Abstract
BACKGROUND
Compelling evidence about the differences in the biology and behavior of invasive breast cancer between African-American (AA) and White-American (WA) women motivate inquiry into comparing the clinicopathology of non-invasive breast cancer (ductal carcinoma in situ, DCIS).
METHODS
AA and WA women diagnosed with their first primary DCIS between 1990 and1999 were identified from the institutional tumor registry. Data on method of presentation, treatment, pateint characteristics were retreived from electronic medical records. Patients were followed up through the medical records until the diagnosis of a subsequent cancer or the last-day of contact with the institution.
RESULTS
A total of 100 (29.6%) AAs and 236 (70.4%) WAs with the mean age of 60 (SD±13) and 57 (SD±12), respectively, contributed to this study. DCIS was detected during routine screening mammography for 81% (n=81) of AAs and 88.4% (n=206) of WAs (P=0.073). Differences in the distributions of grade, margin status, necrosis, or treatment modalities were not statistically significant between AAs and WAs. Analysis of competing risks Cox proportional hazard multivariate modeling yielded a significant 8-year cumulative risk of a second cancer for AAs but only in the ipsilateral breast (HR=3.96, 95% CI 1.42–11.04, P=.01).
CONCLUSION
Despite comparable clinical presentation and treatment, 8 years after the initial treatment, AAs expereinced a higher risk of second breast cancer in ipsilateral but not in the contralateral breast. The observed excess risk of a second cancer in the ipsilateral breast may suggest of intrinsic differences in the biology of cancer.
Keywords: Ductal Carcinoma in Situ, Second Breast Cancer, African-American, White-American, African-Ancestry
INTRODUCTION
Extensive population-based and clinical correlative studies confirm that African American women are more likely to be diagnosed with early-onset, high-grade invasive cancers that are negative for expression of the estrogen and progesterone receptors and the HER2/neu marker. [1–3] These adverse prognostic indicators have been proposed as one of the underlying factors in the observed higher disease-specific mortality in African-American women. Few studies have reported on racial differences in histopathology and treatment outcome of ductal carcinoma in situ (DCIS) of the breast.
In 2007, the incidence of DCIS in African-Americans ages 50 and older was 90.2 per 100,000 and in white Americans 87.8 per 100,000. For younger women, this incidence was 10.6 per 100,000 for African-American and 10.6 per 100,000 for White-American women. [4] These comparable incidence rates underscore the importance of equal access to health care in closing the breast cancer disparity gap across racial lines and socioeconomic strata; however, elimination of disparity expands beyond the concept of health care access but implies identification of disease-specific risk for different sub-populations. [5,6] Published findings comparing the treatment outcome of DCIS between African-American and White-American are inconclusive. Studies that have adjusted for pathologic prognostic indicators, i.e. size in greatest dimension, grade, or presence of necrosis have not identified significant difference in the risk of a second breast cancer. [7–9] However, excess risk of any second breast cancer in African-American women after a diagnosis of DCIS has been reported when age at the initial clinical presentation of the disease, year of diagnosis, geographic site and treatment modality, but not for pathologic prognostic indicators, were considered. [10,11] The compelling epidemiologic and clinical evidence about differences in the biology and behavior of invasive breast cancer and the inconclusive findings on the long-term risk difference after DCIS motivated our research team to compare the pathologic prognostic indicators of DCIS and the long-term rates of any second breast cancers between African-American and White-American women.
PATIENTS AND METHODS
The present study, retrospective and longitudinal in design, was restricted to one institution, Henry Ford Health System (HFHS), Detroit, MI. This health care institution is a comprehensive, self contained system that is organized so that persons in the system receive every level of care from preventive and primary to subspecialty services. When a patient is first seen at any of HFHS facilities for any reason, he/she is assigned a permanent and unique medical record number and is entered into the Master Patient Index database that resides within a larger relational database. This database serves as the central repository for data on patient encounters and includes information on the date and time of service, the name of provider at the clinical encounter, the place of encounter, laboratory services data, surgical, pathology, radiology, oncology diagnostic information.
The institutional review board at HFHS approved this study protocol (IRB 2403). This study is in compliance with the US Congress Health Insurance Portability and Accountability Act of 1996.
Study Sample Ascertainment
A total of 750 women who were diagnosed with their first primary non-infiltrating breast cancer (ductal carcinoma in situ, DCIS) between January 1990 and December of 1999 were identified from the institutional tumor registry and were validated against medical records. The date of surgery was defined as the date of initial diagnosis. After reviewing the medical records a total of 316 women were excluded because of a concurrent or previous diagnosis of invasive carcinoma, or diagnosis of lobular carcinoma in situ or Paget’s disease of the breast. Of the remaining 434 women, we were able to retrieve the diagnostic slides for a total of 336 women. Women were followed up by reviewing their electronic health records until the last date of contact with the HFHS, for any reason, or the date of a second breast cancer (Figure 1).
Figure 1.
Consort Diagram of Patient Flow.
