Abstract
Objective
To investigate the effect of nutrient withdrawal on human intestinal epithelial barrier function (EBF). We hypothesized that unfed mucosa results in decreased EBF. This was tested in a series of surgical small intestinal resection specimens.
Design
Small bowel specifically excluding inflamed tissue, was obtained from pediatric patients (aged 2 days to 19 years) undergoing intestinal resection. EBF was assessed in Ussing chambers for transepithelial resistance (TER) and passage of FITC-Dextran (4kD). Tight junction and adherence junction proteins were imaged with immunofluorescence staining. Expression of Toll like receptors (TLR) and inflammatory cytokines were measured in loop ileostomy takedowns in a second group of patients.
Results
Because TER increased with patient age (p<0.01), results were stratified into infant versus teenage groups. Fed bowel had significantly greater TER versus unfed bowel (p<0.05) in both age populations. Loss of EBF was also observed by an increase in FITC-Dextran permeation in nutrient-deprived segments (p<0.05).
Immunofluorescence staining showed marked declines in intensity of ZO-1, occludin, Ecadherin and Claudin-4 in unfed intestinal segments, as well as a loss of structural formation of tight junctions. Analysis of cytokine and TLR expression showed significant increases in TNF-α and TLR4 in unfed segments of bowel compared to fed segments from the same individual.
Conclusion
EBF declined in unfed segments of human small bowel. This work represents the first direct examination of EBF from small bowel derived from nutrient-deprived humans and may explain the increased infectious complications seen in patients not receiving enteral feeds.
Keywords: epithelial barrier function, intestinal epithelial cells, parenteral nutrition, zonula occludens-1, occludin
INTRODUCTION
Parenteral nutrition (PN) is used commonly as treatment for many patients, ranging from short-term use in those with gastrointestinal dysfunction (1) to long-term use with short bowel syndrome (2). PN is administered in over 350,000 patients within the U.S. each year, and the usage has almost doubled in the last two decades (3). While life-saving for many, PN use is associated with numerous harmful sequelae, comprising a loss of immune reactivity, organ failure (4, 5), and an increased frequency of infectious complications (6–8). While investigations continue, the etiology of the increased prevalence of perioperative infectious complications is unknown.
Despite sustaining the host organism with sufficient energy and nutrient needs, total PN (TPN) places the intestine in an abrupt state of nutrient deprivation. Previous studies from our laboratory and others have shown substantial physical changes and immunologic imbalances in the intestinal mucosa using a murine model of TPN (9–14). Immunologically, a pro-inflammatory state develops within the gastrointestinal tract, including increased mucosal and intraepithelial lymphocyte-derived tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) (15, 16). More recently, using this same mouse model, our group has shown a marked increase in the abundance of Toll-like receptors (TLRs) within the lamina propria of the small bowel, and that these factors may be a critical signaling pathway for the mediation of this pro-inflammatory state (17).
The consequences of this pro-inflammatory state may be a critical element in the development of a variety of complications observed clinically in patients on PN, including infectious complications as well as secondary cholestatic changes (18, 19). In our mouse model, TPN administration results in distinct changes in the expression and distribution of tight junctional proteins (16, 20). The intercellular junctions formed by these proteins are crucial for maintenance of epithelial barrier function (EBF) as well as other functions, including modulating passage of electrolytes and nutrients (21–26). Changes in EBF are well described in enterally-deprived rodents receiving TPN, however, changes in EBF in humans maintained on TPN are poorly understood and scarcely documented. Buchman, van der Hulst and D’Antiga and colleagues (27)(28)(29) have each shown that the intestine undergoes mucosal atrophy in humans receiving TPN, although apparently at lesser rates than in rodent models. Buchman, et al. also demonstrated moderate increases in urinary lactulose-mannitol ratios that approached significance with a group of healthy adult volunteers receiving short-term TPN (27). While suggesting a loss of EBF, this important study failed to demonstrate the site at which this loss of EBF occurred. Additionally, the study did not examine the potential mechanisms which led to this increase in sugar permeation.
Other than these few studies, there is a paucity of literature describing EBF in humans receiving TPN. Furthermore, a direct investigation of the intestinal mucosa in such patients has not been performed. Such direct examination of the intestine is essential in order to gain mechanistic insight into any aberrant EBF in patients on TPN. Even though hard evidence of a decrease in EBF in humans is lacking, the detrimental impact of the loss of EBF has been well described (6, 8,30–32).
In the present work, we posited that enteric nutrient deprivation leads to loss of EBF in human small bowel. To study this, operative specimens from pediatric patients undergoing small bowel resections were collected, and a comparison between those segments which were exposed to enteral nutrients and those which were isolated from such nutrients was performed. EBF, as well as the changes in expression and distribution of tight junction and adherens junction proteins, were investigated. The study represents the first demonstration of a loss of the junctional integrity of the cell with nutrient deprivation. To further explore the role that intestinal inflammation plays in decreasing EBF, TLRs and pro-inflammatory cytokines, which could be produced secondary to TLR stimulation, were also measured. Despite inter-individual variability, the study shows the novel finding of a striking increase in mucosal TNF-α and TLR4 with nutrient deprivation, potentially representing a mechanistic path which might lead to changes in the tight junction found in this study.
Methods
Inclusion Criteria and Handling of Human Tissue
Experiments were done in accordance with University of Michigan Hospital Institutional Review Board, IRB #_HUM00024263. All pediatric patients with planned or emergent small bowel resections were considered. Patients with active inflammation (including Crohn’s disease), necrotizing enterocolitis, and acute ischemia or perforation were excluded, because each of these pathologies may intrinsically change EBF unrelated to their nutrient delivery (33–36). Feeding status was determined by chart review and discussion with family and the surgical team. Unfed specimens were labeled as such only if they were chronically devoid of enteral feeds (>14 days). Unfed specimens comprised two patient populations. The first were those who were unable to eat and thus were TPN-dependent. All patients in this group were on TPN for a minimum of 2 weeks, but in most cases greater than 2 weeks, before the small bowel specimens were obtained. The other unfed specimens were obtained from children who had either small bowel enterostomies or mucus fistulae in which the distal limb was isolated from enteric flow. Patients achieving nutritional goals through a combination of the enteral and parenteral route were excluded due to the degree of difficulty in stratifying partially-fed patients. Importantly, to avoid the inherent differences in EBF between jejunum and ileum, samples were limited to portions taken from mid-small bowel to terminal ileum.
