Abstract
Surfactant Protein D (SP-D) is critical for maintenance of lung homeostasis and provides a first line of defense to pathogens at mucosal surfaces. Polymorphisms in the SP-D-encoding gene SFTPD have been associated with chronic obstructive pulmonary disease and ulcerative colitis. Identification of the immunoreceptors that bind SP-D is essential for understanding its contribution to lung homeostasis and mucosal defense. We located a putative binding motif for the Osteoclast-associated Receptor (OSCAR) within the SP-D collagenous domain. An OSCAR-Fc fusion protein specifically bound to the collagenous region of recombinant SP-D and captured native SP-D from human bronchoalveolar lavage. OSCAR localized in an intracellular compartment of alveolar macrophages together with SP-D. Moreover, we found OSCAR on the surface of interstitial lung and blood CCR2+ inflammatory monocytes, which secreted TNF-α when exposed to SP-D in an OSCAR-dependent fashion. OSCAR and SP-D did not exclusively co-localize in lung as they were also highly expressed in atherosclerotic plaques of human aorta supporting a role for this interaction in atherosclerosis. Our results identify the OSCAR-SP-D interaction as a potential therapeutic target in chronic inflammatory diseases of the lung as well as other diseases involving tissue accumulation of SP-D, infiltration of inflammatory monocytes and release of TNF-α.
INTRODUCTION
Surfactant Protein D (SP-D) is a member of the collagenous lectins (collectins), which provide a first line of humoral innate immune defense (1–3). The collectin family also includes, but is not limited to, Mannan-binding lectin (MBL) and Surfactant Protein A (SP-A). Collectins are soluble proteins, which are structurally characterized by an N-terminal collagenous region, a flexible coiled coil ‘neck’ (N) region and a C-terminal Carbohydrate Recognition Domain (CRD), which binds various sugars in a calcium-dependent fashion (4). The hydrophobic N-terminal region of the SP-D polypeptide encodes two cysteine (Cys) residues (Cys15 and Cys20). Multimeric SP-D dodecamers can be formed through N-terminal disulfide bonding of trimeric SP-D monomers. Within the collectin family the formation of dodecamers is unique to SP-D, which can be observed as the characteristic cruciform structures by electron microscopy (5). The collectin family are expressed in a range of different mucosal tissues where they are thought to play important tissue-specific roles in the innate immune response (4).
SP-D is predominantly secreted by alveolar type II epithelial cells, but is also produced outside of the lung, in the gastrointestinal and genital mucosae, salivary glands, prostate, kidney, pancreas, skin and endothelial cells (6). SP-D can act as a pattern recognition receptor through binding of the CRD to evolutionary conserved glycolipids and glycoproteins associated with infectious agents, such as LPS from certain bacterial species or viral envelope glycoproteins. SP-D can thus opsonize, neutralize and agglutinate infectious microorganisms predisposing to elimination by phagocytes. In the lung, SP-D also plays an important homeostatic role through CRD-dependent scavenging of surfactant phospholipids by alveolar macrophages (7, 8). SP-D deficient (Sftpd−/−) mice developed accumulation of surfactant phospholipids in the lungs, as well as infiltration of monocytes and the pro-inflammatory activation of alveolar macrophages, leading to chronic inflammation, emphysema and fibrosis (9, 10). Correction of some, but not all, of the pulmonary abnormalities in Sftpd−/− mice required the transgenic expression of SP-D with an intact collagenous domain (11). SP-D deficient children were susceptible to more frequent pneumonias and long-term outcome was worse than SP-D sufficient control children (12).
Human SFTPD genotype can influence the assembly, concentration and biological function of SP-D in vivo (13). Interestingly, polymorphisms in SFTPD have been associated with susceptibility to chronic and infectious lung diseases, such as chronic obstructive pulmonary disease (COPD) (14, 15), emphysema (16), pneumococcal lung disease (17), tuberculosis (18) and may even influence clinical outcome following lung transplantation (19). Serum SP-D levels have been associated with lung function or health status in patients with severe COPD (20). SFTPD genotype has also been associated with inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis (21). SP-D is also produced by vascular endothelial cells and has been implicated in lipid homeostasis and vascular lipid deposition. Sftpd−/− mice were protected from diet-induced atherosclerosis (22), whereas a polymorphism of SFTPD has been associated with coronary artery disease (23). These data suggest an important role for SP-D in regulating pulmonary homeostasis in addition to roles in the gut and vascular system, although the molecular basis for this remains ill defined.
Identification of the immunoreceptors that capture SP-D and transduce intracellular signaling is therefore essential for understanding how SP-D contributes to lung homeostasis and innate mucosal defense and SFTPD disease associations (3, 24). OSCAR is an activating receptor for collagen expressed by osteoclasts that co-stimulates osteoclastogenesis (25). OSCAR transmits intracellular signals through the associated adapter FcRγ (26), which contains an Immunoreceptor Tyrosine-based Activation Motif (ITAM) that recruits the protein tyrosine kinase Syk. While in mouse OSCAR is exclusively expressed in osteoclasts, human OSCAR was reported to be also expressed on monocytes, macrophages, neutrophils and dendritic cells (DC) (26) and shown to enhance the pro-inflammatory response of monocytes, although the monocyte subset that specifically expressed OSCAR was not described (26, 27). The wider expression of OSCAR by human myeloid immune cells suggested to us that OSCAR might play a role in innate immunity in addition to the reported role in osteoclastogenesis. Here, we identified SP-D as a candidate ligand for OSCAR by a bioinformatics search for proteins encoding OSCAR-binding motifs (25). We further demonstrated that OSCAR and SP-D colocalize in alveolar macrophages and that the OSCAR:SP-D interaction triggers TNF-α production by inflammatory monocytes.
MATERIALS AND METHODS
Bioinformatics
To initially identify putative OSCAR-binding candidate proteins for further scrutiny, an exhaustive protein BLAST search was carried out using all permutations of the 9 amino acid ‘minimum’ OSCAR-binding motif and side chain binding variants defined previously (25): G[A|P|G]PG[P|A][A|S]G[F|D|S|Y][A|R|P|Q]. In addition, a less stringent but longer 12 amino acid ‘consensus’ OSCAR-binding sequence, derived from the same data and used to generate the minimum OSCAR-binding sequence, was also used: GXPGPX′GFX′GXP (where X is any amino acid). BLAST searches were done with an expect threshold of 20000, a word size of 2 and using the PAM30 matrix, as recommended by NCBI when searching for short, nearly exact matches. Candidate motifs in both collagen and non-collagen proteins were identified. For non-collagen proteins, putative motifs were selected where protein BLAST provided an alignment of 80% or better, with some leeway for the substitution of physicochemically similar amino acids. As an additional crucial criterion, putative motifs were also required to lie within a collagenous domain, as had previously been identified either experimentally or through computational approaches. For collagens, an 8/9 amino acid sequence alignment with one of the minimum OSCAR-binding motif variant sequences was required for a motif to be recognized, although for the vast majority a perfect alignment was obtained. In the non-collagen proteins, the biological roles of each protein could be broadly classified into receptor proteins, secreted proteins, or (extracellular matrix) ECM proteins.