Data Collection
Diagnostic pathology slides were retrieved and two of the investigators (RS and AR), masked to the racial heritage of the women, reviewed the slides and documented the architectural patterns, nuclear grade, absence or presence of necrosis with sub-classification, margin, and multi-focality using College of American Pathologists checklist guidelines. Demographic data (date of birth and self-identified race/ethnicity) were retrieved from the tumor registry and validated against medical records. Clinical data, (menopausal status, co-morbid conditions, height, weight, use of post-menopausal hormone replacement therapy, first degree family history of breast cancer, and method of detection) were collected from physician notes at the time of the initial consultation. Radiology data were reviewed to confirm the validity of method of detection of the cancer. For patients with missing information for height and weight at the time of consultation, we reviewed anesthesiologists’ or cardiologists’ notes prior to surgery. We had defined menopause as a minimum 6 continuous months of absence of menses. Therefore, for women who were classified as peri-menopausal, we compared their last reported date of menses with their date of surgery; if a minimum of 6-months had elapsed, then they were classified as post-menopausal. Otherwise, they were classified as pre-menopausal. We defined the treatment variables as the first course of treatment up to four months after the diagnosis. Surgery was characterized as mastectomy, capturing simple uni-/bilateral mastectomy and modified radical mastectomy, or breast conserving surgery (BCS) with the later group including segmental mastectomy, partial mastectomy, or lumpectomy.
Statistical Analysis
Descriptive statistics were used to summarize demographic, clinical and histopathology information of the women. The variable age at the time of diagnosis and lesion size, in the greatest dimension, were included as continuous variables. Body mass index (BMI) was calculated using the formula: weight (kg)/[height (meter)]2 and was considered both as a continuous and a categorical variable in regression models. BMI was categorized as BMI ≤18.5 or underweight, 18.6 ≤ BMI ≤24.9 or normal/ideal body weight, 25.0 ≤ BMI≤ 29.9 or overweight, 30.0 ≤ BMI or obese. Because a small number of women (n=5) had BMI ≤18.5, the BMI categories of “underweight” and “normal/ideal” body weight were collapsed into one group for statistical analysis.
Women with bi-/unilateral simple mastectomy and modified radical mastectomy were classified into one group because of small number in each sub-classification. Women then were classified according to their surgical treatment into mastectomy vs. BCS and whether they received radiation treatment (yes vs. no). Thus, women were classified into one of the three combined surgical and radiation treatment based on their final treatment: mastectomy, BCS with radiation, and BCS without radiation. Finally, we reviewed pathology and surgical oncology records to ascertain the number of re-excisions because of positive or negative but <1 mm margin.
We applied Cox proportional hazard statistics to estimate the longitudinal risk of any second breast cancer, stratified by race and treatment. [12] In developing the best-fitted model, we first estimated the individual effect of each variable on the outcome, any second breast cancer. Variables were evaluated because of their prognostic effect (histologic grade, calcification, margin, necrosis and lesion size) or clinical effect (diabetes, and Charlson’s co-morbidity index) or demographic influence (age, menopausal status, race and BMI). Correlations between different variables were estimated and multi-collinearity was prevented by including in the model only variables with coefficient values of 0.7 or less. [13] Variables with a p-value of <0.10 from the univariate analysis were considered candidate variables. The interaction between variables also was tested at a significant level of 0.1. Two variables, age at diagnosis and menopausal status, were highly correlated (p<0.001); thus, the variable age at diagnosis was included in the final model because, it has more diagnostic significance. The final model contained only variables and interaction terms with p-value ≤ 0.05.
In our second analysis, we estimated the longitudinal risk of a second breast cancer in either the ipsilateral or contralateral breast, stratified by initial treatment and by the race of women using the competing risks Cox proportional hazard modeling approach. This approach was justified because of the assumption of independence of probability progression of the first primary DCIS to ipsi-/contralateral breast. Furthermore, the risk of a second cancer in either ipsi-/contral-lateral breast was the outcome of equal interest in our analysis. [14] We proceeded with developing the final model as described previously; however, we were not able to include in the final model the two variables, use of post-menopausal hormone replacement therapy and Charlson’s co-morbidity index because of missing values for a considerable number of women as shown in Table 1.
Table 1.
Demographic and pathologic prognostic indicators of the study participants
| Variable | All Women N= 335 (%) |
African-American N= 100 (%) |
White-American N=235 (%) |
P-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 58 (± 12.5) | 60 (± 13) | 57 (± 12.0) | .017 |
| BMI in Kg/m2 (mean ± SD) | 28.6 (± 6.8) | 30.0 (± 6.6) | 27.8(± 6.8) | .009 |
| Post-menopausal Hormone Therapy | .021 | |||
| Yes | 108 (36.4) | 25 (26.9) | 83 (40.8) | |
| No | 189 (63.6) | 68 (73.1) | 121 (59.2) | |
| Missing | 38 | 7 | 31 | |
| First Degree Family History of Breast Cancer | .44 | |||
| Yes | 75 (25.9) | 25 (28.7) | 50 (24.6) | |
| No | 217(74.1) | 62 (71.3) | 155(75.4) | |
| Missing | 43 | 13 | 30 | |
| Charlson Co-morbidity Index | .010 | |||
| 0 | 268 (83.6) | 74 (75.5) | 194 (87.1) | |
| 1 ≤ | 53 (16.4) | 24 (24.5) | 29 (12.9) | |
| Missing | 14 | 2 | 12 | |
| Clinical Presentation | .073 | |||
| Screening Mammography | 287 (86.2) | 81 (81.0) | 206 (88.4) | |
| Palpation or Nipple Discharge | 46 (13.8) | 19 (19.0) | 27 (11.6) | |
| Missing | 2 | 0 | 2 | |
In our data, one African-American and one White-American were diagnosed with second breast cancers in both breasts during the follow-up time. We opted to remove these two women from the analysis because of the small number. All statistical tests were two-sided and analyses were performed using SAS v. 9.2. (SAS Institute, Cary, NC)
RESULTS
A total of 335 women of whom 29.6% (n=100) were African-American and 70.4% (n=235) White-American contributed to this study. The mean age at the time of initial clinical presentation of the disease for African-American women was 60 (±13) years compared with 57 (±12.4) years for White-American women (p=.022). (Table 1) African-American women, with mean BMI of 30.0±6.6 Kg/m2, were heavier than White-Americans, mean BMI of 27.8 ±6.7 Kg/m2 (P=.009) and a higher proportion of them had reported health conditions secondary to adiposity, including type II diabetes and hypercholesteremia. Use of hormone replacement therapy was more prevalent among the White-American women (P=.027). There were no statistically significant differences (P=.44) in the proportions of women who had reported a history of breast cancer in a first degree family member. The majority of women (86.2%) were diagnosed based on screening mammography. A total of 19 (19%) of African-American and 27 (11.6%) of White-American women were referred to the breast clinic by their primary care physicians either because of a palpable mass or nipple discharge (P=.073).