In all cases, demographic data were recorded as well as whether or not the small bowel segment was in contact with enteral nutrients. All specimens were taken fresh to the Pathology Department from the operating room by the primary author. After brief evaluation by the pathologist, a portion of the specimen was cut and placed into RPMI 1640 with glutamine (Invitrogen, Carlsbad, CA) for immediate transport to the laboratory on ice. The tissue was divided into sections for analysis as permitted by the size of the tissue sample. For physiologic assessment of EBF, a fresh segment was selected and further divided for Ussing chamber mounting as described previously (37). A second segment underwent mucosal scrapping for mRNA analysis. A third segment was placed into optimum cutting temperature embedding compound (PELCO International, Redding, CA) and snap-frozen for subsequent immunofluorescence staining. A final segment was embedded in paraffin for histologic examination.
Physiologic EBF studies
Transepithelial resistance (TER) across the mucosal surface of the small bowel was assessed. A submucosal dissection was performed to strip the muscularis propria from the specimens. This mucosal/submucosal specimen was then mounted on P2307 Ussing chamber slider with a 0.03cm2 area and measured using a modified Ussing chamber (Physiologic Instruments, San Diego, CA). The tissue was allowed to equilibrate in 37°C Krebs buffer as described previously (38). TER was analyzed using instrument-associated software Acquire & Analyze v2.3 (Physiologic Instruments). To further investigate EBF, paracellular passage of fluorescein isothiocyanate (FITC)-Dextran (4000 MW, Sigma-Aldrich Co. LLC, St Louis, MO) was also quantified using previously described methods (39). Briefly, in addition to the buffer solution, 150µl of FITC-Dextran was added to the chamber interfaced with the mucosa. Samples (500µl) from the chamber interfaced with the submucosa were taken at 60, 90 and 120 min and snap frozen for later measurement of concentration using the SpectraMax M5 Multi-Mode Microplate Readers (Molecular Dynamics LLC, Sunnyvale, CA). Volume was replaced with fresh Krebs buffer.
Histology and Immunofluorescence microscopy
Paraffin-embedded specimens underwent hematoxylin and eosin (H&E) staining for histologic examination. To confirm a perceived increased density of goblet cells, we also performed period acid-Schiff (PAS) Alcian blue stains on selected samples. Immunofluorescence of OCT-embedded human tissue were challenging due to a rapid degradation and loss of immunofluorescence staining. To address this, tissues were embedded immediately in OCT compound and snap-frozen in liquid nitrogen. A modification of previous techniques allowed for an improvement in the detection of the immunofluorescence signal. Cryosections (8–10µm) were fixed, soaked in 2% formalin for 15 min, and washed three times with 1× Phosphate Buffered Saline (PBS) for 10 min. The samples were then incubated in blocking buffer (10% goat serum) for 1 h at room temperature, and incubated with primary antibodies diluted in 2% goat serum (mouse monoclonal anti-ZO-1 1:100, anti-occludin 0.6:100, anti-claudin-4 at 0.5:100 and anti-E-cadherin at 0.3:100 (Life Technologies, Invitrogen™, Carlsbad, CA) at 4°C overnight. After 3 additional 10 min washes in PBS, slides were incubated with corresponding secondary antibodies for 1 h and mounted with ProLong® Gold antifade reagent with 4’6-diamidino-2-phenylindole (DAPI) (Life Technologies). Slides were visualized using a Nikon A-1 Confocal fluorescence microscope.
Reverse transcriptase polymerase chain reaction (PCR) and Quantitative real-time PCR
Expression of Toll-like receptors (TLRs) and their eventual down-stream effector cytokines, TNF-α, IFN-γ, IL-1β and IL-6, were measured to assess inflammatory signaling within harvested mucosa. Relative expression of mRNA was measured using real time quantitative PCR as described previously (10, 40). Fold changes of target genes were calculated using comparative quantification to high abundance 18S rRNA. Primers were developed using basic local alignment search tool (BLAST) software by NCBI.
A group of 12 patients undergoing loop ileostomy takedown were studied for expression of TLRs and selected cytokines. This approach allowed the comparison of fed and unfed bowel from the same individual, thus subjected to the identical systemic scenario. The only difference in these paired samples was the exposure to luminal nutrition. The inflammatory data were averaged as a whole group, but inter-individual differences in expression became quickly apparent. Given the variability between patient samples, results are reported by paired analysis, thereby comparing fed versus unfed segments of the small intestine from the same patient.
Statistical Analysis
Results are expressed as mean ± standard deviation unless otherwise specified. Comparison between two groups used the unpaired, one-tailed t-test. For TLR and cytokine expression, a paired t-test was used. Linear regression analysis was used when comparing EBF with age in segments in contact with nutrition and those that were eternally deprived.
RESULTS
Demographics
Specimens were collected from January, 2011 to March, 2014. A complete summary of the patient demographic information is given in Table 1. A total of 31 patients, 22 male and 9 female, with a mean age of 11.5±10.4 (range 4 months to 39 years), resulted in 48 samples which were appropriate and sufficient for analyses. This comprised 27 fed samples and 21 unfed samples. The underlying pathology in the group included uninvolved small bowel from patients with ulcerative colitis (none with backwash ileitis, n=12), previous cases of necrotizing enterocolitis (all specimens were 2 months or more after their acute event and without active disease, n=6), Hirschsprung disease (n=4), gastroschisis (n=3), intestinal atresia (n=3), trauma (n=2), and malrotation with volvulus (n=1).
Table 1.
