Abstract
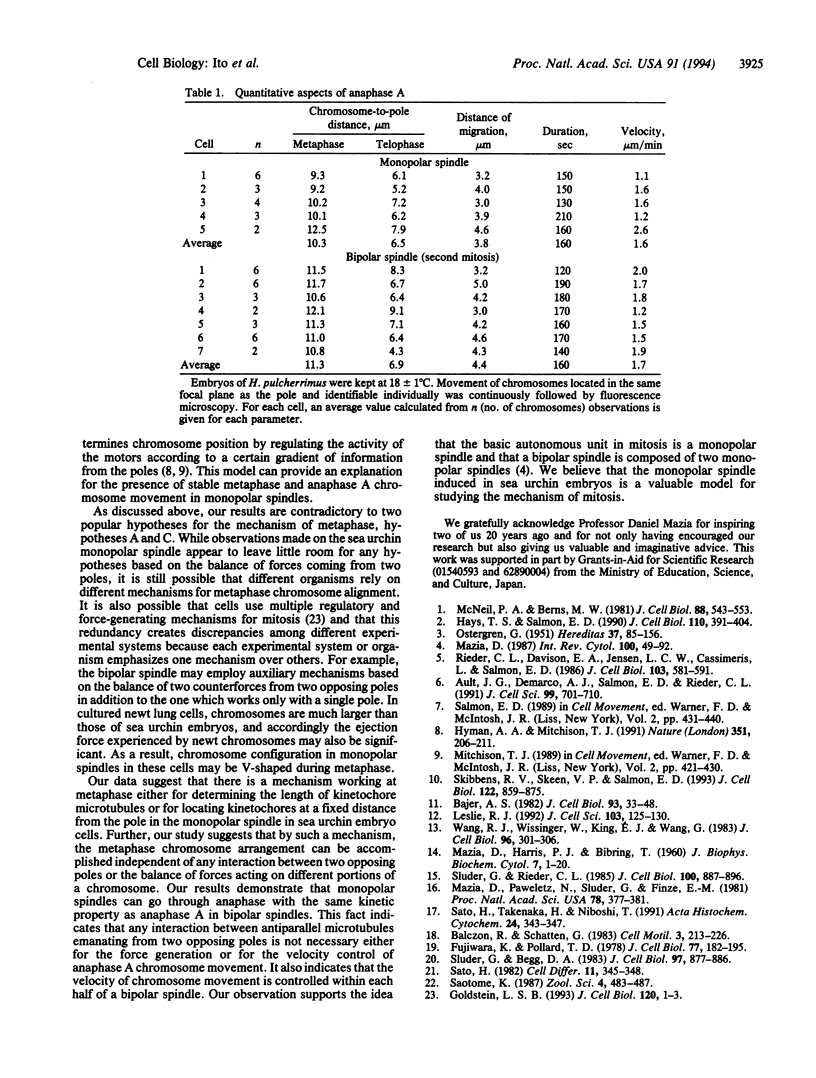
By using monopolar spindles artificially induced in sea urchin embryos, we examined whether or not the presence of two opposing poles was an indispensable condition for keeping chromosomes at a fixed distance from the pole at metaphase and for the anaphase chromosome movement. Chromosomes were stained with Hoechst dye 33342 and their behavior was followed in the monopolar and the control bipolar spindles. In the monopolar spindle, chromosomes were first arranged on a curved metaphase plate and then spread on a part of the imaginary surface of a sphere whose center was the monopole. The estimated chromosome-to-pole distance was similar to that of bipolar spindles at metaphase and remained fixed until chromosomes started to move toward the pole. The average duration of metaphase in the monopolar spindle was 6 times longer than that in the bipolar spindle. The poleward movement of chromosomes in the monopolar spindle was similar to the anaphase A (chromosome-to-pole movement) in the bipolar spindle with respect to the velocity, duration, distance, and synchronization of migration. These results show that even half of the normal spindle has capacities for the arrangement of chromosomes at metaphase and for the anaphase A chromosome movement. Based on these results, we were able to exclude some existing theories of metaphase, such as the one based on the balance of forces between the two poles.