Pathologic features of the DCIS in African-American and White-American women are presented in Table 2. Differences in the distributions of the primary prognostic indicators, margin, grade and necrosis did not reach the level of statistical differences. (Table 2) About 45 (46.4%) African-American and 113 (48.5%) of White-American were diagnosed with high histologic grade. Necrosis was identified in lesions of 27 (32.5%) of African-American and 56 (28.6%) of White-American women (P=.51). (Table 2)
Table 2.
Comparison of pathologic characteristics of ductal carcinoma in situ lesions and treatment modalities between African American and White-American women
| Variable | All Women N= 335 (%) |
African-American N= 100 (%) |
White-American N=235 (%) |
P-Value |
|---|---|---|---|---|
| Histologic Grade | ||||
| 1 | 78(23.7) | 30 (30.9) | 48 (21.4) | .140 |
| 2 | 92 (27.9) | 22 (22.7) | 70 (29.9) | |
| 3 | 158 (47.9) | 45 (46.4) | 113 (48.5) | |
| Missing | 7 | 3 | 4 | |
| Calcification | ||||
| Present | 246 (89.5) | 66 (86.8) | 180 (90.9) | .376 |
| Absent | 28 (10.5) | 10 (13.2) | 18 (9.1) | |
| Missing | 61 | 24 | 37 | |
| Margin | ||||
| Negative | 215 (69) | 64 (69.6) | 151 (68.8) | .918 |
| Negative < 1mm | 68 (22.0) | 20 (21.7) | 48 (22.2) | |
| Positive, focal | 22 (7.0) | 7 (7.6) | 15 (6.8) | |
| Positive, multifocal | 2 (0.6) | 0 | 2 (.9) | |
| Positive, extensive | 4 (1.3) | 1 (1.1) | 3 (1.4) | |
| Missing | 24 | 8 | 16 | |
| Lesion Size in Greatest Dimension in cm (mean± SD) | 1.99 (± 1.9) | 2.13 (± 1.8) | 1.90 (± 1.9) | .362 |
| Necrosis | ||||
| Positive | 83 (30.2) | 27 (32.5) | 56 (29.7) | .590 |
| Negative | 196 (69.8) | 56 (67.5) | 140 (70.7) | |
| Missing | 56 | 17 | 39 | |
| Architectural Pattern | ||||
| Mixed | 122 (46.2) | 35 (44.9) | 87 (46.8) | .440 |
| Solid | 20 (7.6) | 5 (6.4) | 15 (8.1) | |
| Cribriform | 42 (15.9) | 17 (21.8) | 24 (13.4) | |
| Micropapillary | 13 (4.9) | 1 (1.3) | 12 (6.4) | |
| Papillary | 2 (0.8) | 0 | 2 (1.1) | |
| Clinging | 2 (0.8) | 0 | 2 (1.1) | |
| Comedo | 62 (23.1) | 20 (25.6) | 42 (22.6) | |
| Missing | 73 | 22 | 51 | |
Breast conserving surgery was the choice of treatment for 62 (63.9%) of African-American and 146 (63.7%) of White-American women. Unilateral simple mastectomy was selected by 26 (26.8%) of African-American and 51 (22.3%) of White-Americans. Eight (8.2%) African-American and 28 (12.3%) of White-American women had opted for modified radical mastectomy. Finally, a total of five women, one (1%) African-American and four (1.7%) White-American had chosen bilateral simple mastectomy (p=.879). Among women who had opted for BCS, a total of 67 women were offered the option of re-exision because of positive or negative but < 1mm margin. Of these, 6 (12.8%) White-American and 1(5.0%) African-American underwent the re-excision (P=.341). Finally, a total of 13 (14.0%) African-American and 31 White-American (14.0%) had opted for tamoxifen adjuvant therapy (P=.997) (Table 3).
Table 3.