Demographics of 31 study patients.
| Number | Percent age |
Range | |
|---|---|---|---|
| Sex | |||
| Female | 9 | 29.0 | |
| Male | 22 | 71.0 | |
| Age | 11.5 ± 10.4 years | 4 months – 39 years | |
| Diagnosis | |||
| Ulcerative colitis (UC) | 12 | 38.7 | |
| Necrotizing enterocolitis (NEC) | 6 | 19.4 | |
| Hirschsprung disease (HD) | 4 | 12.9 | |
| Gastroschisis | 3 | 9.7 | |
| Intestinal atresia | 3 | 9.7 | |
| Trauma | 2 | 6.5 | |
| Volvulus | 1 | 3.2 | |
| Time Since Treatment of Primary Disease | 13.1 ± 26.7 months | 6 weeks – 11 years | |
Due to the limited size of each surgical sample, tissue was dedicated to either EBF studies or TLR and cytokine analysis. Among samples collected for EBF analysis, four of the unfed patients were TPN-dependent and devoid completely of enteral nutrition. Five patients had an ileostomy (with complete distal diversion) and were undergoing operation to close their stoma. This approach resulted in more samples than patients and allowed in several patients for direct comparison of fed and unfed segments in the same individual at the same time point. All samples collected for cytokine and TLR abundance measurement were obtained from takedown of a loop ileostomy which proved particularly important due to individual variability in the expression of these factors. The proximal stoma was in continuity with enteric flow, whereas the distal segment (mucus fistula) was isolated from enteric contents. In these latter cases, the bowel was defined as unfed, or enterally-deprived. A description of these latter patients is given in Table 2.
Table 2.
Patients with Loop Ileostomies for Paired Analysis
| Patient Number | Age | Sex | Diagnosis |
|---|---|---|---|
| 1 | 18 years | male | UC |
| 2 | 39 years | male | UC |
| 3 | 17 years | male | trauma |
| 4 | 17 years | male | UC |
| 5 | 39 years | female | UC |
| 6 | 11 years | female | UC |
| 7 | 18 years | male | UC |
| 8 | 19 years | female | UC |
| 9 | 18 years | male | HD |
| 10 | 4 months | male | NEC |
| 11 | 12 months | female | gastroschisis |
| 12 | 6 months | male | HD |
Studies of Epithelial Barrier Function
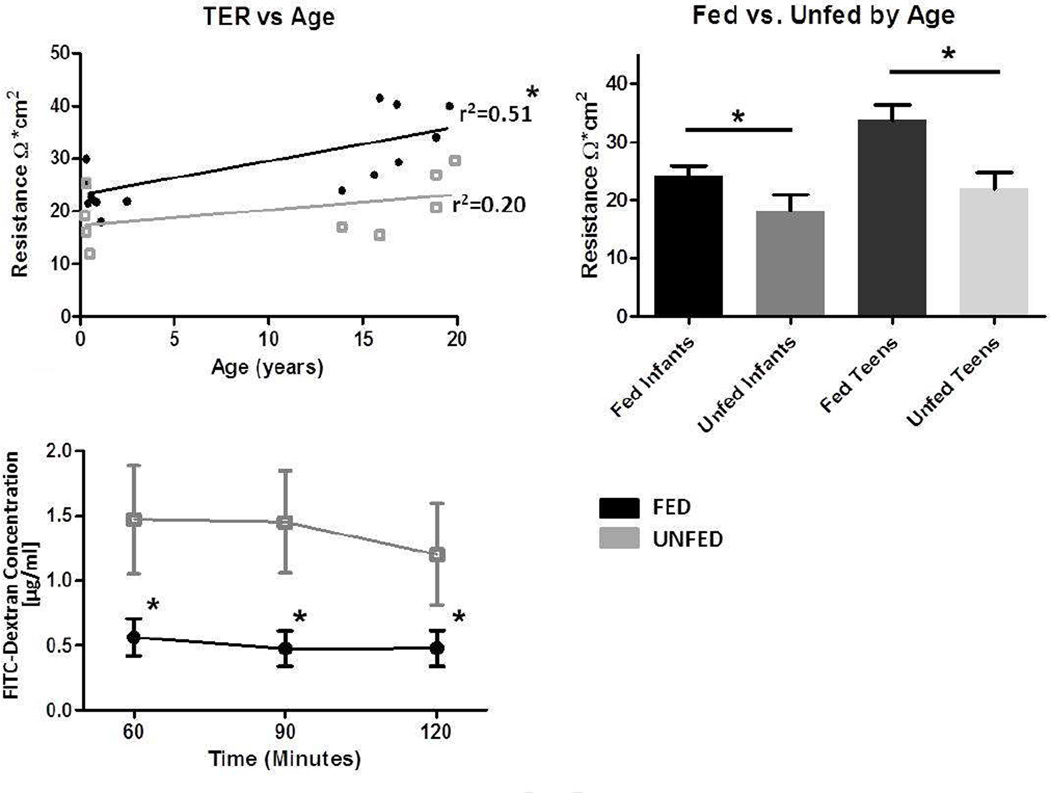
Several approaches were taken to examine EBF in our human specimens to ensure detection of changes at the physiologic level as well as the distribution and amount of selected junctional proteins. First, the intestinal epithelial barrier of the small bowel was assessed by measuring TER in fed and unfed segments using Ussing chambers. Because of a wide variation, and an observed greater TER in specimens from older children, we first plotted TER against patient age. Figure 1A shows that in fed samples, TER significantly increased with patient age (r2=0.51). This also trended in a similar manner, but to a lesser degree, for unfed bowel (r2=0.20). This observation has been reported by others (41), and greatly influenced our subsequent analysis of EBF results. To address this age-related change in TER, the results were then stratified by patient age into two groups: infants (2 months to 2.5 years) or teenagers (13–19 years). Figure 1B shows TER data as stratified by these two age groups. This analysis showed increased TER with age, but more importantly, the results demonstrated that enterally-fed small bowel segments had greater TER compared to unfed bowel segments in both the infant (24.2±4.4 vs. 18.1±5.6 Ohms• cm2) and teenager (33.7±7.10 vs. 22.0±6.2 Ohms•cm2) groups (p<0.05).
Figure 1.
Physiologic measurement of distal small bowel barrier function was performed in Ussing chambers. Mounted mucosa and submucosa specimens (post-dissection of the muscularis propria) were studied in triplicate, and a minimum of N=4 humans were studied for each group. A. Measurements of transepithelial resistance (Ω*cm2) of epithelial barrier function in Fed (dark gray upper line) vs. Unfed (light gray lower line) small bowel groups. Regression lines show a significantly increased resistance in fed patients with increasing age, and a similar trend toward increased resistance in unfed specimens from older patients. B. Age-stratified transepithelial resistance in Fed vs. Unfed small bowel (*P<0.05 by t test). C. FITC-Dextran-4000 paracellular permeability was assessed by placement of the tracer molecule on the mucosal side with interval sampling from the submucosal side (muscularis and serosa were stripped). Results are shown in subgroups (N=6/group) from fed and unfed segments of matched patients. Note the greater permeation (P<0.05) in unfed segments at each time point measured.
