Abstract
Enzymatic supramolecular hydrogelation is a simple, controllable, and novel strategy for preparation of soft colloidal materials, which allows the integration of self-assembly with enzyme associated biological processes. The development of more enzymes involve in hydrogelation is a subject of developing useful soft colloids. In this work, a new enzyme, CD73, was found to trigger the formation of nanofibers as matrices of hydrogels. CD73 is an important cell surface enzyme which converts extracellular adenosine monophosphate (AMP) to adenosine. It is broadly expressed in many cancer cells and participates in tumor growth. The successful application of CD73 in self-assembly and hydrogelation provides new strategies for CD73-guided materials and therapies.
Keywords: enzyme, hydrogel, CD73, AMP
Introduction
Supramolecular hydrogels resulting from self-assembly of small molecules in water, usually in the form of nanofibers, are emerging as an important class of biomaterials. They have been explored extensively in the past decade, and promise many useful applications such as drug delivery,[1–3] tissue engineering,[4–6] and cancer therapy.[7, 8] The formation of supramolecular hydrogels requires a triggered phase change. There are several ways to successfully trigger the formation of supramolecular hydrogels, such as changes in temperature and pH, increase of ionic strength, and sonication. However, these methods are less compatible with cells which are the main target of the applications of supramolecular hydrogels. Therefore, the use of inherent biological processes to generate supramolecular hydrogels would be more suitable to promote hydrogel application in a biological environment. One successful approach is to use enzymatic transformation. Enzymes, serving as macromolecular biological catalysts, can catalyze the generation of hydrogelators that self-assemble in water, thus providing an effective method to form hydrogels under in vitro and in vivo conditions, intracellularly and extracellularly. Moreover, selective enzymatic reactions are able to offer precise and localized control of hydrogelation. However, to the best of our knowledge, there are only a handful of enzymes reported for enzymatic supramolecular hydrogelation, such as alkaline phosphatase,[1, 9–11] matrix metalloprotease,[12] protease,[13] esterase,[14] and β-Lactamase.[15] Thus, it is worthwhile to explore more enzymes that trigger hydrogelation to further develop elaborate applications of supramolecular hydrogels in biomedicine. Here we report the use of an ectonucleotidase, CD73, for generation of supramolecular hydrogels.
CD73, ecto-5’-nucleotidase, is a cell-membrane localized enzyme that catalyzes the dephosphorylation of extracellular adenosine monophosphate (AMP) to adenosine. It is expressed in many cell types, including vascular endothelial cells (EC) and certain subtypes of lymphocytes.[16] Recently, some studies indicated that CD73 is highly expressed in many cancer cells[17–21] and promotes tumor growth by suppressing T cells[22, 23]. Due to the important role of CD73 in cancer cells, the use of CD73 to trigger the hydrogelation of small molecules would be highly desirable because the formation of nanofibers/hydrogel on cancer cell surface represent a new paradigm for developing anticancer therapy.[24] Due to CD73’s effect on AMP, we chose AMP as the building block to construct substrates for enzymatic molecular self-assembly and subsequent hydrogelation.[25]
Based on the successful enzymatic transformation of the precursor naphthalene-L-Phe-L-Phe-L-Lys(AMP) to hydrogelator naphthalene-L-Phe-L-Phe-L-Lys(adenosine) by alkaline phosphatase to form supramolecular hydrogel in our group recently,[10] we focus on the AMP-containing peptide. Because L-peptides tend to be degraded by proteases in vivo, which limits long-term applications of supramolecular hydrogels we choose to employ D-peptides due to their expected biostability.[26, 27] Therefore, we use D-amino acids to make naphthalene-D-Phe-D-Phe-D-Lys(AMP) (1) as the CD73 substrate and the precursor of the corresponding hydrogelator naphthalene-D-Phe-D-Phe-D-Lys(adenosine) (2). We use solid phase peptide synthesis (SPPS) to make both 1 and 2 according to reported synthetic procedures,[10] but utilizing D-amino acids (i.e., D-phenylalanine and D-lysine).