Treatment modalities and recurrence outcomes
| Variable | All Women N= 335 (%) |
African-American N= 100 (%) |
White-American N=235(%) |
P-Value |
|---|---|---|---|---|
| Surgery | .615 | |||
| Bilateral Simple Mastectomy | 5 (1.8) | 1 (1.0) | 4 (2.2) | |
| Unilateral Simple Mastectomy | 77 (23.5) | 26 (26.8) | 51 (22.1) | |
| Modified Radical Mastectomy | 36 (11.0) | 8 (8.2) | 28 (12.2) | |
| Breast Conserving Surgery | 208 (63.6) | 62 (63.9) | 146 (75.7) | |
| Missing | 9 | 3 | ||
| Radiation | .612 | |||
| Yes | 143 (45.1) | 44 (47.3) | 99 (44.2) | |
| No | 172 (54.9) | 49 (52.7) | 123 (55.8) | |
| Missing | 20 | 7 | 13 | |
| Final Treatment | .736 | |||
| Mastectomy | 118 (37.2) | 35 (37.2) | 83 (37.2) | |
| BCS with radiation | 135 (42.2) | 42 (44.7) | 93 (41.1) | |
| BCS without radiation | 66 (20.6) | 17 (18.1) | 48 (21.7) | |
| Missing | 17 | 6 | 11 | |
| Re-Excision | .342 | |||
| Yes | 7(10.5) | 1 (5.0) | 6 (12.8) | |
| No | 60 (89.5) | 19 (95.0) | 41 (87.2) | |
| Tamoxifen | .997 | |||
| Yes | 44 (14.0) | 13 (14.0) | 31 (14.0) | |
| No | 269 (86.0) | 80 (86.0) | 189 (86.0) | |
| Missing | 22 | 7 | 15 | |
| Follow-up Time in Years (mean± SD) | 8.10 (± 3.2) | 9.25 (± 4.0) | 9.00(± 4.4) | .710 |
| Second Breast Cancer | .003 | |||
| Yes | 34 (10.4) | 18 (17) | 16 (7.2) | |
| No | 301 (89.6) | 82 (83) | 219 (92.8) | |
| Diagnosis of Second Cancer | .154 | |||
| Invasive | 19 (55.9) | 8 (44.4) | 11(68.7) | |
| In Situ | 15 (44.1) | 10 (55.6) | 5(32.3) | |
| Site of Second Cancer | .388 | |||
| Ipsilateral | 19 (54.3) | 12 (66.7) | 7 (41.2) | |
| Contralateral | 13 (40.0) | 5 (27.8) | 8 (52.9) | |
| Both breasts | 2 (5.7) | 1 (5.6) | 1 (5.9) | |
The mean follow-up time for African-American women was 10.6 (±4.5) years and for White-American women was 10.4 (± 4.9) years (P=.71). During the follow-up time, 18 (18%) of African-American and 16 (6.8%) of White-American women, experienced second breast cancers (P=.003). Among White-American women, 11 (68.7%) were diagnosed with invasive breast cancer and 6 (32.3%) with DCIS. A total of 8 (44.4%) African-American women were diagnosed with invasive breast cancer and with10 (55.6%) with DCIS. The difference in this distribution did not reach the level of statistical significance (P=.154). During the follow-up period, 12 (66.7%) African-American women experienced a second cancer in the ipsilateral breast, 5 (27.8%) in the contralateral breast and one women (5.5%) was diagnosed with cancer in her both breasts. During the same time period, a total of 7 (43.7%) White-American women were diagnosed with ipsilateral cancers, 8 (50.0%) with contralateral cancers, and one woman (6.3%) in both breasts (P=.387).
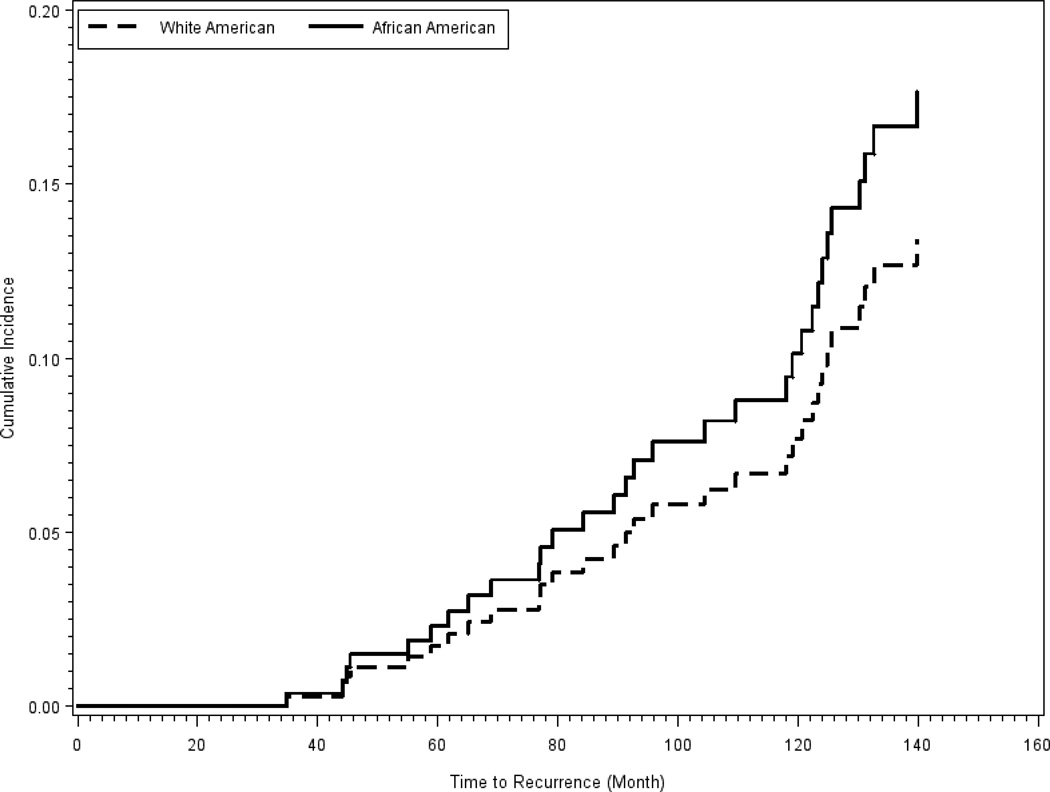
Results from the multivariate Cox proportional hazard statistics are presented in Table 4. During the follow-up time, African-American women experienced more than 2.5 fold increased in risk (HR=2.61, 95% CI 1.27–5.36, P=.01) of a second breast cancer. This risk was adjusted for treatment, marginal status of the lesion, age and BMI at the time of diagnosis. In Figure 2, we have presented the race stratified cumulative risk for any second breast cancer during the follow-up time. We did not detect any significant difference in the cumulative risk between African-American and White-American women during the first 60 months post the initial treatment. African-American women, however, experienced an increased in risk of any second cancer 80 months after the initial treatment, with the level of this risk reaching statistical significance.