Plasmids
The human OSCAR-Fc construct has been described before (25). The extracellular domain of human Leukocyte Associated Ig-like Receptor (LAIR)-1 was amplified from plasmid DNA containing the human LAIR-1 cDNA with Platinum Pfx DNA Polymerase (Invitrogen) using the following forward 5′-catcctcgagcaggaggaagatctgcccag-3′ and reverse 5′-gaattctagaatgctcagctttcaggccttg-3′ primers (Xho I and Xba I restriction sites underlined). Xho I and Xba I restricted PCR products were cloned into the Signal Ig plus vector, which encoded the LAIR-1 ectodomain in frame with human IgG1 Fc. Cloning and expression of immunoglobulin-like transcript (ILT)-1, 3 and -7 and Triggering Receptor expressed on myelod cells (TREM)-1 and -2 Fc-fusion proteins were as described (28).
Cell culture, expression and purification of recombinant Fc-fusion proteins
293T were transiently transfected with 48μg of plasmid DNA/10cm dish using Lipofectamine 2000 diluted in Optimem (both Invitrogen), according to the manufacturer’s instructions. Fc-fusion proteins were purified from serum-free culture supernatants by Protein A affinity chromatography.
Recombinant collectin and collectin-like molecules
The cloning and expression of recombinant full length human SP-D and the full-length and mutant isoforms of rat SP-D have been described in detail before (5, 29). Briefly, S15, 20 is a recombinant rat SP-D mutant with a substitution of serine for Cys15 and Cys20 that is assembled exclusively as SP-D trimers (29). Mini SP-D is a mutant form of rat SP-D that lacks two internal exons (3 and 4) encoding the SP-D collagenous domain. The Neck Carbohydrate Recognition Domain (NCRD) (trimeric neck + CRD) is a rat SP-D truncation mutant lacking the N-terminal peptide and collagen domains that retains lectin activity (5). Recombinant human Mannan-binding lectin (MBL) was purchased from Sino Biological Inc. (Beijing, China) and Complement component 1q (C1q) purified from human serum was purchased from Complement Technology Inc. (Texas, USA). The endotoxin level in our stock (112μg/ml) human SP-D reagent was determined at 120EU/ml by Limulus amebocyte lysate (LAL) assay (Lonza).
Immobilization of antibodies, proteins and peptides
Collagen I from rat tails and BSA were purchased from Sigma. BSA (2%), Collagens (2.5μg/ml), recombinant SP-D and isoforms (2.5μg/ml) were immobilized onto either tissue culture or ELISA plates overnight in 0.01M Acetic acid at 4°C. All peptides were certified LPS-free by LAL assay (Lonza). For soluble-phase SP-D binding asays, 0.25μg/ml of each Fc-fusion protein was immobilized to plates in 100μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) overnight at 4°C. All wells were washed 3 times in TBS to remove excess proteins before blocking in 5% BSA in TBS for 1 hour at 37°C prior to performing binding assays.
Solid- and soluble-phase SP-D binding assays
For solid-phase SP-D binding assays, 5μg/ml of each Fc-fusion protein in 100μl of TBS binding buffer: 10mM Tris.HCl pH7.5, 150mM NaCl + 0.1% BSA + 0.05% Tween-20 ± 5mM CaCl2 (TBS ± 5mM Ca2+) were incubated at room temperature for 1 hour in ELISA plates. Wells were then washed five times in TBS ± 5mM Ca2+ before incubating with 1:5000 goat anti-human Ig-HRP conjugate (Southern Biotech) for 1 hour in TBS ± 5mM Ca2+. Wells were then washed a further five times in TBS ± 5mM Ca2+ before developing with O-Phenylenediamine dihydrochloride and the absorbance at 490nM recorded. For soluble-phase SP-D binding assays, two-fold dilutions of either recombinant human SP-D dodecamer starting 125ng/ml or human bronchoalveolar lavage (BAL) (starting at ¼ dilution) were made in TBS and incubated in Fc-fusion coated wells for 3h. Captured SP-D was detected using 2μg/ml of the anti-human SP-D mAb (Hyb-246-04) for 1 hour at room temperature, followed by rabbit anti-mouse IgG-HRP (Dako).
Generation and purification of monoclonal antibodies
To generate blocking mAbs to the OSCAR ectodomain, NMRI mice were immunized subcutaneously three times with 20 μg of human OSCAR-Fc. For generation of mAbs to the OSCAR cytoplasmic tail, mice were immunized with a synthetic peptide, representing the last 14 C-terminal residues (Cys-DWRSQNRAPAGIRP) of human OSCAR. The synthetic peptides were coupled to diphtheria toxoid via the cysteine using amine-to-sulfhydryl crosslinker (Thermo Scientific). Three days prior to the fusion the mice received an intravenous injection with 50 μg of immunogen administered together with adrenalin. The fusion and selection were done essentially as described (30). The SP2/0-AG14 myeloma cell line was used as fusion partner. Positive clones for the OSCAR ectodomain were selected by differential screening of OSCAR-Fc versus an irrelevant Fc-fusion by ELISA. Positive clones for the OSCAR C-terminal cytoplasmic tail were identified by screening to the peptide used for immunization, described above. Cloning was performed by limited dilution. Single clones were grown in culture flasks in RPMI + 10% FCS and mAbs were purified from culture supernatant by Protein A affinity chromatography.
Antibodies
Goat anti-human OSCAR polyclonal antibody was purchased from Santa Cruz Biotechnologies (25). The SP-D mAb 245-01 was purchased from Enzo Life Sciences. The anti-SP-D mAb 246-04 was purchased from Bioporto Diagnostics, Gentofte, Denmark. The mAb KP1, which recognizes human CD68, was purchased from Serotec. Donkey anti-goat IgG Alexa 568 and donkey anti-mouse IgG Alexa 488 were purchased from Invitrogen. Purified and PE-conjugated anti-human OSCAR mAb 11.1CN5 was purchased from Becton Coulter. The HLA-DR-APC, CD16-FITC and CD56-PE-Cy7 anti-human antibodies, used to define human monocytes, granulocytes and Natural Killer cells, respectively, were purchased from BD Pharmingen and the CD14-Pacific Blue, CD45-AF700, CCR2-PerCP-Cy5.5 anti-human antibodies were all purchased from BioLegend. Mouse mAb to thyroid transcription factor 1 (TTF1), clone SPT24, was from Novocastra Laboratories Ltd. Mouse mAb to human CD163, which recognizes cells of the monocyte/macrophage lineage, was from Neomarkers. MAb clone 13-9-17 was used in immunostainings for the OSCAR C-terminus. Rabbit anti SP-D clone H-120 was from Santa Cruz. Rabbit isotype control antibody was from Cell Signaling and mouse IgG1 isotype control was from BD Biosciences. For immunohistochemistry, primary antibodies were revealed with Envision Dako Polymer HRP.
Microscopy
Paraffin embedded human lung sections from COPD and normal control subjects were deparaffinized using citrisolve (Vector laboratories). Sections were then rehydrated in a graded ethanol series before antigen retrieval by boiling in antigen retrieval solution (Vector labs) in a microwave, according to the manufacturer’s instructions. Sections were blocked in fish gel (Sigma) for 1 hour before incubating with primary antibodies diluted in fish gel overnight at 4°C. Slides were then washed three times in large volumes of PBS before incubating individually with secondary antibodies, respectively. Tiramide enhancement of fluorescent immunostaining was performed after each individual secondary antibody step, according to the manufacturer’s instructions (Invitrogen). For confocal microscopy, lung sections were mounted with vectashield containing DAPI, which stained nuclei blue (Vector labs), and analyzed on an Olympus Fluoview™ FV1000 confocal microscope. For light microscopy, sections stained immunohistochemically were photographed using the DP-70 Olympus digital camera mounted on the Olympus BX60 microscope.