Results and discussion
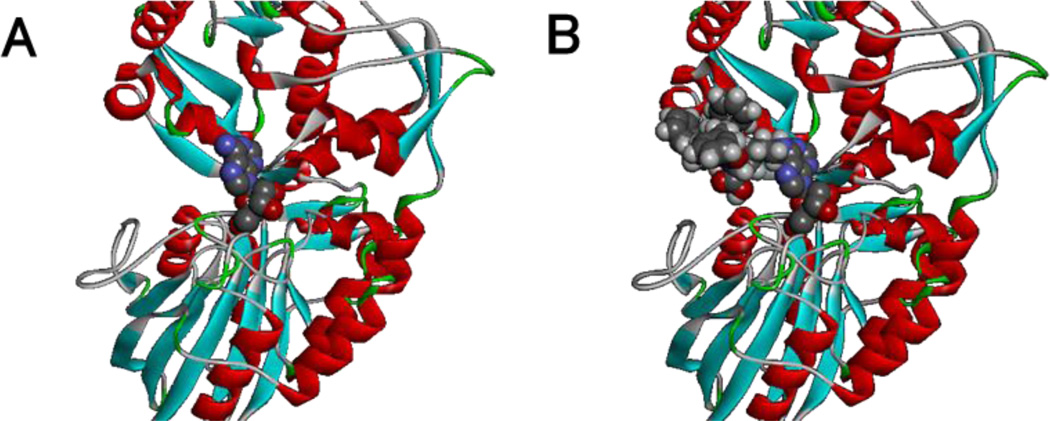
Before testing enzymatic hydrogelation, we examined the interaction of hydrogelator 2 and CD73 based on the crystal structure of CD73.[28] Figure 1A shows the binding of adenosine with CD73 enzyme, adenosine embeds into CD73 with the amine group on the purine ring toward the open side of CD73. This orientation makes modification of the amine on the adenosine purine ring possible without affecting dephosphorylation by CD73. Figure 1B shows the binding model of hydrogelator 2 with CD73, indicating that the bulky naphthalene-D-Phe-D-Phe-D-Lys motif connected to adenosine does not affect binding with CD73.
Figure1.
The reported binding (A) of Adenosine and simulated binding (B) of 2 with CD73 enzyme (ligands as CPK model and CD73 as ribbons).
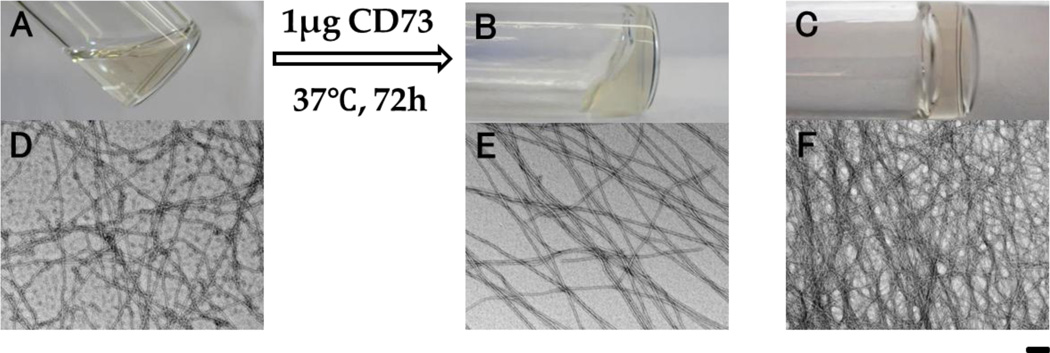
After the peptides synthesis, we tested the self-assembly properties of the precursor (1). 1 affords a transparent solution (Fig. 2A) at pH 7.0 and 2.0 wt % concentration. Transmission electron microscopy (TEM) images of the solution indicate that 1, at 2.0 wt % concentration, already starts to self-assemble in water to form low density nanofibers with diameters of 8 ± 1 nm, in addition to some colloids. Adding 1 μg of CD73 to the solution of 2 wt % 1 (0.5 mL) and keeping the solution at 37 °C and pH 7.0 resulted in hydrogel formation after three days. TEM reveals long, uniform, and flexible nanofibers (8 ± 1 nm in diameter, Fig. 2E) as the matrices of the hydrogel. In contrast to the solution of 1, the fibers of the enzymatic hydrogel are straight and some tend to form parallel alignment and intertwine with each other, likely due to interfiber interactions resulting from the presence of CD73. As a hydrogelator, 2 can self-assemble in water to form transparent hydrogel at pH 7.0 and 2.0 wt % concentration (Fig 2C) via pH adjustment. TEM shows that the widths of the nanofibers formed by the direct self-assembly of 2 are 5 ± 1 nm, and that density of nanofibers appears to be higher than in the enzyme induced hydrogel.
Figure 2.