Full text
PDF

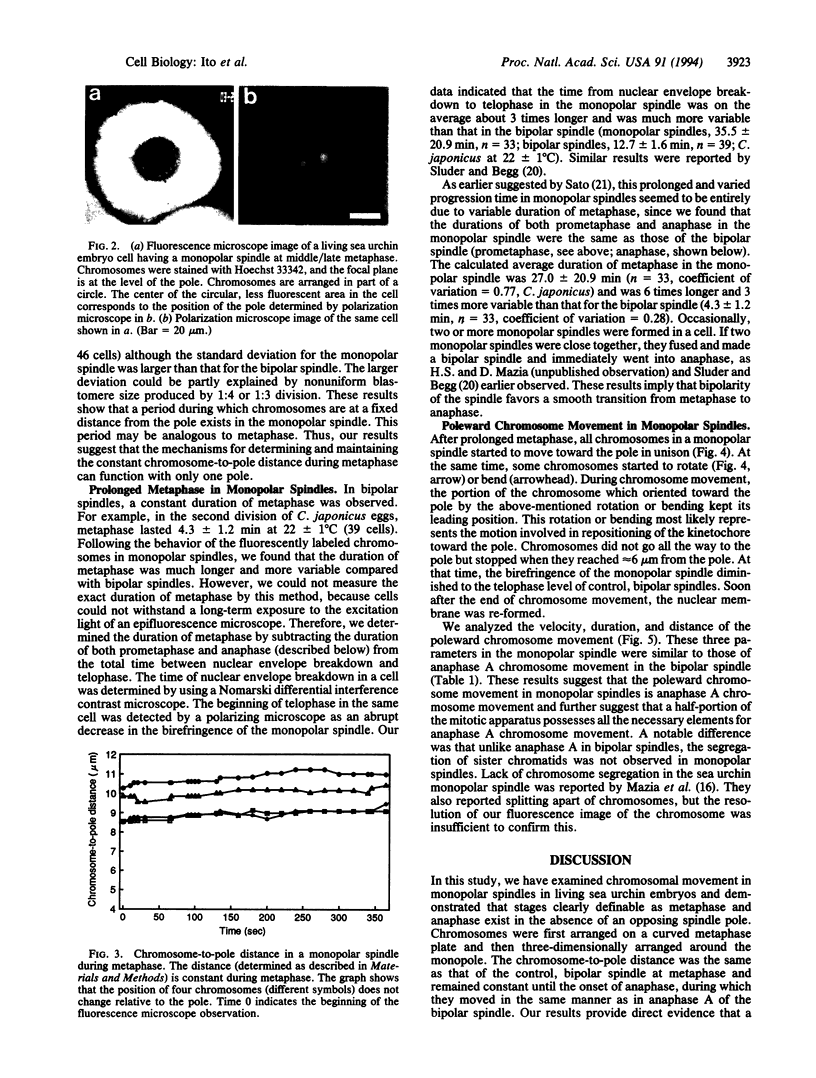
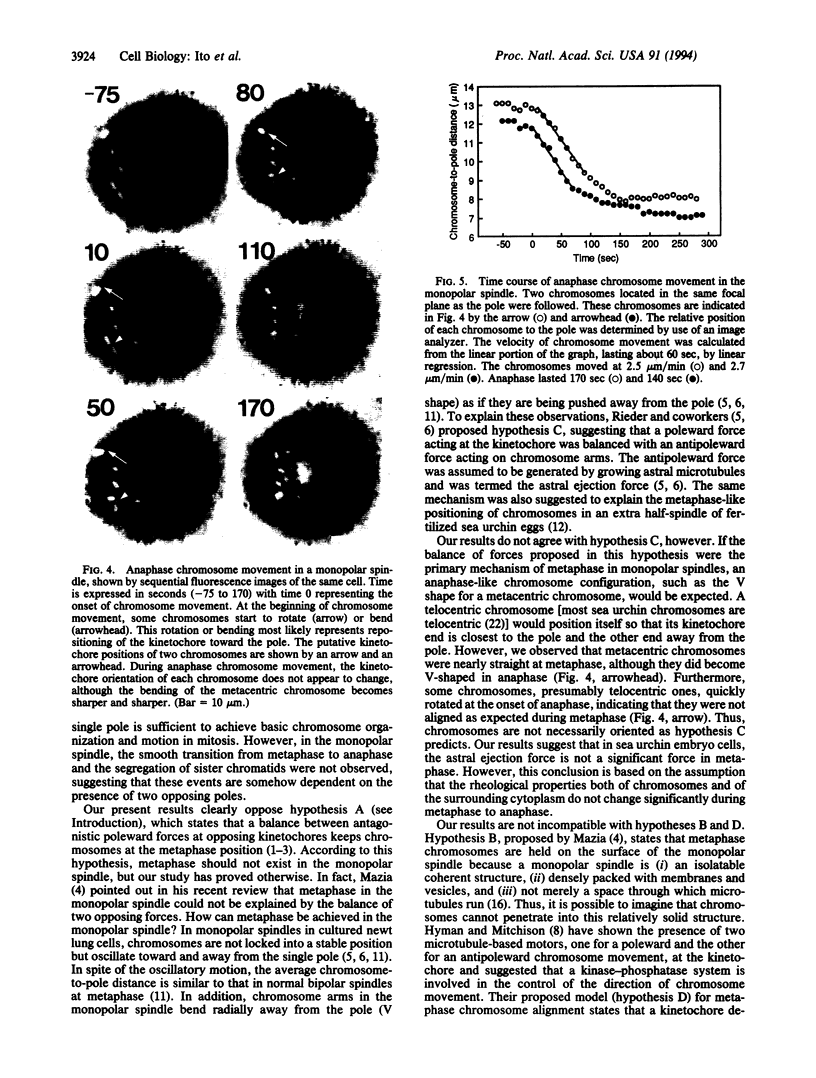
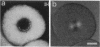
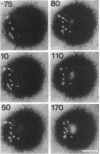
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault J. G., DeMarco A. J., Salmon E. D., Rieder C. L. Studies on the ejection properties of asters: astral microtubule turnover influences the oscillatory behavior and positioning of mono-oriented chromosomes. J Cell Sci. 1991 Aug;99(Pt 4):701–710. doi: 10.1242/jcs.99.4.701. [DOI] [PubMed] [Google Scholar]
- Bajer A. S. Functional autonomy of monopolar spindle and evidence for oscillatory movement in mitosis. J Cell Biol. 1982 Apr;93(1):33–48. doi: 10.1083/jcb.93.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Simultaneous localization of myosin and tubulin in human tissue culture cells by double antibody staining. J Cell Biol. 1978 Apr;77(1):182–195. doi: 10.1083/jcb.77.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. S. Functional redundancy in mitotic force generation. J Cell Biol. 1993 Jan;120(1):1–3. doi: 10.1083/jcb.120.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays T. S., Salmon E. D. Poleward force at the kinetochore in metaphase depends on the number of kinetochore microtubules. J Cell Biol. 1990 Feb;110(2):391–404. doi: 10.1083/jcb.110.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A., Mitchison T. J. Two different microtubule-based motor activities with opposite polarities in kinetochores. Nature. 1991 May 16;351(6323):206–211. doi: 10.1038/351206a0. [DOI] [PubMed] [Google Scholar]
- Leslie R. J. Chromosomes attain a metaphase position on half-spindles in the absence of an opposing spindle pole. J Cell Sci. 1992 Sep;103(Pt 1):125–130. doi: 10.1242/jcs.103.1.125. [DOI] [PubMed] [Google Scholar]