Human subjects and BAL isolation
Human clinical samples were obtained from lung transplant donors and recipients, as described previously (31). In brief, lung explants were harvested at the time of lung transplantation from COPD recipients (n=2) with very severe disease (GOLD Stage 4). Control samples were obtained from normal donors (n=3) using lung explants that were otherwise not useable for transplantation. BAL was performed under general anesthesia using standard procedures: briefly, the bronchoscope was placed in the right middle lobe and the lavage was performed using 3 × 50 ml sterile saline and aspirated under low pressure. The concentration of SP-D in BAL was determined at 3495ng/ml, as previously described (32).
Flow cytometry
Cells were prepared from human lung explant tissue using protease digestion as described previously (31). Single cell suspensions were generated from minced lung tissue that was subjected to collagenase (Liberase Blendzyme III, Roche), hyaluronidase (Sigma), and DNAse I (grade II, Roche) digestion for 45 min at 37 °C. For flow cytometry, frozen aliquots of cell preparations were thawed and washed in complete medium before analysis. Following FcR blockade, cell suspensions were incubated with labeled antibodies on ice prior to flow cytometric analysis. For peripheral blood leukocytes, granulocytes were defined by electronic gating as SSCHiCD45+CD56−CD16+ cells and monocytes as SSCLoCD45+HLA-DR+CD56−. Monocytes were further distinguished based on expression of CD14, CD16 and CCR2 (27).
OSCAR internalization assays
Purified monocytes were left untreated (0 mins) or treated with 2.5ug/ml recombinant human SP-D at 37°C for 5, 15, 30 and 60 mins, respectively. Monocytes were then placed on ice and washed twice with ice cold PBS before fixing with 2% paraformaldehyde for 10 mins on ice. After washing 3 times in PBS, OSCAR internalization was determined by flow cytometry as a reduction in monocyte cell surface staining with non-blocking anti-OSCAR mAb, 11.1CN5.
Monocyte isolation
Human monocytes were isolated from 50 ml of blood using the Monocyte Negative Isolation Kit (Invitrogen).
Cytokine analysis
100,000 monocytes in a flat-bottomed 96-well plates were stimulated with 2.5μg/ml recombinant hSP-D in the presence or absence of 5μg/ml purified mAbs, TNF-α in tissue culture supernatants was determined using the BD™ CBA human inflammatory cytokine kit (Becton Dickinson).
Statistical analysis
Statistical significance was determined using GraphPad Prism 6.0. Statistical differences were determined by 2-tailed Student’s t test. A P value of <0.05 was considered significant.
Study approval
All studies were reviewed and approved by institutional human and animal studies committees and all experiments were carried out according to national and regional legislation and regulations. Informed consent was obtained from all subjects included in this study.
RESULTS
OSCAR is a receptor for SP-D
We hypothesized that OSCAR may interact with collagenous molecules other than ECM collagens, which may have immunological roles. We conducted a bioinformatics search using two partially overlapping linear amino-acid sequences (see Materials and Methods) derived from the previously defined triple-helical OSCAR-binding motif (25). The search yielded twenty-two non-collagen based sequences (Table I) and sixteen putative collagen based OSCAR-binding motifs (Supplemental Table I). The collectins SP-A, SP-D and MBL, and collectin-like molecules, such as C1q (Table I), represented good candidates for interacting with OSCAR-expressing leukocytes because they play central roles in tissue-specific immunity (1). Focusing on SP-D, the ‘minimum’ 9 amino acid OSCAR-binding motif used in the bioinformatics search aligned with 77.8% direct amino-acid sequence identity to the SP-D sequence 106–114 (GPPGPPGVP) (Supplemental Fig. 1A). The 12 amino acid ‘consensus’ OSCAR-binding motif used in the bioinformatics search aligned with 83.3% direct amino acid identity with the SP-D sequence 106–117 (GPPGPPGVPGPA) (Supplemental Fig. 1B). The putative OSCAR-binding-motifs are encoded by exon 3 of human SFTPD, which encodes part of the SP-D collagenous domain (Supplemental Fig. 1A). Since the 9 and 12 amino acid search motifs are largely overlapping, we conclude that each trimeric sub-unit of the SP-D monomer encodes one triple-helical OSCAR-binding sequence. Dodecameric SP-D, comprised of four trimeric sub-units, would thus be expected to encode four triple-helical OSCAR-binding motifs.
Table I.
Amino-acid sequences and start positions of putative OSCAR-binding motifs within collagenous proteins (non-collagens).
| Biological class | Protein Name | Uniprot | Sequence | Position | Notes |
|---|---|---|---|---|---|
| ECM and adhesion | EMILIN-1 | Q9Y6C2 | GPPGPPGLQGPP GPPGPAGPPGSP |
820 829 |
Secreted adhesive protein, involved in elastic fiber anchoring |
| EMILIN-2 | Q9BXX0 | TVPGAEGFAGAP | 876 | Secreted adhesive protein, involved in elastic fiber anchoring/vessel assembly | |
| Protein sidekick-2 | Q58EX2 | GAPGPPGVP | 1823 | Adhesion protein (neuronal development) | |
|
| |||||
| Receptors | Collectin-12 (Scavenger receptor with C-type lectin type I) | Q5KU26 | GPPGPAGER GVPGPRGLPGLP |
476 557 |
Pathogen recognition/phagocytosis |
| Macrophage receptor with collagenous structure (MARCO) | Q9UEW3 | GPPGLAGFP | 286 | Pattern recognition receptor – binds bacteria | |
| Scavenger receptor class A member 3 (SCARA3) | Q6AZY7 | GAPGPPGPR | 595 | Scavenges oxidative species | |
| Scavenger receptor class A member 5 (SCARA5) | Q6ZMJ2 | GPPGPKGDQ | 315 | Ferritin receptor - non transferrin-dependent delivery of iron | |
|
| |||||
| Secreted | C1q-related factor | O75973 | GPPGPPGDP | 79 | Implicated in excitatory synapse regulation on hippocampus neurons |
| Cell proliferation-inducing protein 41 | A1KY36 | GLPGPPGFPGIG | 523 | - | |
| Collagen and calcium-binding EGF domain-containing protein 1 | Q6UXH8 | GAPGPRGSPGPP | 321 | Angiogenic and lymphangioblastic growth during embryogenesis | |