Table 4.
Adjusted risk for any second breast cancer: multivariate Cox proportional hazard statistics
| Variable | Women with second breast cancers N |
Women without second breast cancers N |
HR (95% CI)1 |
P-Value | ||
|---|---|---|---|---|---|---|
| BCS + Radiation relative to Mastectomy |
BCS w/o Rad relative to mastect3 |
BCS + Radiation relative to mastect2 |
BCS w/o Rad relative to mastect3 |
|||
|
Race4 African-American vs. White-American |
12/2 vs. 6/4 |
4/2 vs. 6/4 |
30/32 vs. 87/79 |
14/32 vs. 42/79 |
2.61 (1.27–5.36) |
.01 |
|
Margin4 Positive vs. Negative |
7 vs. 11 |
6 vs. 10 |
20 vs. 53 |
59 vs. 141 |
.83 (.46–1.50) |
.53 |
|
Presentation5 Mammography vs. Palpation |
3 vs. 15 |
4 vs. 12 |
66 vs. 16 |
194 vs. 23 |
0.59 (.25–1.40) |
.23 |
Hazard ratio; 95% Confidence Interval;
Breast conserving surgery with radiation vs. Mastectomy;
Breast conserving surgery without radiation vs. Mastectomy;
Adjusted for margin, clinical presentation of cancer, age at the time of diagnosis and body mass index;
Adjusted for final treatment, clinical presentation of cancer, age at the time of diagnosis and body mass index
Adjusted for final treatment, age, body mass index and margin
Figure 2.
Cumulative risk of any second breast cancer, stratified by race
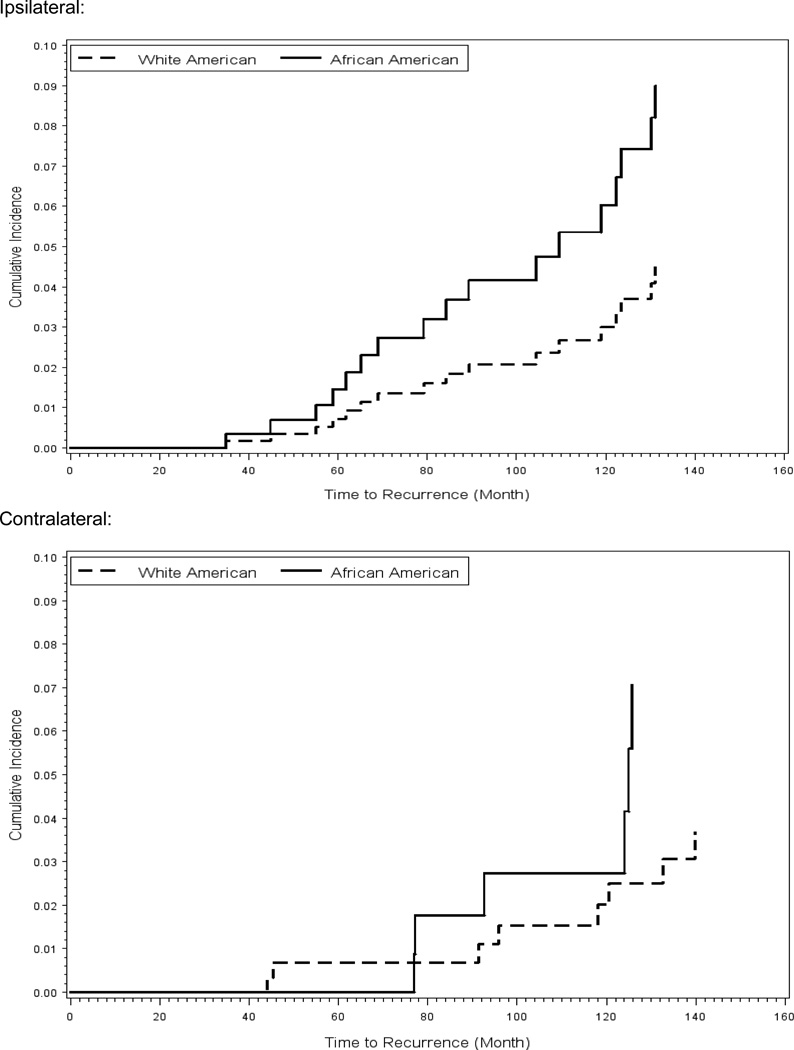
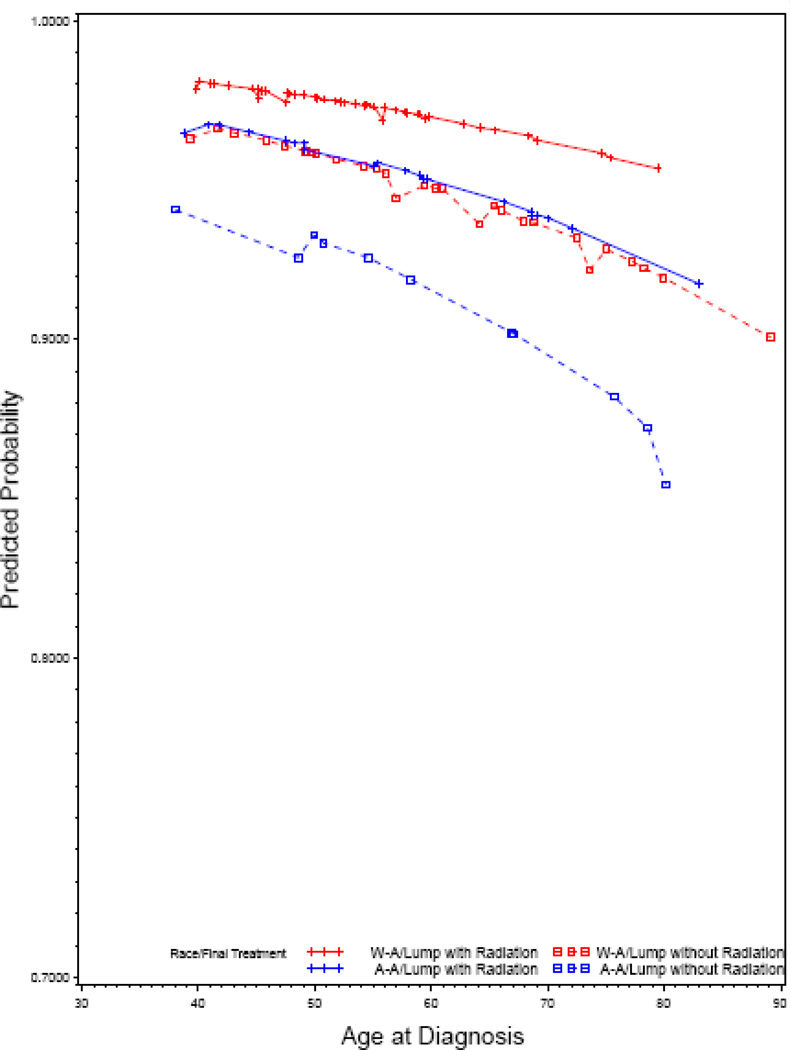
In our second analysis, using the multivariable competing risk Cox modeling approach, we estimated the risk of a second cancer in ipsi- or contra-lateral breasts. (Table 5) Race was associated with an increased risk of a second cancer only ipsilateral breast. The risk of a second cancer in ipsilateral breast, adjusted for age, marginal status (negative vs. positive), method of detection (screening mammography vs. palpation) was four-times higher for African-American women (HR=3.96, 95% CI 1.42–11.04, P=.01) compared with White-American women. In Figure 3, we have presented race-stratified cumulative risk for a second cancer in ispilateral and contralateral breasts. The difference in the cumulative risk of a second breast cancer between African-American and White-American women reached the level of statistical significance 8 years after the initial treatment. The age-adjusted 8-year disease-free probability in the ipsilateral breast, stratified by race and treatment, for women with negative margin status is presented in Figure 4. African-American women who were treated with BCS without radiation had the lowest while White-American women treated with BCS with radiation had the highest disease-free probability. The disease free probability was similar for African-American women treated with BCS with radiation and White-American women treated with BCS without radiation.