- Mazia D., Paweletz N., Sluder G., Finze E. M. Cooperation of kinetochores and pole in the establishment of monopolar mitotic apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):377–381. doi: 10.1073/pnas.78.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. The chromosome cycle and the centrosome cycle in the mitotic cycle. Int Rev Cytol. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- McNeill P. A., Berns M. W. Chromosome behavior after laser microirradiation of a single kinetochore in mitotic PtK2 cells. J Cell Biol. 1981 Mar;88(3):543–553. doi: 10.1083/jcb.88.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Davison E. A., Jensen L. C., Cassimeris L., Salmon E. D. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J Cell Biol. 1986 Aug;103(2):581–591. doi: 10.1083/jcb.103.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbens R. V., Skeen V. P., Salmon E. D. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J Cell Biol. 1993 Aug;122(4):859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Begg D. A. Control mechanisms of the cell cycle: role of the spatial arrangement of spindle components in the timing of mitotic events. J Cell Biol. 1983 Sep;97(3):877–886. doi: 10.1083/jcb.97.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Rieder C. L. Centriole number and the reproductive capacity of spindle poles. J Cell Biol. 1985 Mar;100(3):887–896. doi: 10.1083/jcb.100.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Wissinger W., King E. J., Wang G. Studies on cell division in mammalian cells. VII. A temperature-sensitive cell line abnormal in centriole separation and chromosome movement. J Cell Biol. 1983 Jan;96(1):301–306. doi: 10.1083/jcb.96.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]