Optical images and the corresponding transmission electron microscope (TEM) images of (A & D) the solution of 1, (B & E) the hydrogel formed by adding CD73 to the solution of 1, and (C & F) the hydrogel of 2. All samples are at pH 7.0 and 2.0 wt % concentration. Scale bar is 100 nm.
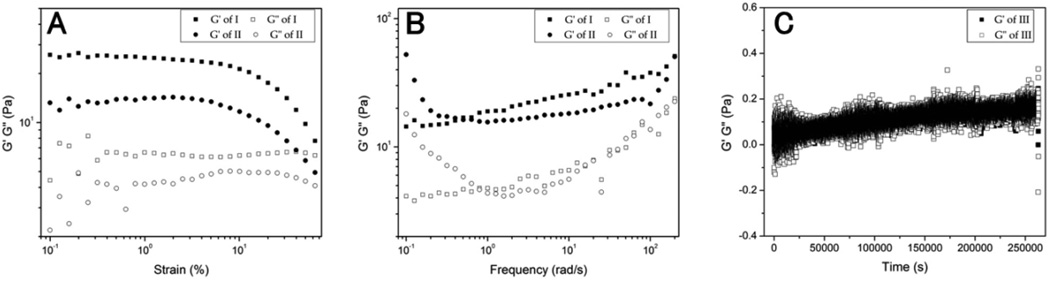
After obtaining the enzymatically formed hydrogel (I) and the hydrogel of 2 (II) made by changing pH, we used rheometry to compare the mechanical properties of the two hydrogels. As shown in Fig 3, the values of storage moduli G’ are always bigger than those of loss moduli G” for both dynamic strain sweeps and frequency sweeps, suggesting that both hydrogels behave as solid-like materials. Dynamic strain sweeps show that the storage modulus G’ of hydrogel I is slightly larger than that of hydrogel II. Both gels possess comparable critical strains: 2.6% and 4.4% for I and II, respectively (Fig. 3A), representing relatively strong networks in both hydrogels. The frequency sweep G’ exhibits slight dependence on frequency (from 0.1 rad/s to 200 rad/s) for the two gels, indicating that the hydrogel matrices exhibit good tolerance to external shear forces. These results indicate that although different self-assembly pathways were used, the resulting hydrogels have similar rheological properties. The concentration dependence rheometry data show that, even at the concentration of 1 wt%, both the solution of 2 and CD73-treated solution of the precursor (1) hardly exhibit the storage moduli G’ to dominate the loss moduli G”, suggesting the liquid-like viscoelastic properties (Fig. S1). These results indicate that both the self-assembly pathways, that is, the pH-adjustment of 2 and CD73-catalyzed conversion of 1, require sufficient concentration of the building block to form the matrices of hydrogels. To confirm the stability of the precursor in serum, we used time-dependent rheometry to monitor the mechanical property change of the solution of 1 in human serum at pH 7.0 and 2.0 wt % concentration. As the result shown in Figure 3C, the storage moduli G’ and loss moduli G” always overlap with each other with the experiment duration (72 hours), indicating that the serum containing 1 behaves as liquid-like material all the time. This result confirms that 1 is stable in human serum.
Figure 3.
Strain dependence (A) and frequency dependence (B) of the dynamic storage moduli (G’) and loss moduli (G”) of (I) the CD73-mediated enzymatic hydrogel, (II) the hydrogel of 2 formed by changing pH; Time dependence of G’ and G” (C) of (III) the solution of 1 in serum. All samples are at pH 7.0 and 2.0 wt % concentration.