We next examined paracellular permeation using the tracer molecule dextran-4000 labeled with FITC. Interestingly, unlike TER, FITC-Dextran permeation did not correlate with the age of the patient. The enterally-deprived samples had consistently greater FITC-Dextran permeation compared to those exposed to luminal nutrients (Figure 1C; 60 minutes 0.56±0.14 vs. 1.47±0.42 µg/ml, P=0.03; 90 minutes 0.48±0.14 vs. 1.45±0.39 µg/ml, P=0.02; and 120 minutes 0.48±0.14 vs. 1.20±0.39 µg/ml, P=0.04).
Microscopy
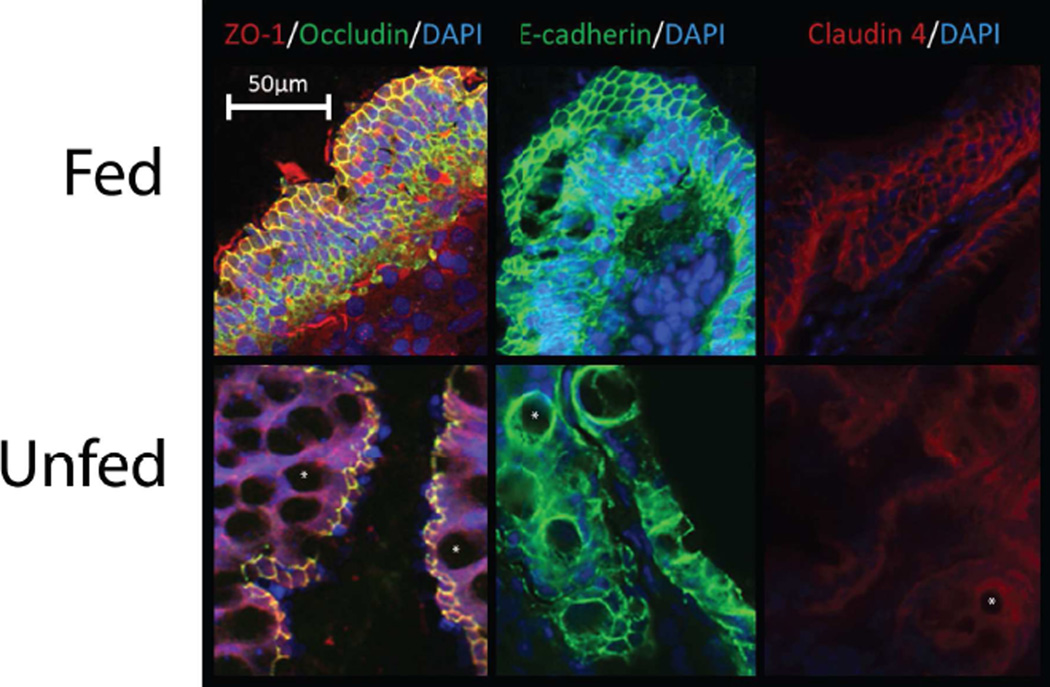
To better examine the structural distribution and integrity of the tight junction and adherens junction in our surgical specimens, a series of immunofluorescence stainings was performed. Representative immunofluorescence images are shown in Figure 2. Enterally-fed specimens showed consistently staining of selected tight junction and adherens junction proteins to the apical/lateral portion of the cell membrane, forming a tight lattice appearance, demonstrating an intact epithelial barrier. Marked decreases in the intensity of ZO-1 and occludin staining were observed in unfed samples. Interestingly, at greater magnification, an internalization of ZO-1 protein was noted within the cytoplasm of unfed specimens. A similar loss of staining intensity was seen with E-cadherin and Claudin-4 proteins. Thus, this marked deterioration in the microscopic integrity of both tight junction and adherens junction correlated closely with our physiologic measures. We also noted a loss of villus height and an increase in the abundance of goblet cells in the immunofluorescence specimens. To address this, we examined standard microscopic specimens.
Figure 2.
Representative immunofluorescence images from samples taken from Fed and Unfed portions of the small bowel for ZO-1 and Occludin, E-Cadherin, and Claudin-4, with DAPI nuclear counterstain. Note the loss of the tight lattice formation in Unfed segments, along with an internalization of ZO-1 into the cytoplasm. Note also the relative increase in goblet cells within the epithelial lining (some of which are marked with white asterisks).
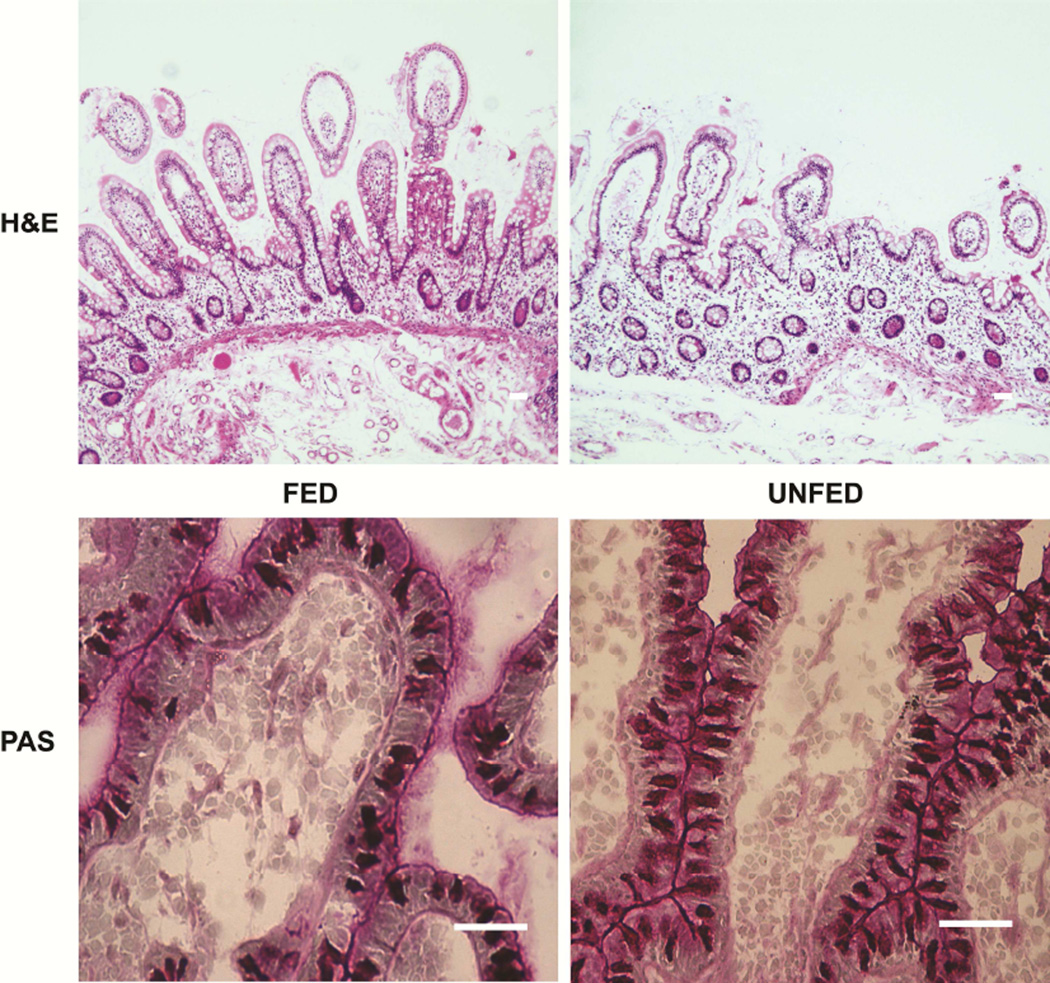