| Complement C1q-like protein 4 | Q86Z23 | GPPGPPGPRGPP | 80 | Implicated in excitatory synapse regulation on hippocampus neurons | |
| Complement C1q subcomponent subunit B | P02746 | GGPGAPGAP GAPGAPGPK |
99 102 |
Part of the complement cascade | |
| Complement C1q subcomponent subunit C | P02747 | GVPGPMGFPGEP | 97 | Part of the complement cascade | |
| Complement C1q tumor necrosis factor-related protein 2 | Q9BXJ5 | GAPGPSGMMGRM GPPGRTGNRGKP |
49 82 |
- | |
| Ectodysplasin-A (EDA) | Q92838 | GPPGPPGPQGPP | 189 | Epithelial-mesenchymal signaling during morphogenesis | |
| Ficolin-2 | Q15485 | GPPGPNGAP | 84 | Liver secreted, innate immunity protein | |
| Ficolin-3 | O75636 | GAPGPQGPP | 57 | May activate lectin complement pathway | |
| Mannose-binding protein C | P11226 | GNPGPSGSPGPK | 80 | Innate immune calcium-dependent lectin | |
| Surfactant Protein A | Q8IWL2 | GPPGPMGPP | 55 | Collectin, found in the lungs, many roles | |
| Surfactant protein D | P35247 | GPPGPPGVP GPPGPPGVPGPA |
106 106 |
Collectin, found in the lungs, many roles | |
|
| |||||
| Other | Gliomedin | Q6ZMI3 | GPPGPPGPPGPP | 236 | Membrane protein involved in formation of the nodes of Ranvier |
| pre-mRNA 3′ end processing protein WDR33 | Q9C0J8 | GPPGPRGMQGPP | 742 | Cleavage and polyadenylation of pre-mRNA 3′ ends (nuclear protein) | |
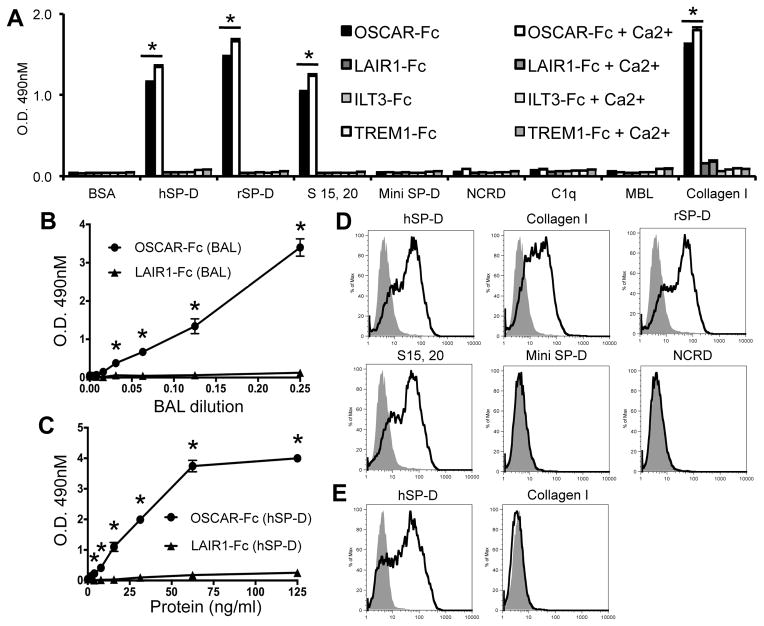
We next determined whether OSCAR could bind to some of the collectin or collectin-like molecules identified from the motif search (Table I). We assayed for binding of a human OSCAR Fc-fusion protein (OSCAR-Fc) to recombinant forms of SP-D, in addition to C1q and MBL proteins (Fig. 1). OSCAR-Fc bound to full-length dodecameric human and rat SP-D and trimeric sub-units of rat SP-D (Fig. 1A). OSCAR-Fc did not bind to ‘Mini SP-D’, a recombinant rat SP-D lacking the SP-D collagenous domain encoded by exons 2 and 3 of the SP-D gene (5), or a recombinant protein encoding the Neck-CRD (NCRD) region of rat SP-D (Fig. 1A). OSCAR-Fc binding was specific for SP-D since OSCAR-Fc did not bind to either C1q or MBL (Fig. 1A), even though these proteins were predicted to encode putative OSCAR-binding motifs (Table I). OSCAR-Fc binding to full-length dodecameric or trimeric SP-D or collagen I was significantly increased by, but not wholly dependent on, the presence of 5 mM Ca2+ (Fig. 1A). Since collagen lacks lectin activity and neither recombinant lectin domains (NCRD) nor ‘Mini SP-D’, which encodes an intact lectin domain, bind to OSCAR (Fig. 1A), we conclude the small increase in calcium-dependent binding of OSCAR-Fc to SP-D or collagen must be mediated through OSCAR binding to collagen domains and not the SP-D lectin domain. SP-D binding was not observed for Fc-fusion proteins that encoded the extracellular domains of human LAIR1 or ILT3, which are known to bind collagen (33, 34), or TREM1, TREM2, ILT1 and ILT7 (Fig. 1A and data not shown). Although SP-D was recently shown to bind LAIR1 (35), we only detected SP-D binding to mouse LAIR1 but not human LAIR1 (data not shown). This discrepancy may depend on the sensitivity of our assay and the relative affinities of mouse and human LAIR1 for SP-D. In conclusion our results show that OSCAR can bind specifically to the SP-D collagenous domain.
FIGURE 1.
OSCAR is a novel SP-D receptor. (A) OSCAR-Fc, LAIR1-Fc, ILT3-Fc and TREM1-Fc binding to different human (h) and rat (r) SP-D isoforms and collagen I (± 5mM Ca2+) in solid-phase (*, P <0.05). (B) Capture of Hyb-246-04 mAb reactive protein from human BAL (n=1 donor) or (C) soluble recombinant hSP-D dodecamer by plate-immobilized OSCAR-Fc (circles) or LAIR1-Fc (triangles) (*, P <0.05). (D) GFP expression from OSCAR-CD3ζ NFAT-GFP reporter cells incubated with recombinant SP-D isoforms in solid- or (E) soluble-phase. Data were performed in triplicate and are representative of three independent experiments.
Since SP-D is secreted as a soluble protein associated with lung surfactant, we next determined whether OSCAR could bind to soluble SP-D, as might reflect conditions in vivo. We incubated dilutions of human BAL (Fig. 1B) in wells coated with OSCAR-Fc or LAIR1-Fc. The amount of protein captured by the immobilized Fc-fusion proteins was then detected using the Hyb-246-04 mAb, which is specific for the SP-D CRD (6). Dose-dependent binding of a protein reactive with the Hyb-246-04 mAb was observed to immobilized OSCAR-Fc, but not to LAIR1-Fc (Fig. 1B). These results suggest OSCAR-Fc can capture native SP-D from human BAL. However, since we have detected a putative OSCAR-binding motif in SP-A (Table 1) and SP-A has been shown to bind to immunoglobulins (36), it may be possible that solid-phase OSCAR-Fc could also be capturing SP-A from BAL. Captured SP-A may, in turn, bind to the Fc portion of the secondary antibodies used to detect bound SP-D. We therefore determined whether immobilized OSCAR-Fc could capture soluble recombinant human SP-D dodecamer in the absence of SP-A. Solid-phase OSCAR-Fc, nut not LAIR1-Fc, captured soluble recombinant human SP-D dodecamer (Fig. 1C). These results show that OSCAR can bind to soluble SP-D dodecamer.
To test whether OSCAR could act as a receptor for SP-D, we performed a sensitive GFP-inducible assay using NFAT-GFP reporter cells, which have been successfully used to determine immunoreceptor:ligand interactions (37) (Fig. 1D). In this assay, clustering of the OSCAR ectodomain fused to the T cell receptor CD3ζ signaling chain will result in ITAM signaling and transactivation of a transgene encoding GFP (25, 37). GFP was induced by OSCAR-CD3ζ/NFAT-GFP reporter cells cultured on tissue culture plates coated with human SP-D, collagen I, recombinant rat dodecameric or trimeric (S15, 20) SP-D, but not BSA, Mini SP-D or NCRD (Fig. 1D). OSCAR-CD3ζ/NFAT-GFP reporter cells also expressed GFP when cultured with soluble human SP-D dodecamer, but not soluble collagen I (Fig. 1E). These results show that OSCAR is a receptor for dodecameric SP-D either as a plate-bound ‘aggregated’ ligand or as soluble SP-D dodecamer.