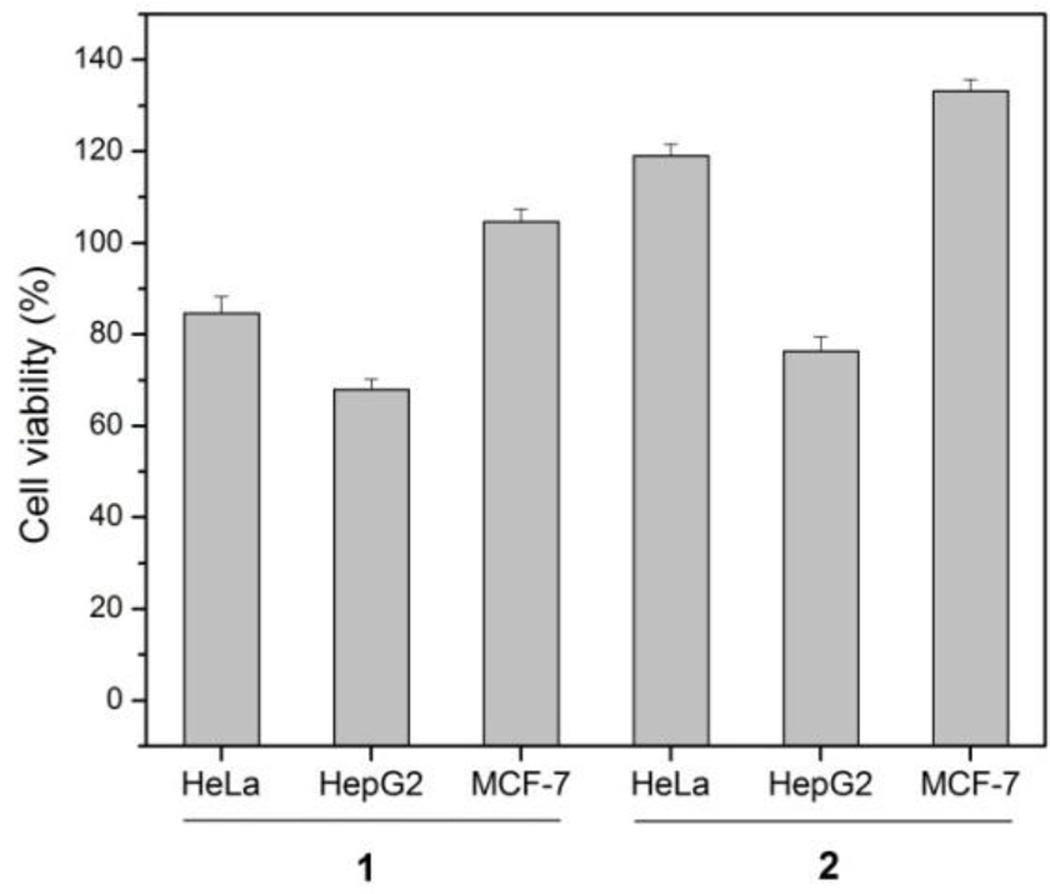
We also tested the cell viabilities of three types of mammalian cells (i.e., HeLa, HepG2, and MCF-7) upon treatment with precursor (1) and hydrogelator (2). As shown in Figure 4, after treatment of the cells with 1 (500 μM) for three days at 37°C, the viabilities of HeLa, HepG2, and MCF-7 cells are around 85%, 70% and 110%, respectively. Meanwhile, after the treatment of the cells with 2 (500 μM) for three days at 37°C, the HeLa, HepG2, and MCF-7 cells show different response to 2 with the cell viabilities around 120%, 75% and 130%, respectively. Since both 1 and 2 exhibit more inhibition to HepG2 cells that to other two cell lines, it appears that the inhibition to HepG2 cells is independent to ectoenzymes. The inhibition of HeLa cells by 1 likely is resulted from ectoenzymes because 2 hardly inhibit HeLa cells. Both 1 and 2, at 500 μM, are innocuous to MCF-7 cells, suggesting that MCF-7 may express lower level of ectoenzymes and carry few receptors for 2 to induce inhibition.
Figure 4.
Cell viabilities of HeLa, HepG2, and MCF-7 cells incubated with 500 μM of 1 or 500 μM of 2 for 72 h.
Conclusion
In summary, enzymatic regulation of the formation of soft colloidal materials as hydrogel is a useful approach for integration of intrinsic biological processes with self-assembly of nanostructures. Exploration of more enzymes to trigger and control the self-assembly of small molecules for hydrogelation, would greatly expand the sophistication of soft biomaterials as smart therapeutics. As shown in the case of CD73 catalyzed hydrogelation, similar strategies for hydrogel formation may offer alternative platforms for application of soft colloidal materials toward CD73 over-expressing cancer cell targeted therapies.
Supplementary Material
Scheme 1.
Adenosine monophosphate (AMP) D-peptide precursor 1 and the corresponding hydrogelator 2.
Highlights.
The first example of the use of CD73 to instructed self-assembly of small molecules.
The first example of the use of CD73 for enzymatic formation of soft colloids.
The first example of the biocompatibility of hydrogelators formed upon the action of CD73.
Illustrated the use of ectoenzymes for generating soft materials via molecular self-assembly.
Acknowledgement
This work was supported by National Institutes of Health (NIH R01CA142746). WDD thanks Mr. Richard Haburcak for helping on English.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material: Experimental Section, 1H NMR and 31P NMR spectra of 1, and 1H NMR spectra of 2.