Morphologically, villus atrophy was seen in unfed samples on standard hematoxylin and eosin staining (Figure 3). Morphologic measurements of crypt depth, did not significantly differ (96±23 µm versus 80±25 µm, fed versus non-fed, respectively, P>0.05). Villus height was not measured because consistently well-oriented villi could not be identified adequately, but Figure 3 shows a decline in villus height which appeared to be present subjectively in all unfed specimens. To examine the perceived increase in goblet cells, Periodic acid-Schiff staining was performed. Although not quantified due to the relatively small number of samples, it was also interesting to note a large increase in the number of goblet cells relative to other epithelial cells in unfed specimens compared to fed ones (Figure 3).
Figure 3.
Representative histologic sections of Fed and Unfed sections of bowel. Hematoxylin and eosin (H&E) staining demonstrates villus flattening in unfed intestine, while periodic acid-Schiff (PAS) staining shows an increase in goblet cells. Scale = 50 µm.
Toll-Like Receptor and Inflammatory Cytokine Abundance
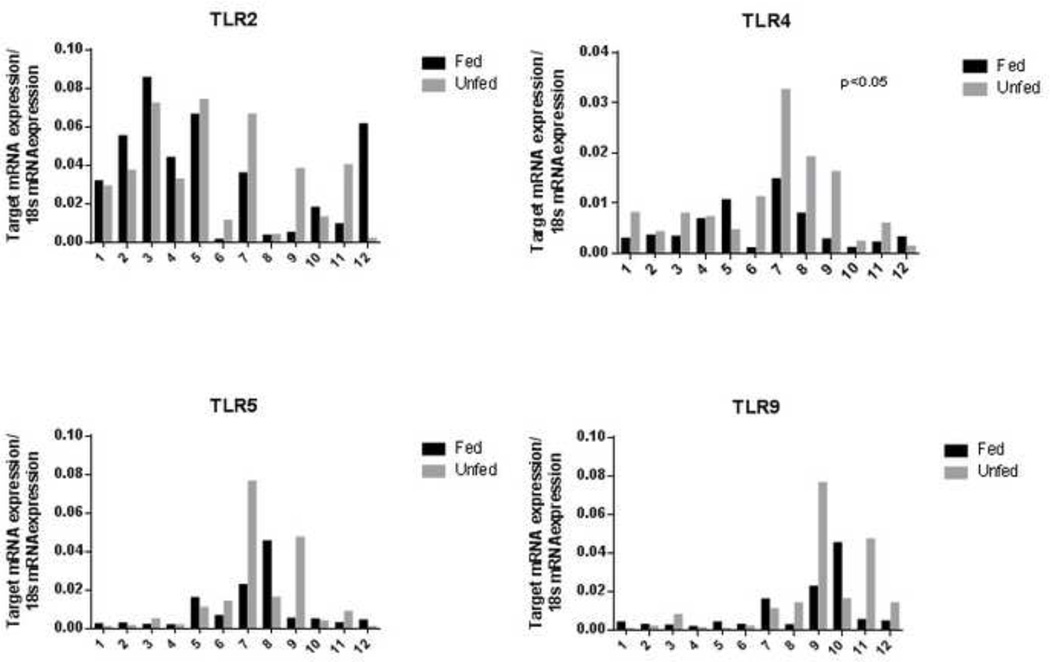
TNF-α and other pro-inflammatory cytokines have been shown to contribute to loss of EBF through the activation of myosin light chain kinase and disruption of several tight junction molecules from the junctional complex (42–45). Because a major signaling pathway for the activation of these pro-inflammatory cytokines is via TLRs, we examined if the breakdown of the intestinal EBF was associated with changes in TLR and pro-inflammatory cytokine abundance. mRNA expression of TLR2, TLR4, TLR5, and TLR9 were measured in fed and unfed mucosal samples. Initial analysis comparing mean abundances between these two groups showed no significant differences (TLR2: 0.034±0.028 vs 0.035±0.025, P=0.99; TLR4: 4.9E-3±4.2E-3 vs 9.9E-3±8.9E-3, P=0.09; TLR5: 9.3E03±0.013 vs 0.015±0.023, P=0.45; TLR9: 9.2E-3±0.013 vs 0.016±0.023, P=0.39; fed and unfed groups, respectively). It was clear, however, that there was a substantial variation in the expression of these receptors between individual patients. Therefore, a sub-analysis was performed on 12 patients (Table 2) from which both a fed and unfed portion of bowel was available, allowing paired t-test comparison (Figure 4). Although there was wide variation between patients in the expression of the genes that encode the TLRs, paired analysis revealed marked relative increases in TLR expression in unfed bowel relative to patient-matched fed samples. The mean difference in expression of TLRs was 2.0E-4±2.5E-2, 5.0E-3±6.9E-3, 5.9E-3±2.2E-2, and 6.6E-3±2.2E-2 for TLR-2, -4, -5 and -9 respectively. Within this paired analysis group, an increase in expression was found for TLR-4 (P=0.03), while TLR-2 (P=0.98), TLR-5 (P=0.37), and TLR-9 (P=0.32) remained non-significant statistically.