OSCAR is expressed in intracellular compartments of human alveolar macrophages that contain SP-D
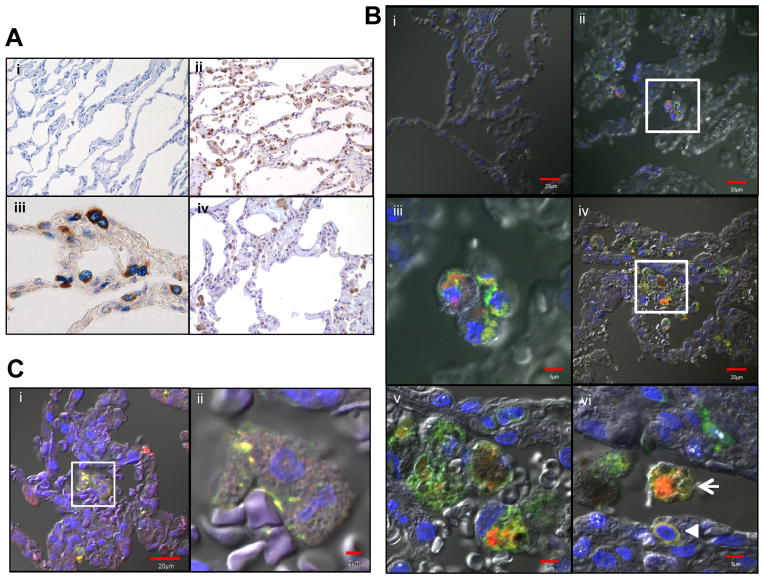
Human OSCAR has previously been located by immunohistochemistry in osteoclasts and their precursors but not in monocytes or macrophages in tissues outside of bone (25). Since SP-D is secreted in lung surfactant where it is readily taken up by alveolar macrophages and monocytes exhibit greater infiltration of inflamed lung airways (38), we next determined OSCAR and SP-D expression in lungs from control and COPD subjects. OSCAR was expressed in alveolar macrophages with a seemingly intracellular granular pattern, whereas SP-D was predominantly expressed in alveolar type II epithelial cells (Fig. 2A). Immunofluorescence analysis for OSCAR and the monocyte/macrophage marker CD68 confirmed the localization of OSCAR in intracellular compartments of CD68+ alveolar macrophages in both normal and COPD lung sections (Fig. 2B). A few infiltrating CD68+ interstitial monocytic cells displayed a membrane pattern of OSCAR staining in COPD sections (Fig. 2B). Since immunohistochemical and immunocytochemical electron microscopy studies had found SP-D localized to endocytic compartments and associated with phagolysosomal structures in human and rat alveolar macrophages (6, 39), we hypothesized that OSCAR and SP-D may co-localize in the same intracellular compartments of alveolar macrophages. Accordingly, double immunofluorescence for OSCAR and SP-D in lung sections demonstrated intracellular co-localization of OSCAR and SP-D in alveolar macrophages (Fig. 2C). These data show that OSCAR interacts with SP-D in alveolar macrophages and OSCAR is expressed by tissue infiltrating monocytes in COPD.
FIGURE 2.
OSCAR expression in lung resident immune cells. (A) Representative light microscopy sections of normal lung immunostained with: (i) rabbit (brown) and mouse IgG1 (blue; x200 objective) control antibodies; (ii) rabbit anti-SP-D (brown; x200 objective); (iii) rabbit anti-SP-D and TTF1 mAb (blue; x600 objective); or (iv) OSCAR C-terminus mAb (brown; x200 objective). (B) Representative confocal sections of normal (i–iii) or COPD (iv–vi) lungs immunostained with: (i) goat (red) and mouse IgG2a (green) control antibodies; (ii–vi) goat anti-OSCAR (red) and CD68 mAb (green; co-localization, yellow), Bar=20μM; (iii & v) Higher magnification confocal microscopy of areas demarcated by white squares in (ii) and (iv), respectively, Bars=5μM. (vi) Alveolar macrophage (arrow) and smaller interstitial lung monocyte (arrowhead), Bar=5μM. (C) Representative confocal sections of normal lungs immunostained with: (i) SP-D mAb 245-01 (green) and goat anti-OSCAR (red), Bar=20μM. (ii) Higher magnification confocal microscopy of area demarcated by white square in (i), Bar=2μM.
Lung interstitial myeloid cells and blood monocytes express OSCAR on the cell surface
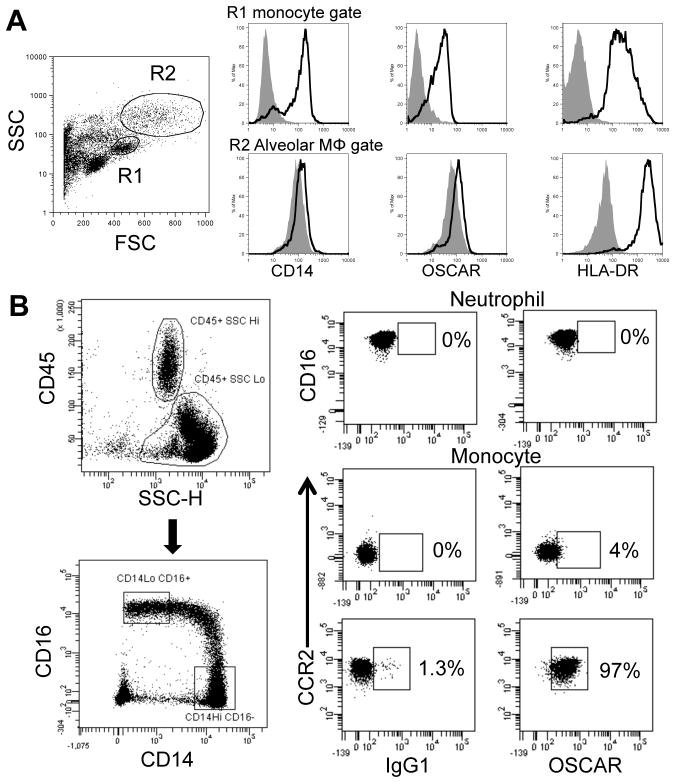
Immunofluorescence analysis of lung tissues showed that OSCAR was expressed not only in alveolar macrophages but also in few CD68+ interstitial myeloid cells (Fig. 2B). Interestingly, in these cells OSCAR displayed a membrane pattern. Flow cytometric analysis revealed a marked OSCAR surface expression also on monocytes (Fig. 3A, upper histograms), whereas alveolar macrophages showed poor OSCAR surface expression, consistent with a predominant intracellular distribution (Fig. 3A, lower histograms). Although OSCAR had been previously found on blood monocytes and neutrophils (26), it was unclear whether it was expressed on a specialized monocyte subset. A recent mRNA profiling of human monocyte subsets suggested that OSCAR maybe expressed on the CCR2+ ‘inflammatory’ monocytes but not CCR2− ‘patrolling’ monocytes (40). We conclusively demonstrated that OSCAR was preferentially expressed on CCR2+ ‘inflammatory’ monocytes by flow cytometry (Fig. 3B). In contrast to a previous report using the anti-human OSCAR mAb 11.1CN5 (26), we did not detect OSCAR staining on neutrophils (Fig. 3B). We conclude that OSCAR has two distinct patterns of expression. In alveolar macrophages OSCAR is predominantly found in an intracellular compartment; in lung interstitial myeloid cells and blood inflammatory monocytes OSCAR is mainly expressed on the cell surface.
