggregate formation and thrombocytopenia. Interestingly CMV does not replicate in platelets and CMV-mediated activation of platelets does not lead to apoptosis. Ultimately, platelet presence throughout the course of CMV infection leads to increased host survival, increased IgG levels, decreased viral load, and decreased severity of myocarditis.
The concept of platelet-neutrophil interactions during viral infections, while not new, is an underappreciated and not a well understood process. Neutrophils recruited to the site of infection in combination with platelets protect against poxviral challenge by inducing NET-formation [12]. Further, platelet neutrophil interactions during EMCV infection are plateletmediated [6]. In vitro studies with HIV and human neutrophils have shown that neutrophils can form NETs and reduce infectious efficiency of HIV [13]. The study of Negrotto et al. [11] provides further proof that platelet-neutrophil interactions are central during viral infections. It also shows that viral load is simply reduced by platelet presence and suggests that viruses do not replicate in platelets. However, further work is necessary to elucidate if platelets are important in infections due to other viruses such as DNA viruses, if platelets are the universal mediators of platelet-neutrophil interactions, and if these interactions with neutrophils lead to pro-thrombotic events or are controlled initial steps toward viral clearance. It continues to be unclear if select viruses have evolved to deactivate platelets as they reproduce in the host. Finally, with respect to improving clinical outcome in the setting of viral infections, it remains undetermined if there is a safe way to manipulate and enhance platelet immune response vs. platelet pro-thrombotic response.
Viral Infections have long been associated with thrombocytopenia, hemorrhagic shock and potential coagulopathies. Certain viral infections have been associated with pro-thrombotic complications and it has been shown that flu pandemics are associated with increased occurrences of myocardial infarctions and strokes. As a growing number of studies are showing, platelets are possibly important mediators in the intersection between cardiovascular and immune events. It remains unclear how much of the platelets’ immune function affects the cardiovascular outcome as opposed to being the consequence of the activated immune system. Finally, it remains to be explored if the targeting of the platelets’ immune function would have a beneficial or detrimental effect on vascular patency and host survival.
Footnotes
Authors state that they have no conflicts of interests.
References
- 1.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. 10.1038/nri3345 nri3345 [pii]. [DOI] [PubMed] [Google Scholar]
- 2.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. 10.1038/nri2956 nri2956 [pii]. [DOI] [PubMed] [Google Scholar]
- 3.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. 10.1189/jlb.0907615jlb.0907615 [pii]. [DOI] [PubMed] [Google Scholar]
- 4.Shi G, Field DJ, Ko KA, Ture S, Srivastava K, Levy S, Kowalska MA, Poncz M, Fowell DJ, Morrell CN. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J Clin Invest. 2014;124:543–552. doi: 10.1172/JCI71858. 10.1172/JCI7185871858 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun. 2013;4:2657. doi: 10.1038/ncomms3657. 10.1038/ncomms3657 ncomms3657 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. 10.1182/blood-2013-11-536003 blood-2013-11-536003 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannacone M, Sitia G, Isogawa M, Whitmire JK, Marchese P, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets prevent IFN-alpha/beta-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc Natl Acad Sci U S A. 2008;105:629–634. doi: 10.1073/pnas.0711200105. 10.1073/pnas.0711200105 0711200105 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danon D, Jerushalmy Z, De Vries A. Incorporation of influenza virus in human blood platelets in vitro. Electron microscopical observation. Virology. 1959;9:719–722. doi: 10.1016/0042-6822(59)90168-0. [DOI] [PubMed] [Google Scholar]
- 9.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 10.Noisakran S, Gibbons RV, Songprakhon P, Jairungsri A, Ajariyakhajorn C, Nisalak A, Jarman RG, Malasit P, Chokephaibulkit K, Perng GC. Detection of dengue virus in platelets isolated from dengue patients. Southeast Asian J Trop Med Public Health. 2009;40:253–262. [PubMed] [Google Scholar]
- 11.Negrotto S, de Giusti C, Rivadeneyra L, Ure A, Schattner M, Gomez R. Platelts interact with Coxsckieviruses B and have a critical role in the pathogenesis of virus-induced myocarditis. Journal of Thrombosis and Haemostasis. 2014 doi: 10.1111/jth.12782. [DOI] [PubMed] [Google Scholar]
- 12.Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, McFadden G, Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. 10.1016/j.chom.2013.01.005 S1931-3128(13)00036-X [pii]. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. 10.1016/j.chom.2012.05.015 S1931-3128(12)00201-6 [pii]. [DOI] [PubMed] [Google Scholar]