Figure 4.
mRNA levels of Toll-like receptors (TLRs) from 12 individuals from whom fed and unfed specimens were collected. When grouped analysis was performed, no statistical significance was achieved between patient, however, paired analysis, as shown, demonstrated a significant increase in TLR4 expression in unfed bowel.
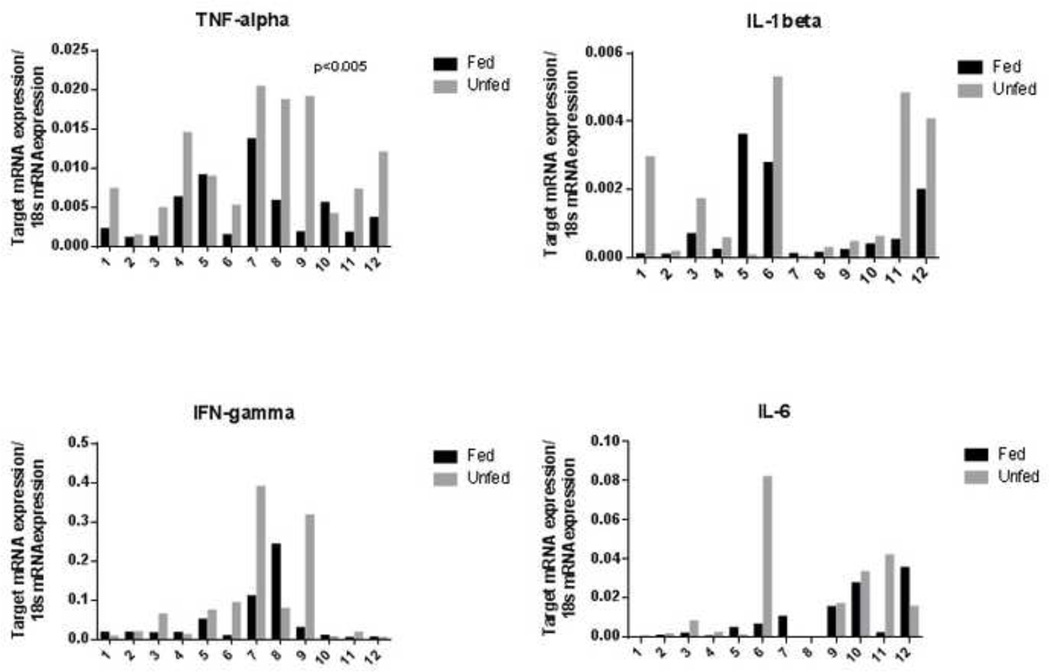
We next examined pro-inflammatory cytokines potentially elicited by TLR signaling. Congruent with the TLR findings, marked variability existed between patients in cytokine expression. Nonetheless, mean TNF-α abundance was increased in unfed compared to fed bowel on unpaired analysis (4.4E-3±3.8E-3 vs. 10.3E-3±6.4E-3, P=0.01; fed vs. unfed). Unpaired analysis of remaining cytokines did not show significant differences between groups (IFN-γ: 0.043±0.069 vs. 0.089±0.13, P=0.28; IL-6: 8.6E-3±0.012 vs. 0.017±0.025, P=0.31; and IL1β: 8.9E-4±1.2E-3 vs. 1.7E-3±2.0E-3, P=0.22; fed and unfed groups, respectively). A paired analysis was again performed on patientmatched fed and unfed portions of bowel (Figure 5). Although there were notable inter-individual differences in cytokine levels, paired analysis, as with the TLR data, revealed marked relative differences in cytokine expression in unfed bowel relative to patient-matched fed samples. The mean increase in expression of cytokines was 5.9E-3±5.4E-3 (P=0.003) for TNF-α; 4.6E-2±1.3E-1 (P=0.23) for IFN-γ; 8.2E-3±2.5E-2 (P=0.29) for IL-6; and 8.5E-4±2.0E-3 (P=0.16) for IL-1β. While TNF-α demonstrated a significant increase in unfed samples, significance was not reached in the paired analyses of other cytokines.
Figure 5.
mRNA levels of inflammatory cytokines from 12 patients from whom fed and unfed specimens were collected. Substantial inter-individual variability is apparent. Unfed bowel segments demonstrated a significant increase in TNF-α expression versus patient-matched fed segments. IL-1β and IFN-γ expression was increased in 10 of the 12 patients, but not in 2 patients (patients 6 and 8). See Table 2 for individual patient characteristics.
DISCUSSION
In the present study, we demonstrated a significant loss of intestinal EBF in nutrient-deprived pediatric small intestine using a number of different approaches. First, we measured TER in specimens from patients receiving enteral nutrition and compared these to segments of bowel that were devoid of luminal nutrients. We found an overall decrease in TER in the samples deprived of enteral nutrition compared to enterally-fed samples when matched for age. This finding correlated with increased paracellular passage of the tracer molecule FITC-Dextran. The decline in EBF was thought to be due to an aberrant function of tight junction and adherence junction proteins and was examined via immunofluorescence staining. Immunofluorescence images showed that the distribution and intensity of these proteins were decreased markedly in unfed samples.
This is the first study to definitively demonstrate mechanistic evidence and solid proof in humans that small bowel EBF is lost with the absence of enteral nutrition. The loss of the integrity and structure of the tight and adherens junctions in unfed segments correlated quite closely with the physiologic measures performed. Although not studied in this work, it may well be that this loss of EBF contributes to the known increased rate of bacterial translocation and increased infectious complications seen in patients receiving TPN (6, 27,29, 38, 46). Importantly, we observed this loss of EBF in patients on TPN as well as in segments of small bowel that were isolated from the normal continuity of enteric flow. Thus, this investigation supports the theory that the removal of enteral nutrients, rather than the administration of TPN, is responsible for the decrease in EBF. Again, this decrease in EBF was demonstrated most clearly in patients with loop enterostomies in which fed and unfed samples could be studied from the same patient. Based on the differential function of the epithelial barrier in the same patient, it appears that intraluminal factors rather that systemic signaling are responsible for the decline in EBF.