FIGURE 3.
Lung and blood monocytes express cell surface OSCAR. (A) Representative histogram plots for lung cells triple-stained with mAbs to CD14, OSCAR and HLA-DR (open histograms) compared to isotype control mAb (gray histograms) for low (Lo) Forward Scatter (FSC)/side scatter (SSC) lung tissue monocytes (Gate R1, upper histogram panels) or high (Hi) FSC/SSC ‘alveolar macrophages’ (Gate R2, lower histogram panels). (B) Representative plots for OSCAR immunostaining of peripheral blood SSCHiCD45+CD56−CD16+ granulocytes (upper panels). Within the SSCLoCD45+CD56−HLA-DR+ monocytic population, CCR2+CD14HiCD16− ‘inflammatory’ monocytes express OSCAR (bottom panels) but not CCR2−CD14LoCD16Hi ‘non-inflammatory’ monocytes (center panels) (n=3 donors).
OSCAR stimulates the secretion of TNF-α by CCR2+ inflammatory monocytes
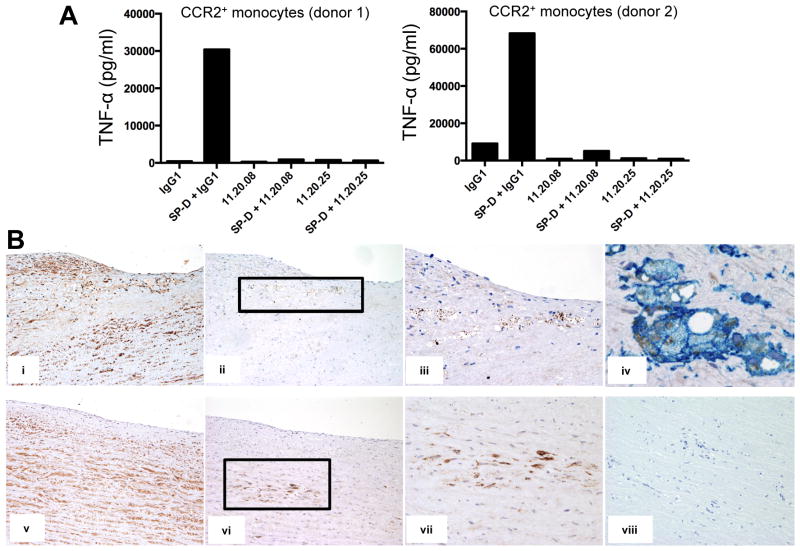
To study the impact of OSCAR in monocyte functions, we immunized mice with our human OSCAR-Fc and generated a panel of novel mAbs to human OSCAR (Supplemental Fig. 2). Amongst several mAb that stained OSCAR-CD3ζ/NFAT-GFP reporter cells (Supplemental Fig. 2A) and human blood monocytes (Supplemental Fig. 2B), we selected mAb that could block binding of OSCAR-Fc to collagen I (Supplemental Fig. 3A). Using this approach, we identified four blocking mAbs (11.20.02, 11.20.06, 11.20.08 and 11.20.25), which were subsequently purified and titrated based on their ability to block OSCAR-CD3ζ/NFAT-GFP reporter cell signaling on plates coated with either collagen I (Supplemental Fig. 3B), rat SP-D (Supplemental Fig. 3C) or human SP-D (Supplemental Fig. 3D). Two mAbs, 11.20.08 and 11.20.25, were most effective in blocking GFP expression in reporter cells with peak blocking activities observed at 5μg/ml. We next determined whether SP-D could stimulate the release of pro-inflammatory cytokines by human blood CCR2+ monocytes through engagement of OSCAR. A recombinant human SP-D dodecamer stimulated TNF-α release by blood CCR2+ monocytes from two different donors, which was blocked by the anti-human OSCAR mAbs 11.20.08 and 11.20.25, respectively (Fig. 4A). OSCAR was not internalized by monocytes exposed to SP-D (Supplemental Fig. 3E). We conclude the SP-D/OSCAR interaction triggers a pro-inflammatory response in CCR2+ monocytes.
FIGURE 4.
SP-D functionally interacts with OSCAR. (A) TNF-α release by human CCR2+ monocytes from two different donors treated with soluble SP-D in the presence of either IgG1 or anti-OSCAR blocking mAbs, 11.20.08 or 11.20.25. (B) Representative sections of atherosclerotic lesions (tunica intima, i–iv; tunica media, v–viii) from human aorta immunostained with: (i & v) rabbit anti-SP-D (brown; x100 objective); (ii & vi) anti-OSCAR C-terminal mAb 13-9-17 (brown; x100 objective); (iii & vii) x200 objective of areas demarcated by black rectangles in (ii) and (vi), respectively; (iv) OSCAR C-terminal mAb 13-9-17 (brown) and anti-CD163 mAb (blue, x600 objective); (viii) rabbit (brown) and mouse IgG1 (blue) control antibodies (x200 objective).
OSCAR is expressed in lipid-laden macrophages infiltrating atherosclerotic plaques
Although SP-D has been implicated in atherosclerosis (22, 23), the mechanism is poorly understood. We hypothesized that SP-D may engage OSCAR in the atherosclerotic plaque, promoting inflammation. To test this hypothesis, we investigated OSCAR expression in atherosclerotic lesions of human aorta. OSCAR was strongly expressed in numerous lipid-laden macrophages infiltrating the tunica intima and in fewer macrophages penetrating the tunica media. SP-D was found in the vascular endothelium as well as in many smooth muscular cells of the tunica media, consistent with previous reports (22, 41) (Fig. 4B). These data suggest that SP-D may contribute to atherosclerosis by binding OSCAR on inflammatory monocytes that infiltrate the atherosclerotic plaque and stimulating the release of TNF-α, which will exacerbate the vascular lesion.
DISCUSSION
In humans and mice, OSCAR is expressed by osteoclasts and their precursors and can bind to collagens exposed on bone surfaces to co-stimulate osteoclastogenesis (25). In humans, OSCAR is additionally expressed on monocytes, macrophages and DC (26), suggesting OSCAR may play a role in the immune response. Motivated by this hypothesis, we designed a bioinformatics search for OSCAR-binding motifs in collagenous molecules that might provide insight into OSCAR immunobiology. We identified putative OSCAR-binding motifs in the collectins, MBL, SP-A and SP-D, and collectin-like molecules, such as C1q, and ficolins, that all play important roles in innate immunity (1). Of the putative ligands tested, we detected binding of OSCAR-Fc to SP-D but not to MBL or C1q. OSCAR may not bind to C1q because we only detected OSCAR-binding motifs in the B and C chains of C1q and none in the A chain (Table 1). Since OSCAR only binds to triple-helical motifs in collagenous molecules and not to linear motifs (25), C1q therefore lacks a motif in the A chain to form a triple-helical sequence capable of binding OSCAR. Further structural and biophysical studies are required to determine the precise molecular binding specificities of OSCAR for collectin and collectin-like molecules.