Table 5.
Adjusted risk for a second cancer in ipsilateral breast: Competing Risks Cox Proportional Hazard
| Variable | HR1 | 95% CI2 | P-value |
|---|---|---|---|
| Race | |||
| African-American vs. White-American | 3.96 | 1.42–11.04 | 0.01 |
| Final Treatment | |||
| Lumpectomy without radiation vs. mastectomy | 7.18 | 1.42 –36.36 | 0.01 |
| Lumpectomy with radiation vs. mastectomy | 4.08 | 0.85–19.44 | 0.08 |
| Age at diagnosis | 1.03 | 0.99–1.07 | 0.14 |
| Margin | |||
| Positive vs. negative | 0.96 | 0.44–2.07 | 0.91 |
| Presentation | |||
| Screening mammography vs. palpation/nipple discharge | 0.48 | 0.16–1.40 | 0.17 |
| Body Mass Index | 1.04 | 0.97–1.11 | 0.31 |
Figure 3.
Cumulative risk of a second cancer in ipsilateral and contralateral breasts, stratified by race
Figure 4.
Race and treatment stratified, predicted 8-year disease free probability in ipsilateral breast among women diagnosed with negative marginal status
DISCUSSION
The difference in the long-term risk of a second breast cancer between African-American and White-American women after the treatment of the first primary DCIS has been studied; however, findings have been conflicting due to several factors such as geographic location, institutional variations in sources of data and inadequate follow-up time. [7–9, 15–17] The patients in this study were diagnosed and treated by practitioners and procedures and policies within one single medical group. Our findings of comparable pathologic prognostic indicators between African-American and White-American women concur with previous reports. [7–10] However, this observation contradicts the overwhelming evidence that African-American women are diagnosed with more aggressive clinical and biological behavior of invasive breast cancer. [2, 3, 18] Most likely this reason is multifold. First, socioeconomic status and access barriers to health care may be one reason. [19, 20] More than 80% of the African-American and White-American women who contributed to this study were diagnosed with DCIS during their routine annual screening mammography. It is well accepted that tumors detected during screening mammography have more favorable pathologic prognostic indicators. Second, breast cancer in older women, generally, is considered as sporadic with more favorable histopathologic features. African-American women who contributed to this study were older with the majority was diagnosed at age 59 years.