The mechanisms by which the removal of nutrients leads to a loss of EBF are unclear. To address this, we examined the TLR expression, because TLR signaling is one of the major pathways by which the intestinal mucosa senses changes in the luminal environment (47). Further, there is an emerging theory that shifts in the microbiota in unfed portions of intestine lead to inflammation and perhaps barrier breakdown. Recently, we reported that humans with enteral nutrient deprivation showed a change in the microbial population compared to fed segments of small bowel (48), including an expansion of many Gram-negative organisms which could well signal via TLR-4. While we did not report the microbial data from this group of patients, the finding of an increased expression of TLR-4 would be consistent with our previous publication. Thus, we speculate that an altered microbial population may result in signaling via TLRs in unfed small bowel segments. Further, we showed recently in a mouse model of TPN that blockade of MyD88 signaling led to a marked improvement in EBF, suggesting the importance of this signaling pathway in this process (17).
Our investigation of TLR and cytokine expression in fed and unfed specimens first revealed marked baseline variation in these inflammatory mediators between patients. This is a key finding, because these changes have ramifications in the interpretation cytokine and TLR data from human samples in diverse inflammatory diseases – where pooling samples may hide significant differences due to inter-patient variability (49). Upon paired analysis, we found significantly increased expression of TLR-4 and TNF-α in unfed samples compared to matched fed samples. Notable trends toward increased expression in unfed limbs were evident, however – especially for IL-1β and IFN-γ, where all but one patient demonstrated increases in unfed bowel. However, the values were not significant. As well, the demographic data showed that there were no differences in age, sex, or diagnosis. Therefore these patients were not excluded from analysis. Clearly additional analysis of larger samples will need to be done to better understand these cytokine changes.
An up-regulation of TNF-α and IFN-γ have been well recognized in a mouse model of TPN to drive a loss of EBF by disrupting the integrity of junctional proteins as well as the internalization of tight junction proteins (50–52). This study provides evidence that such an increase in pro-inflammatory cytokines may contribute to the loss of EBF observed in human patients.
Admittedly, enteral contents contain more than bacteria that may signal the epithelium. Sodhi et al. noted that bile acids play a critical role in cell lineage differentiation and gut health (53). Other dietary nutrients such as glutamine, the preferred fuel source of enterocytes, and polyunsaturated fats are absent or decreased in these enterally-deprived segments, and these changes may well contribute to the loss of EBF (54, 55). While hormonal mechanisms may also affect intestinal atrophy with nutrient deprivation, the fact that we observed a similar decrease in EBF in samples from TPN-dependent patients as well as in unfed distal segments from fed patients suggests nutrient deprivation to a particular segment of the intestine may be more important mechanistically. Our observation of an increase in goblet cells with nutrient deprivation may represent an atrophy of other epithelial cells or may be a true expansion of goblet cells potentially to serve to promote additional barrier function which is lost at the tight junctional level. Future studies are required to quantify the extent of goblet cell expansion. Interestingly, this same observation was found in a pig model of TPN (56).
The current study does have several limitations. As with many pediatric human studies, this is a relatively small and heterogeneous patient group. Each disease process, however, was selected carefully to exclude confounding conditions which would affect EBF, including active obstruction or inflammatory conditions. Concrete conclusions from the TLR and cytokine analysis might be inappropriate, but the observation of a nearly universal increase, albeit selectively, in the inflammatory signaling in paired samples supports our hypothesis. Additionally, although we did not investigate the microbiota, using 454 pyrosequencing of the mucosally associated bacteria because it would require larger tissue specimens, we have recently shown with other clinical specimens that enteral nutrient deprivation produces significant changes to the human microbiome (48). Bacterial translocation in the human samples was also not assessed, because mesenteric lymphoid tissue was typically not available for testing. Finally, the limited amount of tissue available (often less than 1 cm2) prevented us from understanding which cell populations within the small bowel expressed the observed changes in either TLRs or cytokines. Future studies will be necessary to delineate further the underlying mechanisms driving these changes in TLR and cytokine expression, as well as downstream intracellular signaling. Such studies will hopefully allow us to better understand the complex mechanisms which drive this loss of EBF with the absence of enteral nutrition.
Acknowledgements
Pele J. Browner and Hong K. Yoon handled many of the samples after initial processing and their contributions are appreciated.
Funding: This research was supported in part by NIH-R01 AI-44076-15 (to DHT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no relevant disclosures.
Authorship contribution (all have given final approval of this draft):
Matthew W. Ralls: Conceptualization, conducted bulk of studies, data interpretation and manuscript preparation.
Farokh R. Demehri: Conducted many of the PCR studies, data interpretation and manuscript preparation
Yongjia Feng: Conducted many of the PCR studies, assisted with manuscript preparation and data interpretation
Kathleen Woods Ignatoski: Study construction and data interpretation.
Daniel H. Teitelbaum: Study conceptualization, data interpretation and manuscript preparation.