Our study demonstrates that human OSCAR is a novel myeloid immunoreceptor for SP-D expressed by alveolar macrophages and inflammatory CCR2+ monocytes, which triggers the release of TNF-α in inflammatory CCR2+ monocytes exposed to SP-D. The identification of SP-D receptors is essential to fully understand the function of SP-D in different tissues and organs in the context of infections and inflammation. Previous studies have shown that SP-D or the closely related collectin SP-A can interact with distinct immunoreceptors resulting in different functional outcomes. When not bound to a pathogen, SP-D and SP-A bind through their lectin domains to SIRP-α, delivering an inhibitory signal, which prevents activation of mononuclear phagocytes and secretion of inflammatory cytokines (24). In contrast, when microrganisms or cell debris are bound to the C-type lectin domain, SP-D and SP-A interact through their free collagen-like domain to CD91/calreticulin, promoting cell activation (24). SP-D also binds to CD14, interfering with the binding of LPS to the CD14-TLR4-MD2 complex, thereby reducing macrophage inflammatory responses elicited by LPS (42, 43). Finally, a recent study showed that the Ig-superfamily receptor LAIR1, which is known to bind collagen, also binds to SP-D, delivering an inhibitory signal that reduces inflammation (35). Since the OSCAR-SP-D interaction promotes activation of inflammatory monocytes, we conclude that the ultimate functional effect of SP-D depends on the balance between inhibitory and activating receptors engaged at any one time in a given tissue.
Our study also demonstrates that in alveolar macrophages OSCAR is predominantly found in an intracellular compartment where it colocalizes with SP-D. This result suggests that OSCAR may capture SP-D in the alveolar space and drive SP-D internalization into a phagolysosomal compartment in alveolar macrophages where SP-D and its cargo are processed and degraded. The internalization of SP-D may be crucial for phagocytosis and killing of pathogens as well as removal of necrotic and apoptotic cells in the lung as well as in various mucosae and organs (2, 44). However, we did not detect any OSCAR internalization in monocytes exposed to SP-D. Since macrophages are more phagocytic than monocytes, it is possible alveolar macrophages are capable of internalizing OSCAR:SP-D complexes. The distribution of OSCAR between the cell membrane and the endosomal compartment in different myeloid cells might also depend on the relative abundance of SP-D to which these cells are exposed in their microenvironment. Alternatively, the contrasting sub-cellular localizations of OSCAR in alveolar macrophages and monocytes could be caused by the alternative splicing of OSCAR transcripts encoding OSCAR isoforms with different sub-cellular trafficking properties (45). Future studies to determine the role, if any, OSCAR isoforms may play in the cell biology and antigen processing of SP-D for alveolar macrophages and dendritic cells are therefore merited.
Previous studies have implicated SP-D in atherosclerosis and coronary heart disease (22, 23, 41). We found that OSCAR is highly expressed in CD163+ monocytes and macrophages infiltrating the atherosclerotic plaques in aorta. We envision that exposure of these OSCAR+ monocytes and macrophages to SP-D produced by endothelial cells in the tunica intima and by smooth muscular cells in the tunica media may lead to production of TNF-α, exacerbating the degenerative process. Similar mechanisms may operate in lung tissue. For example, monocytes can traffic into the lung during the steady state (46) but do not preferentially enter airway mucosal surfaces unless there is a pro-inflammatory or chemotactic stimulus (38). Therefore, CCR2+ monocytes that patrol lung interstitial tissues in the steady state may not become exposed to SP-D secreted in airway lung surfactant. In contrast, monocytes increasingly infiltrate the airways in response to a pro-inflammatory or chemotactic stimuli (38). Thus, upon tissue damage and/or pro-inflammatory stimuli, as would be expected to be operating in pathologies, such as COPD or atherosclerosis, CCR2+ monocytes will traffic from blood to the damaged tissues whereupon they may then become exposed to SP-D and release TNF-α. Thus, our characterization of the OSCAR-SP-D interaction and its functional impact in the secretion of pro-inflammatory cytokines by CCR2+ monocytes recruited to the tissues may have important therapeutic implications not only for inflammatory diseases of the lung and the mucosae but also for atherosclerosis and its complications.
Supplementary Material
Acknowledgments
This work was supported by NIH grant 5R01HL097805 to Marco Colonna and a Marie Curie International Outgoing Fellowship awarded to A.D.B.
We thank Dr Maria G. Quisgaard for kindly providing BAL fluid and Loralyn Benoit, Stoyan Ivanov and Hani Suleiman for scientific discussions and technical assistance.
Abbreviations used in this article
- BAL
Bronchoalveolar lavage
- CRD
Carbohydrate recognition domain
- COPD
Chronic Obstructive Pulmonary Disease
- C1q
Complement component 1q
- Cys
Cysteine
- ECM
extracellular matrix
- Hi
High
- ILT
Immunoglobulin-like Transcript
- LAIR
Leukocyte-Associated Ig-like Receptor
- Lo
Low
- MBL
Mannan-binding lectin
- N
Neck
- OSCAR
Osteoclast-associated receptor
- SP-A
Surfactant Protein A
- SP-D
Surfactant Protein D
- TREM
Triggering Receptor Expressed on Myeloid cells
Footnotes
DISCLOSURES
A.D.B. is a named inventor on a patent application from the University of Cambridge: Modulation of the Activity and Differentiation of Cells Expressing the Osteoclast-associated Receptor, United States patent application number 13122637.
References
- 1.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 2.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 3.Jakel A, Qaseem AS, Kishore U, Sim RB. Ligands and receptors of lung surfactant proteins SP-A and SP-D. Front Biosci Landmark Ed. 2013;18:1129–1140. doi: 10.2741/4168. [DOI] [PubMed] [Google Scholar]
- 4.Seaton BA, Crouch EC, McCormack FX, Head JF, Hartshorn KL, Mendelsohn R. Review: Structural determinants of pattern recognition by lung collectins. Innate Immun. 2010;16:143–150. doi: 10.1177/1753425910368716. [DOI] [PubMed] [Google Scholar]
- 5.White M, Kingma P, Tecle T, Kacak N, Linders B, Heuser J, Crouch E, Hartshorn K. Multimerization of surfactant protein D, but not its collagen domain, is required for antiviral and opsonic activities related to influenza virus. J Immunol Baltim Md 1950. 2008;181:7936–7943. doi: 10.4049/jimmunol.181.11.7936. [DOI] [PubMed] [Google Scholar]
- 6.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol Baltim Md 1950. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 7.Ogasawara Y, Kuroki Y, Akino T. Pulmonary surfactant protein D specifically binds to phosphatidylinositol. J Biol Chem. 1992;267:21244–21249. [PubMed] [Google Scholar]
- 8.Persson AV, Gibbons BJ, Shoemaker JD, Moxley MA, Longmore WJ. The major glycolipid recognized by SP-D in surfactant is phosphatidylinositol. Biochemistry (Mosc) 1992;31:12183–12189. doi: 10.1021/bi00163a030. [DOI] [PubMed] [Google Scholar]
- 9.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci U S A. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikegami M, Na C-L, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L552–561. doi: 10.1152/ajplung.00142.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kingma PS, Zhang L, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. Correction of pulmonary abnormalities in Sftpd−/− mice requires the collagenous domain of surfactant protein D. J Biol Chem. 2006;281:24496–24505. doi: 10.1074/jbc.M600651200. [DOI] [PubMed] [Google Scholar]