Despite similar pattern of method of presentation of cancer and histopathology of cancer, our data suggest that African-American women in this sample experienced higher risk for a second ipsilateral breast cancer relative to White-American women. Lower screening mammography utilization by African-American women, delayed in surgery after the diagnosis of DCIS, and omission of radiation therapy for a higher proportion of African-American relative to White-American have been reported. [21, 22] In the sample of women in our study, about 80% of African-American and White-American women were diagnosed with DCIS during their annual screening mammography, suggesting equal access to screening. The study sample was insured through the capitated payment method (HMO health insurance), which may account for the comparable proportions of mammographically-detected DCIS and histopathologic features between African-American and White-American women. Similar rate of annual screening mammography between African-American and White-American women in other HMO settings also has been reported. [23] Furthermore, we did not find treatment disparities. In fact, the proportion of African-American women who had for radiation therapy following BCS was slightly higher. Yet, results from our analysis revealed that African-American women were at increased risk for a second cancer in the involved breast.
The widening racial disparity in breast cancer treatment out come in African-American women has been attributed to calendar time period, suggesting differential access to more novel and effective medical interventions. [24] Historically, breast cancer has been viewed as a uniform and hormonally responsive disease and therefore, clinical and therapeutic interventions, i.e. tamoxifen, were developed accordingly. [24] Women who contributed to this study were diagnosed with DCIS between 1990 and 1999, during the time period when the assessment of these markers were not recommended for diagnosis of DCIS and consequently were not components of patient care policies and clinical procedures. Limitations of clinical data do not permit making any statement about the underlying molecular perturbations and their potential contribution to the observed higher risk of a second in African-American women although others have reported that the triple negative subtype is identifiable in DCIS. [25]
Positive or close margins also have been reported to increase the risk of ipsilateral recurrences. [26, 27] In our study, we did not include the extent or the number of or the location of positive margins. Because of the small number of women with multifocal and extensive positive margin status, margins were dichotomized as either negative or positive. There is no consensus about the definition of negative margin [28, 29] and we applied the same College of American Pathologists diagnostic criteria to classify margins as either positive or negative; we do not expect differential misclassification of women since the two investigators who reviewed the diagnostic slides were masked to the racial/ethnic heritage of the women. In addition, surgical treatment and omission of radiation therapy also have been reported as important predictors for a second breast cancer. [27] In our analyses, we stratified African-American and White-American women by the type of surgery they received and whether or not they received radiation therapy and tamoxifen. However, for every stratum of treatment, African-American women experienced an elevated risk for a second cancer in ipsilateral breast relative to White-American women.
In a recent communication, Kreiger et al, using US national cancer registry data, demonstrated that the observed high ratio of estrogen receptor negative in African-American women relative to White-American women is a reflection of temporal changes in the use of post menopausal hormone. [30] The authors have argued that difference in the biology of breast cancer between African-American and White-American women should not be assumed as fixed and a reflection of intrinsic biological differences. We certainly do not deny the influence of post menopausal hormones on the incidence ratio of estrogen receptor positive to estrogen receptor negative tumors. However, estrogen receptor is one of the many markers that have been applied to genetically classify breast cancer into different subtypes. [31] Several reports confirm that the prevalence of basal-like subtype of breast cancer is higher in African-American women. [1, 32, 33] Also, the assertion of the higher prevalence of basal-like breast cancer in African-American should not be equated to or assumed as absence of the likelihood for the other subtypes of breast cancer. [21] We agree with the authors’ statement that the societal conditions shape the expression of observed biological characteristics, as it is well demonstrated by the higher prevalence of BRCA1 and BRCA2 breast cancer in Ashkenazi Jewish women. Consanguinity and intra-ethnic marriage, neither exclusive to one group nor to one culture, increases the probability for the aggregation of genetic perturbations and the phenotypic expression of such changes. Finally, the spectrum of genetic characteristics of complex diseases such as breast cancer has not been completely understood.
Our study has several strengths; first, all women were diagnosed and treated in one comprehensive health care system by the same group of practitioners under the same policies and procedures, therefore reduced the potential confounding effect of geographic differences and data variation. Second, because women were insured through the same HMO health insurance influence of access to screening mammography and delay in diagnosis of cancer was reduced. Our study has several limitations. First, because women were diagnosed at the time when evaluation of hormone receptors and HER2 biomarkers was not a component of standard diagnostic requirement, diagnostic data on these markers were not available. Additionally, our dataset was limited in distinguishing the true recurrence from a second primary breast cancer in the ipsilateral breast. Finally, because of the relatively small sample size, we dichotomized marginal status into positive and negative; therefore, we could not assess the extent or the number of or the location of positive margin on the risk of a subsequent second cancer in ipsilateral breast.
In conclusion, our results suggest that African-American women experienced a higher cumulative risk for a second cancer in the ipsilateral but not in contralateral breast. This excess risk in the ipsilateral was observed despite similar clinical presentation, histopathology and treatment. Understanding the molecular markers of DCIS should shed further light on the underlying reasons for the observed excess risk.