REFERENCES
- 1.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378–386. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47(Suppl 1):S33–S36. doi: 10.1097/MPG.0b013e3181819007. [DOI] [PubMed] [Google Scholar]
- 3.(AHRQ) AfHRaQ, editor. 2010. HCUPnet National Center for Health Statistics Data on PN and EN Use. [Google Scholar]
- 4.Torres C, Sudan D, Vanderhoof J, Grant W, Botha J, et al. Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J Pediatr Gastroenterol Nutr. 2007;45:204–212. doi: 10.1097/MPG.0b013e31805905f9. [DOI] [PubMed] [Google Scholar]
- 5.Cowles RA, Ventura KA, Martinez M, Lobritto SJ, Harren PA, et al. Reversal of intestinal failure-associated liver disease in infants and children on parenteral nutrition: experience with 93 patients at a referral center for intestinal rehabilitation. J Pediatr Surg. 2010;45:84–87. doi: 10.1016/j.jpedsurg.2009.10.014. discussion 87–88. [DOI] [PubMed] [Google Scholar]
- 6.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 7.Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition. 1995;11:339–344. [PubMed] [Google Scholar]
- 8.Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, et al. Effect of parenteral and enteral nutrition on gut-associated lymphoid tissue. Journal of Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G833–G841. doi: 10.1152/ajpgi.00030.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–G147. doi: 10.1152/ajpgi.00386.2007. PMID:17991705. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G629–G637. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Sun X, Yang H, Teitelbaum D. Dissociation of E-Cadherin and betacatenin in a mouse model of total parenteral nutrition: A mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol (London) 2009;587:641–654. doi: 10.1113/jphysiol.2008.162719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto K, Fukatsu K, Hashiguchi Y, Ueno H, Shinto E, et al. Lack of Preoperative Enteral Nutrition Reduces Gut-Associated Lymphoid Cell Numbers in Colon Cancer Patients: A Possible Mechanism Underlying Increased Postoperative Infectious Complications During Parenteral Nutrition. Annals of Surgery. 2012 doi: 10.1097/SLA.0b013e31827a0e05. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Teitelbaum DH. Epidermal Growth Factor/TNF-alpha Transactivation Modulates Epithelial Cell Proliferation and Apoptosis in a Mouse Model of Parenteral Nutrition. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Teitelbaum DH. TNF-alpha-Induced Loss of Intestinal Barrier Function Requires TNFR1 and TNFR2 Signaling in a Mouse Model of Total Parenteral Nutrition. J Physiol. 2013 doi: 10.1113/jphysiol.2013.253518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyasaka EA, Feng Y, Poroyko V, Falkowski NR, Erb-Downward J, et al. Total Parenteral Nutrition-Associated Lamina Propria Inflammation in Mice Is Mediated by a MyD88-Dependent Mechanism. J Immunol. 2013;190:6607–6615. doi: 10.4049/jimmunol.1201746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Annals of Surgery. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. discussion 511-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Kasmi KC, Anderson AL, Devereaux MW, Fillon SA, Harris JK, et al. Tolllike receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology. 2012;55:1518–1528. doi: 10.1002/hep.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nose K, Yang H, Sun X, Nose S, Koga H, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res. 2010;30:67–80. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderholm JD, Hedman L, Artursson P, Franzen L, Larsson J, et al. Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol Scand. 1998;162:47–56. doi: 10.1046/j.1365-201X.1998.0248f.x. [DOI] [PubMed] [Google Scholar]
- 23.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 24.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 25.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 26.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185–190. [PubMed] [Google Scholar]
- 27.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Hulst R, Von Meyenfeldt M, Van Kreel B, Thunnissen F, Brummer R, et al. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition. 1998;14:1–6. doi: 10.1016/s0899-9007(97)00385-7. [DOI] [PubMed] [Google Scholar]
- 29.D'Antiga L, Dhawan A, Davenport M, Mieli-Vergani G, Bjarnason I. Intestinal absorption and permeability in paediatric short-bowel syndrome: a pilot study. J Pediatr Gastroenterol Nutr. 1999;29:588–593. doi: 10.1097/00005176-199911000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. discussion 511-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiristioglu I, Antony P, Fan Y, Forbush B, Mosley RL, et al. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Digest Dis Sci. 2002;47:1147–1157. doi: 10.1023/a:1015066813675. [DOI] [PubMed] [Google Scholar]
- 32.Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. Journal of Surgical Research. 1998;79:91–96. doi: 10.1006/jsre.1998.5408. [DOI] [PubMed] [Google Scholar]
- 33.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 34.John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal. 2011;15:1255–1270. doi: 10.1089/ars.2011.3892. [DOI] [PubMed] [Google Scholar]
- 35.Salim SY, Soderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 36.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, et al. The Epithelial Barrier Is Maintained by In Vivo Tight Junction Expansion During Pathologic Intestinal Epithelial Shedding. Gastroenterology. 2011;140:1208-+. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Critical Care Medicine. 2003;31:1118–1125. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 39.Bucker R, Krug SM, Rosenthal R, Gunzel D, Fromm A, et al. Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J Infect Dis. 2011;204:1283–1292. doi: 10.1093/infdis/jir504. [DOI] [PubMed] [Google Scholar]
- 40.Yang H, Gumucio DL, Teitelbaum DH. Intestinal specific overexpression of interleukin-7 attenuates the alternation of intestinal intraepithelial lymphocytes after total parenteral nutrition administration. Ann Surg. 2008;248:849–856. doi: 10.1097/SLA.0b013e31818a1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udall JN, Pang K, Fritze L, Kleinman R, Walker WA. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr Res. 1981;15:241–244. doi: 10.1203/00006450-198103000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 43.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-gamma and TNF-alpha-Induced Intestinal Epithelial Barrier Dysfunction by Berberine via Suppression of MLCK-MLC Phosphorylation Signaling Pathway. PLoS One. 2013;8:e61944. doi: 10.1371/journal.pone.0061944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–G147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 47.Kubinak JL, Round JL. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012;8:e1002785. doi: 10.1371/journal.ppat.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralls MW, Miyasaka E, Teitelbaum DH. Intestinal Microbial Diversity and Perioperative Complications. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113486482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho P, Gelinas L, Corbett NP, Tebbutt SJ, Turvey SE, et al. Association of common single-nucleotide polymorphisms in innate immune genes with differences in TLR-induced cytokine production in neonates. Genes Immun. 2013 doi: 10.1038/gene.2013.5. [DOI] [PubMed] [Google Scholar]
- 50.Li GZ, Wang ZH, Cui W, Fu JL, Wang YR, et al. Tumor necrosis factor alpha increases intestinal permeability in mice with fulminant hepatic failure. World J Gastroenterol. 2012;18:5042–5050. doi: 10.3748/wjg.v18.i36.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, et al. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 52.Ueno C, Fukatsu K, Maeshima Y, Moriya T, Omata J, et al. Arginine-enriched total parenteral nutrition improves survival in peritonitis by normalizing NFkappaB activation in peritoneal resident and exudative leukocytes. Ann Surg. 2010;251:959–965. doi: 10.1097/SLA.0b013e3181d775ea. [DOI] [PubMed] [Google Scholar]
- 53.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143:708–718. e701–e705. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann N Y Acad Sci. 2009;1165:267–273. doi: 10.1111/j.1749-6632.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 55.Nose K, Yang H, Sun XY, Nose S, Koga H, et al. Glutamine Prevents Total Parenteral Nutrition-Associated Changes to Intraepithelial Lymphocyte Phenotype and Function: A Potential Mechanism for the Preservation of Epithelial Barrier Function. J Interf Cytok Res. 2010;30:67–79. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conour J, Ganessunker D, Tappenden K, Donovan S, Gaskins H. Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1185–G1196. doi: 10.1152/ajpgi.00097.2002. [DOI] [PubMed] [Google Scholar]