- 12.Griese M, Steinecker M, Schumacher S, Braun A, Lohse P, Heinrich S. Children with absent surfactant protein D in bronchoalveolar lavage have more frequently pneumonia. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2008;19:639–647. doi: 10.1111/j.1399-3038.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 13.Leth-Larsen R, Garred P, Jensenius H, Meschi J, Hartshorn K, Madsen J, Tornoe I, Madsen HO, Sørensen G, Crouch E, Holmskov U. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. J Immunol Baltim Md 1950. 2005;174:1532–1538. doi: 10.4049/jimmunol.174.3.1532. [DOI] [PubMed] [Google Scholar]
- 14.Van Diemen CC, Postma DS, Aulchenko YS, Snijders PJLM, Oostra BA, van Duijn CM, Boezen HM. Novel strategy to identify genetic risk factors for COPD severity: a genetic isolate. Eur Respir J. 2010;35:768–775. doi: 10.1183/09031936.00054408. [DOI] [PubMed] [Google Scholar]
- 15.Foreman MG, Kong X, DeMeo DL, Pillai SG, Hersh CP, Bakke P, Gulsvik A, Lomas DA, Litonjua AA, Shapiro SD, Tal-Singer R, Silverman EK. Polymorphisms in surfactant protein-D are associated with chronic obsructive pulmonary disease. Am J Respir Cell Mol Biol. 2011;44:316–322. doi: 10.1165/rcmb.2009-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii T, Hagiwara K, Kamio K, Ikeda S, Arai T, Mieno MN, Kumasaka T, Muramatsu M, Sawabe M, Gemma A, Kida K. Involvement of surfactant protein D in emphysema revealed by genetic association study. Eur J Hum Genet EJHG. 2012;20:230–235. doi: 10.1038/ejhg.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingappa JR, Dumitrescu L, Zimmer SM, Lynfield R, McNicholl JM, Messonnier NE, Whitney CG, Crawford DC. Identifying host genetic risk factors in the context of public health surveillance for invasive pneumococcal disease. PloS One. 2011;6:e23413. doi: 10.1371/journal.pone.0023413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silveyra P, Floros J. Genetic variant associations of human SP-A and SP-D with acute and chronic lung injury. Front Biosci Landmark Ed. 2012;17:407–429. doi: 10.2741/3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aramini B, Kim C, Diangelo S, Petersen E, Lederer DJ, Shah L, Robbins H, Floros J, Arcasoy SM, Sonett JR, D’Ovidio F. Donor surfactant protein D (SP-D) polymorphisms are associated with lung transplant outcome. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2013;13:2130–2136. doi: 10.1111/ajt.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DK, Cho MH, Hersh CP, Lomas DA, Miller BE, Kong X, Bakke P, Gulsvik A, Agustí A, Wouters E, Celli B, Coxson H, Vestbo J, MacNee W, Yates JC, Rennard S, Litonjua A, Qiu W, Beaty TH, Crapo JD, Riley JH, Tal-Singer R, Silverman EK ECLIPSE, ICGN, COPDGene Investigators . Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1238–1247. doi: 10.1164/rccm.201206-1013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Arimura Y, Goto A, Hosokawa M, Nagaishi K, Yamashita K, Yamamoto H, Sonoda T, Nomura M, Motoya S, Imai K, Shinomura Y. Genetic variants in surfactant, pulmonary-associated protein D (SFTPD) and Japanese susceptibility to ulcerative colitis. Inflamm Bowel Dis. 2009;15:918–925. doi: 10.1002/ibd.20936. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen GL, Madsen J, Kejling K, Tornoe I, Nielsen O, Townsend P, Poulain F, Nielsen CH, Reid KBM, Hawgood S, Falk E, Holmskov U. Surfactant protein D is proatherogenic in mice. Am J Physiol Heart Circ Physiol. 2006;290:H2286–2294. doi: 10.1152/ajpheart.01105.2005. [DOI] [PubMed] [Google Scholar]
- 23.Berg KK, Madsen HO, Garred P, Wiseth R, Gunnes S, Videm V. The additive contribution from inflammatory genetic markers on the severity of cardiovascular disease. Scand J Immunol. 2009;69:36–42. doi: 10.1111/j.1365-3083.2008.02187.x. [DOI] [PubMed] [Google Scholar]
- 24.Gardai SJ, Xiao Y-Q, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 25.Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, Delaisse J-M, Takayanagi H, Lorenzo J, Colonna M, Farndale RW, Choi Y, Trowsdale J. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J Clin Invest. 2011;121:3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merck E, Gaillard C, Scuiller M, Scapini P, Cassatella MA, Trinchieri G, Bates EEM. Ligation of the FcR gamma chain-associated human osteoclast-associated receptor enhances the proinflammatory responses of human monocytes and neutrophils. J Immunol Baltim Md 1950. 2006;176:3149–3156. doi: 10.4049/jimmunol.176.5.3149. [DOI] [PubMed] [Google Scholar]
- 27.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 28.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 29.Brown-Augsburger P, Hartshorn K, Chang D, Rust K, Fliszar C, Welgus HG, Crouch EC. Site-directed mutagenesis of Cys-15 and Cys-20 of pulmonary surfactant protein D. Expression of a trimeric protein with altered anti-viral properties. J Biol Chem. 1996;271:13724–13730. doi: 10.1074/jbc.271.23.13724. [DOI] [PubMed] [Google Scholar]
- 30.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 31.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard J-P, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, Holtzman MJ. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leth-Larsen R, Nordenbaek C, Tornoe I, Moeller V, Schlosser A, Koch C, Teisner B, Junker P, Holmskov U. Surfactant protein D (SP-D) serum levels in patients with community-acquired pneumonia. Clin Immunol Orlando Fla. 2003;108:29–37. doi: 10.1016/s1521-6616(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L, Barclay AN. New assay to detect low-affinity interactions and characterization of leukocyte receptors for collagen including leukocyte-associated Ig-like receptor-1 (LAIR-1) Eur J Immunol. 2009;39:1167–1175. doi: 10.1002/eji.200839188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyaard L. LAIR and collagens in immune regulation. Immunol Lett. 2010;128:26–28. doi: 10.1016/j.imlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Olde Nordkamp MJM, van Eijk M, Urbanus RT, Bont L, Haagsman HP, Meyaard L. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J Leukoc Biol. 2014;96:105–111. doi: 10.1189/jlb.3AB0213-092RR. [DOI] [PubMed] [Google Scholar]
- 36.Lin PM, Wright JR. Surfactant protein A binds to IgG and enhances phagocytosis of IgG-opsonized erythrocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1199–1206. doi: 10.1152/ajplung.00188.2006. [DOI] [PubMed] [Google Scholar]
- 37.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava M, Jung S, Wilhelm J, Fink L, Bühling F, Welte T, Bohle RM, Seeger W, Lohmeyer J, Maus UA. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol Baltim Md 1950. 2005;175:1884–1893. doi: 10.4049/jimmunol.175.3.1884. [DOI] [PubMed] [Google Scholar]
- 39.Voorhout WF, Veenendaal T, Kuroki Y, Ogasawara Y, van Golde LM, Geuze HJ. Immunocytochemical localization of surfactant protein D (SP-D) in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem Off J Histochem Soc. 1992;40:1589–1597. doi: 10.1177/40.10.1527377. [DOI] [PubMed] [Google Scholar]
- 40.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJR, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder GD, Oberley-Deegan RE, Goss KL, Romig-Martin SA, Stoll LL, Snyder JM, Weintraub NL. Surfactant protein D is expressed and modulates inflammatory responses in human coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2008;294:H2053–2059. doi: 10.1152/ajpheart.91529.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sano H, Chiba H, Iwaki D, Sohma H, Voelker DR, Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem. 2000;275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- 43.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 44.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KBM. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol Baltim Md 1950. 2002;169:2892–2899. doi: 10.4049/jimmunol.169.6.2892. [DOI] [PubMed] [Google Scholar]
- 45.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195:201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DWH, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.