ACKNOWLEDGMENT
Partially was supported by a grant from the National Institute of Health/National Cancer Institute (R01 CA92444) and Geisinger Endowment for Research (ACR 500)
REFERENCES
- 1.Carey LA, Perou CM, Livasy CA, Dressler LG, Gowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston SL, Deming SL, Geradits J, Cheang MCU, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 2.Newman LA, Martin LK. Disparities in breast cancer. Curr Probl Cancer. 2007;31:134–156. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Newman LA, Bunner S, Carolin K, Bouwman D, Kosir MA, White M, Schwartz A. Ethnicity related differences in the survival of young breast cancer patients. Cancer. 2002;95:21–27. doi: 10.1002/cncr.10639. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance Epidemiology and End Results (SEER) [accessed on 5/4/2010]; SEER.cancer.gov/csr/1975_2007/browse_csr.php?section=4&page=sect_04_table.11.html;
- 5.Demicheli R, Retksy MW, Hrushesky WJM, Baum M, Gukas ID, Jatoi I. Racial disparities in breast cancer outcome; insights into host-tumor interactions. Cancer. 2007;110:1880–1888. doi: 10.1002/cncr.22998. [DOI] [PubMed] [Google Scholar]
- 6.Healthy People 2010. Understanding and improving health. Washington DC: US Department of Health and Human Services; 2000. [Google Scholar]
- 7.Nassar H, Sharafaldeen B, Visvanathan K, Visscher D. Ductal carcinoma in situ in African-American versus Caucasian-American women. Cancer. 2009;115:3181–3188. doi: 10.1002/cncr.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernester VL, Stewart K, Chew K, Moore DH, Waldman F. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–1702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 9.Warren JL, Weaver DL, Bocklage T, Key CR, Platz CE, Cronin KA, Ballard-Barbash R, Willey SC, Harlan LC. The frequency of ipsilateral second tumors after breast-conserving surgery for DCIS: a population based analysis. Cancer. 2005;104:1840–1848. doi: 10.1002/cncr.21406. [DOI] [PubMed] [Google Scholar]
- 10.Joslyn SA. Ductal carcinoma in situ; Trends in geographic, temporal, and demographic patterns of care and survival. Breast Journal. 2006;12:20–27. doi: 10.1111/j.1075-122X.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Malone KE, Saltzman BS and Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006;106:2104–2112. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables. J Royal Statist Society (series B, Methodological) 1972;34:187–220. [Google Scholar]
- 13.Hosmer D, Lemeshow S. Applied logistic regression. New York: John Wiley & Son; 1997. [Google Scholar]
- 14.Heckman JJ, Honoré BOE. The identifying of the competing risks models. Biometrika. 1989;76:325–330. [Google Scholar]
- 15.Komenka IK, Martinez ME, Pennington RE, Jr, Hsu CH, Clare SE, Thompson PA, Murphy C, Zork NM, Goulet RJ., Jr Race and ethnicity and breast cancer outcome in an underinsured population. J Natl Cancer Inst. 2010;102:1178–1187. doi: 10.1093/jnci/djq215. [DOI] [PubMed] [Google Scholar]
- 16.Dunn BK, Agurs-Collins T, Browne D, Lubet R, Johnson KA. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat. 2010;121:281–292. doi: 10.1007/s10549-010-0827-x. [DOI] [PubMed] [Google Scholar]
- 17.Innos K, Horn-Ross PL. Recent trends and racial/ethnic differences in the incidence and treatment of ductal carcinoma in situ of the breast in Californian women. Cancer. 2003;97:1099–1106. doi: 10.1002/cncr.11104. [DOI] [PubMed] [Google Scholar]
- 18.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwaetz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 19.Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471–473. doi: 10.1093/jnci/94.7.471. [DOI] [PubMed] [Google Scholar]
- 20.Freeman HP. Poverty, culture and social injustice: Determinants of cancer disparities. CA Cancer J Clin. 2004;54:72–77. doi: 10.3322/canjclin.54.2.72. [DOI] [PubMed] [Google Scholar]
- 21.Brawley OW. Is race really a negative prognostic factor for cancer? J Natl Cancer Inst. 2009;101:970–971. doi: 10.1093/jnci/djp185. [DOI] [PubMed] [Google Scholar]
- 22.Smith GL, Shih YCT, Xu Y, Giordano SH, Smith BD, Perkins GH, Tereffe W, Woodward WA, Buchholz TA. Racial disparities in the use of radiotherapy after breast-conserving surgery: A national Medicare Study. Cancer. 2010;116:734–741. doi: 10.1002/cncr.24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisch LM, Barton MB, Fletcher SW, Kreuter W, Elmore JG. Breast cancer screening use by African-Americans and Whites in an HMO. J Gen Intern Med. 2000;15:229–234. doi: 10.1111/j.1525-1497.2000.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jatoi I, Anderson WF, Rao SR and Devesa SS. Breast cancer trends among Black and White women in the United States. J Clin Oncol. 2005;23:7836–7841. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 25.Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, Tse CK, Nyante S, Millikan RC. Identification of a basal-like subtype of breast ductal carcinoma in situ. Human Pathology. 2007;38:197–204. doi: 10.1016/j.humpath.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Rudloff U, Brogi E, Reiner AS, Goldberg JI, Brockway JP, Wynveen CA, McCormick B, Patill S, Van Zee KJ. The influence of margin width and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Annals of Surgery. 2010;251:583–591. doi: 10.1097/SLA.0b013e3181b5931e. [DOI] [PubMed] [Google Scholar]
- 27.Dick AW, Sorbero MS, Ahrent GM, Hayman JA, Gold HT, Schiffhauer L, Stark A, Griggs JJ. Comparative effectiveness of ductal carcinoma in situ management and the roles of margins and surgeons. J Natl Cancer Inst. 2011;103:92–104. doi: 10.1093/jnci/djq499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margins status after breast conserving therapy. Results of a survey. Ann Surg. 2005;241:629–639. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 30.Krieger N, Chen JT, Waterman PD. Temporal trends in the black/white breast cancer case ration for estrogen receptor status: disparities are historically contingent, not innate. Cancer Causes Control. 2011;22:511–524. doi: 10.1007/s10552-010-9710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implication. PNAS. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millkan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-America and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]