Reference
- 1.Gao Y, Kuang Y, Guo Z-F, Guo Z, Krauss IJ, Xu B. Journal of the American Chemical Society. 2009;131:13576. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]
- 2.Zhao F, Ma ML, Xu B. Chemical Society Reviews. 2009;38:883. doi: 10.1039/b806410p. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Kuang Y, Gao Y, Du X, Shi J, Xu B. Journal of the American Chemical Society. 2013;135:542. doi: 10.1021/ja310019x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Science (Washington, DC, United States) 2008;319:1812. doi: 10.1126/science.1154586. [DOI] [PubMed] [Google Scholar]
- 5.Salick DA, Kretsinger JK, Haines-Butterick LA, Pochan DJ, Schneider JP. Abstracts, 40th Middle Atlantic Regional Meeting of the American Chemical Society; May 17–21 (2008); Queens, NY, United States. MRM; [Google Scholar]
- 6.Zhou M, Smith AM, Das AK, Hodson NW, Collins RF, Ulijn RV, Gough JE. Biomaterials. 2009;30:2523. doi: 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Cheetham AG, Zhang P, Lin Y-a, Lock LL, Cui H. Journal of the American Chemical Society. 2013;135:2907. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Yang Z. Soft Matter. 2012;8:2344. [Google Scholar]
- 9.Yang Z, Liang G, Xu B. Soft Matter. 2007;3:515. doi: 10.1039/b700138j. [DOI] [PubMed] [Google Scholar]
- 10.Du X, Li J, Gao Y, Kuang Y, Xu B. Chemical Communications (Cambridge, United Kingdom) 2012;48:2098. doi: 10.1039/c2cc16723a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Gao Y, Kuang Y, Shi J, Du X, Zhou J, Wang H, Yang Z, Xu B. Journal of the American Chemical Society. 2013;135:9907. doi: 10.1021/ja404215g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss JA, Stokols S, Hixon MS, Ashley FT, Chang JY, Janda KD. Biomacromolecules. 2006;7:1011. doi: 10.1021/bm051001s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toledano S, Williams RJ, Jayawarna V, Ulijn RV. Journal of the American Chemical Society. 2006;128:1070. doi: 10.1021/ja056549l. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Xu K, Guo Z, Guo Z, Xu B. Advanced Materials (Weinheim, Germany) 2007;19:3152. [Google Scholar]
- 15.Yang Z, Ho P-L, Liang G, Chow KH, Wang Q, Cao Y, Guo Z, Xu B. Journal of the American Chemical Society. 2007;129:266. doi: 10.1021/ja0675604. [DOI] [PubMed] [Google Scholar]
- 16.Airas L, Niemela J, Salmi M, Puurunen T, Smith DJ, Jalkanen S. Journal of Cell Biology. 1997;136:421. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadej R, Spychala J, Skladanowski AC. Melanoma Research. 2006;16:213. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 18.Stagg J, Smyth MJ. Oncogene. 2010;29:5346. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 19.Zhou P, Zhi X, Zhou T, Chen S, Li X, Wang L, Yin L, Shao Z, Ou Z. Cancer Biology & Therapy. 2007;6:426. doi: 10.4161/cbt.6.3.3762. [DOI] [PubMed] [Google Scholar]
- 20.Kondo T, Nakazawa T, Murata SI, Katoh R. Histopathology. 2006;48:612. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Zhi X, Zhou P, Chen S, Zhao F, Shao Z, Ou Z, Yin L. Oncology Reports. 2007;17:1341. [PubMed] [Google Scholar]
- 22.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Journal of Immunology. 2011;187:676. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 23.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Journal of Experimental Medicine. 2007;204:1257. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang Y, Shi J, Li J, Yuan D, Alberti KA, Xu Q, Xu B. Angewandte Chemie, International Edition. 2014;53:8104. doi: 10.1002/anie.201402216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Liang G, Xu B. Accounts of Chemical Research. 2008;41:315. doi: 10.1021/ar7001914. [DOI] [PubMed] [Google Scholar]
- 26.Seebach D, Matthews JL. Chemical Communications (Cambridge) 1997:2015. [Google Scholar]
- 27.Li X, Du X, Li J, Gao Y, Pan Y, Shi J, Zhou N, Xu B. Langmuir. 2012;28:13512. doi: 10.1021/la302583a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knapp K, Zebisch M, Pippel J, El-Tayeb A, Mueller CE, Straeter N. Structure (Oxford, United Kingdom) 2012;20:2161. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.